FTO Regulates Apoptosis in CPB2-Treated IPEC-J2 Cells by Targeting Caspase 3 Apoptotic Protein
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Statement of Ethics
2.2. Sample Collection
2.3. Preparation of the CPB2 Toxin Protein
2.4. Cell Culture and Treatment
2.5. Cell Transfection
2.6. Total m6A Measurement
2.7. Determination of Cell Morphology and Viability
2.8. Quantitative Real-Time Reverse Transcription Polymerase Chain Reaction (qRT-PCR) Analysis
2.9. RNA Stability
2.10. Western Blotting
2.11. Flow Cytometry
2.12. Statistical Analysis
3. Results
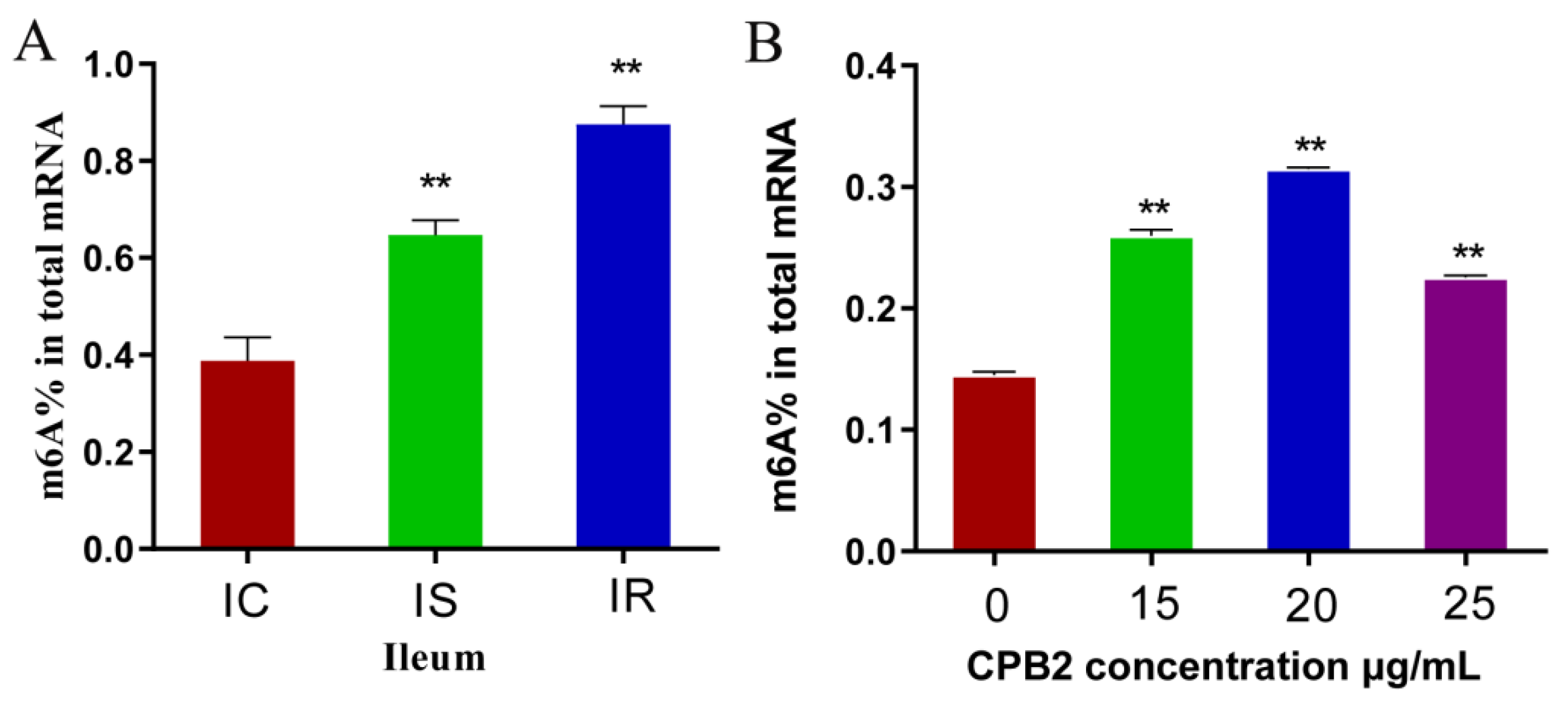
3.1. Total m6A Contents Are Upregulated in Ileal Tissue and IPEC-J2 Cells
3.2. CPB2 Treatment Downregulated FTO in IPEC-J2 Cells
3.3. FTO Influenced the Viability of CPB2-Treated IPEC-J2 Cells
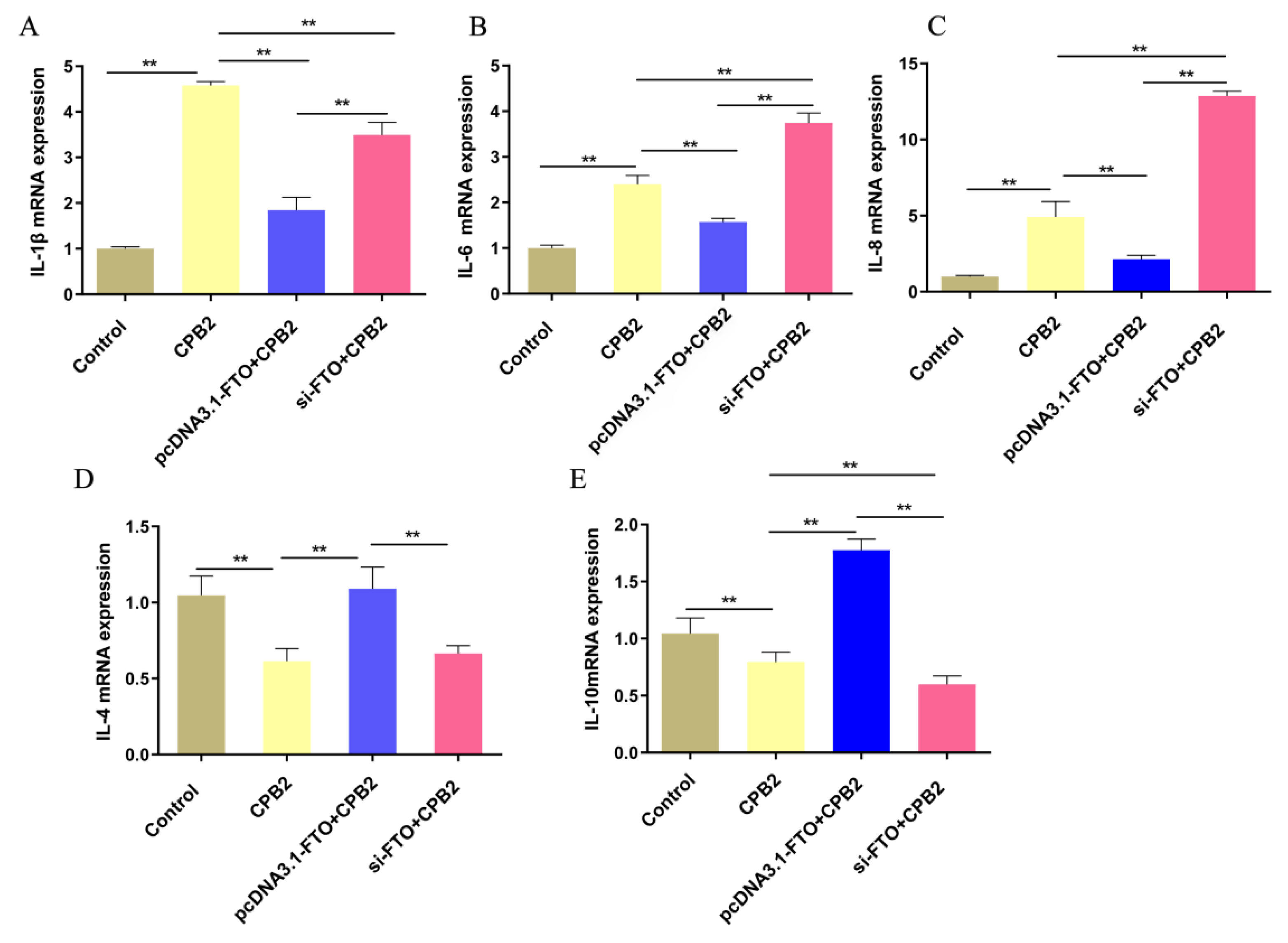
3.4. The Effects of FTO on the Expression of Inflammatory Markers in CPB2-Treated IPEC-J2 Cells
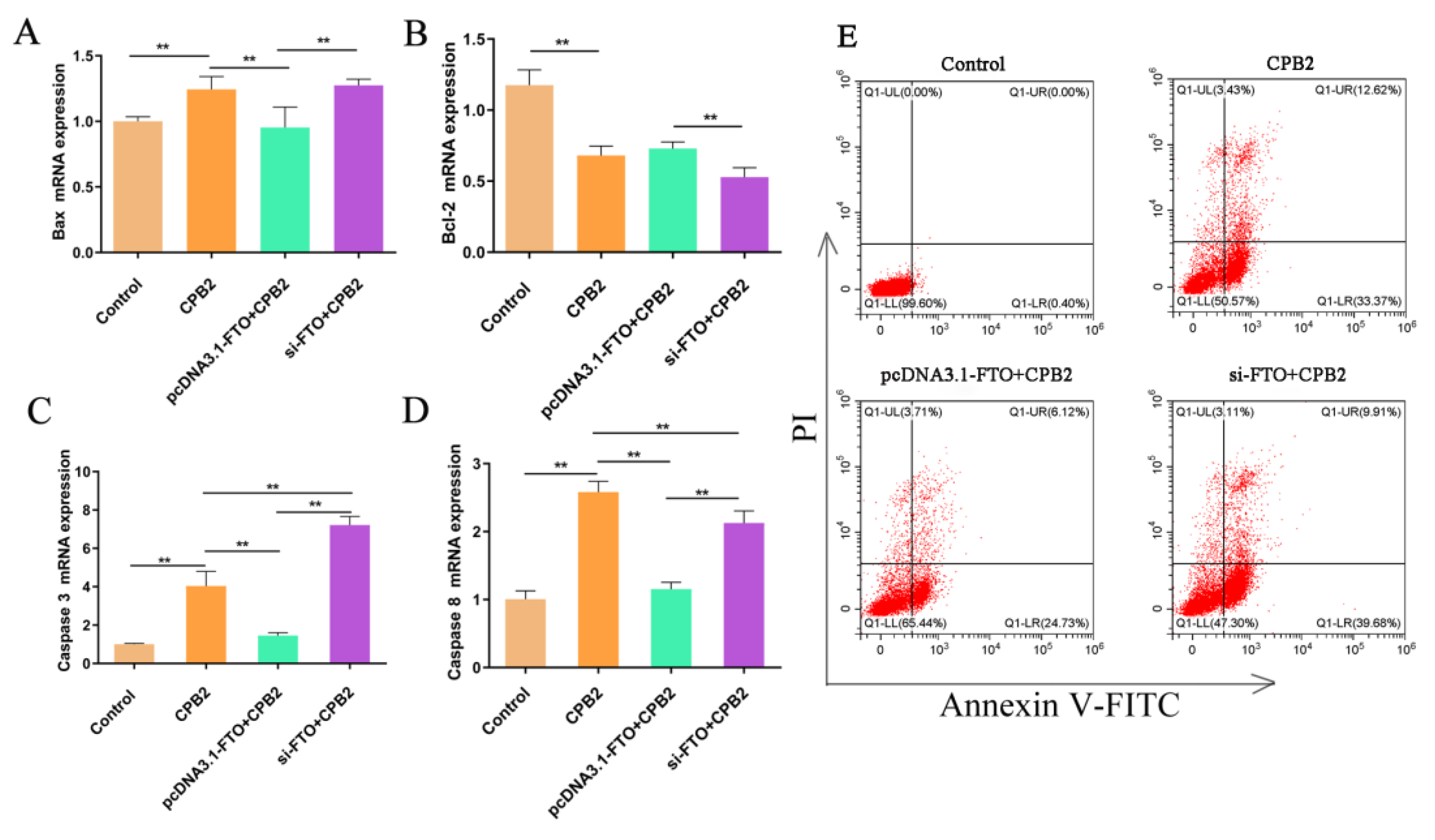
3.5. The Effects of FTO on CPB2-Induced Apoptosis in IPEC-J2 Cells
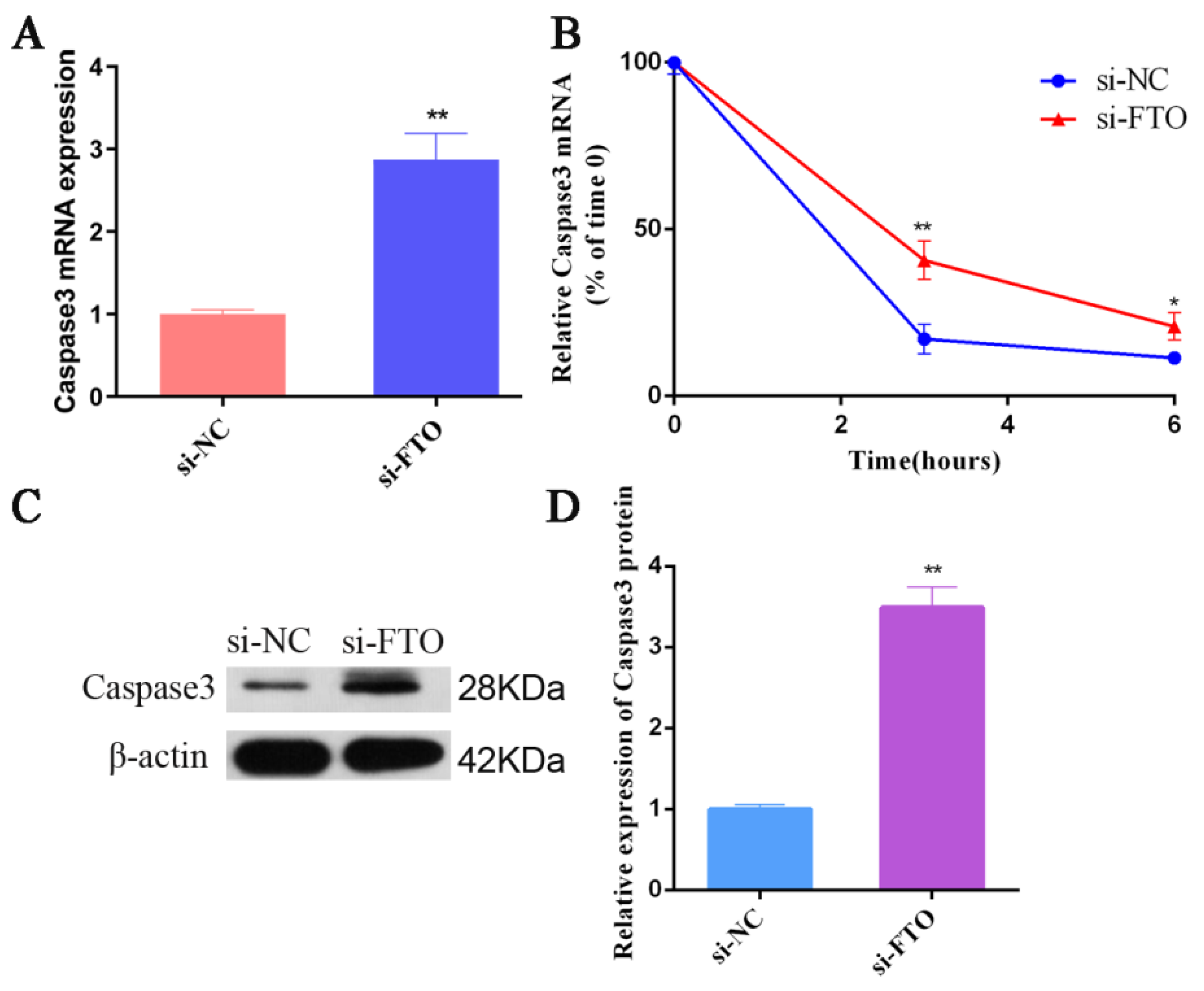
3.6. FTO Targets Caspase 3 in IPEC-J2 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Radulović, J.; Došen, R.; Stojanov, I.; Polaček, V.; Milanov, D.; Pušić, I.; Grubač, S. Neonatal diarrhea in pigs caused by clostridium perfringens. Arh. Vet. Med. 2014, 7, 49–58. [Google Scholar] [CrossRef]
- Chan, G.; Farzan, A.; Soltes, G.; Nicholson, V.M.; Pei, Y.; Friendship, R.; Prescott, J.F. The epidemiology of clostridium perfringens type a on ontario swine farms, with special reference to cpb2-positive isolates. BMC Vet. Res. 2012, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Songer, J.G. Clostridia as agents of zoonotic disease. Vet. Microbiol. 2010, 140, 399–404. [Google Scholar] [CrossRef]
- Songer, J.G.; Uzal, F.A. Clostridial enteric infections in pigs. J. Vet. Diagn. Investig. 2005, 17, 528–536. [Google Scholar] [CrossRef]
- Silva, R.; Junior, C.O.; Guedes, R.; Lobato, F. Clostridium perfringens: A review of the disease in pigs, horses and broiler chickens. Ciência Rural 2015, 45, 1027–1034. [Google Scholar] [CrossRef]
- Zhao, G.; Hou, J.; Xu, G.; Xiang, A.; Sun, S. Cellular microrna mir-10a-5p inhibits replication of porcine reproductive and respiratory syndrome virus by targeting the host factor signal recognition particle. J. Gen. Virol. 2017, 98, 624. [Google Scholar] [CrossRef] [PubMed]
- Lebrun, M.; Filée, P.; Mousset, B.; Desmecht, D.; Galleni, M.; Mainil, J.G.; Linden, A. The expression of clostridium perfringens consensus beta2 toxin is associated with bovine enterotoxaemia syndrome. Vet. Microbiol. 2007, 120, 151–157. [Google Scholar] [CrossRef]
- Karunakarnan, A.C.; Milton, A.; Reddy, A.; Rajendrakumar, A.M.; Abhishek; Verma, M.R.; Kumar, A.; Nagaleekar, V.K.; Agarwal, R.K. Diversity of toxin-genotypes among clostridium perfringens isolated from healthy and diarrheic neonatal cattle and buffalo calves. Anaerobe 2018, 49, 99–102. [Google Scholar]
- Bacciarini, L.N.; Boerlin, P.; Straub, R.; Frey, J.; Grone, A. Immunohistochemical localization of clostridium perfringens beta2-toxin in the gastrointestinal tract of horses. Vet. Pathol. 2003, 40, 376. [Google Scholar] [CrossRef]
- Gao, X.; Yang, Q.; Huang, X.; Yan, Z.; Gun, S. Effects of clostridium perfringens beta2 toxin on apoptosis, inflammation, and barrier function of intestinal porcine jejunum epithelial cells. Microb. Pathog. 2020, 147, 104379. [Google Scholar] [CrossRef]
- Rl, A.; Qy, A.; Xh, A.; Zy, A.; Xg, A.; Wei, W.A.; Kx, A.; Pw, A.; Sga, B. Clostridium perfringens beta2 toxin induced in vitro oxidative damage and its toxic assessment in porcine small intestinal epithelial cell lines. Gene 2020, 759, 144999. [Google Scholar]
- Deng, X.; Chen, K.; Luo, G.Z.; Weng, X.; Ji, Q.; Zhou, T.; He, C. Widespread occurrence of n6-methyladenosine in bacterial mrna. Nucleic Acids Res. 2015, 43, 6557–6567. [Google Scholar] [CrossRef] [PubMed]
- Desrosiers, R.; Friderici, K.; Rottman, F. Identification of methylated nucleosides in messenger rna from novikoff hepatoma cells. Proc. Natl. Acad. Sci. USA 1974, 71, 3971–3975. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Jin, H.; Que, B.; Chao, Y.; Zhang, H.; Ying, X.; Zhou, Z.; Yuan, Z.; Su, J.; Wu, B. A-cdcp1 signaling axis in chemical carcinogenesis. Oncogene 2019, 38, 4755–4772. [Google Scholar] [CrossRef]
- Wang, S.; Chai, P.; Jia, R.; Jia, R. Novel insights on m(6)a rna methylation in tumorigenesis: A double-edged sword. Mol. Cancer 2018, 17, 101. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Fu, Y.; Zhao, X.; Dai, Q.; Zheng, G.; Yang, Y.; Yi, C.; Lindahl, T.; Pan, T.; Yang, Y.G. N6-methyladenosine in nuclear rna is a major substrate of the obesity-associated fto. Nat. Chem. Biol. 2011, 7, 885–887. [Google Scholar] [CrossRef]
- Frayling, T.M.; Timpson, N.J.; Weedon, M.N.; Zeggini, E.; Freathy, R.M.; Lindgren, C.M.; Perry, J.; Elliott, K.S. A common variant in the fto gene is associated with body mass index and predisposes to childhood and adult obesity. Science 2007, 316, 889–894. [Google Scholar] [CrossRef]
- Fan, S.; Wei, H.; Huang, J.T.; Xiong, J.; Yang, Y.; Wu, K.; Jia, G.F.; Chen, J.; Feng, Y.Q.; Yuan, B.F. Decreased n(6)-methyladenosine in peripheral blood rna from diabetic patients is associated with fto expression rather than alkbh5. J. Clin. Endocrinol. Metab. 2015, 100, 148–154. [Google Scholar]
- Elkashef, S.M.; Lin, A.P.; Myers, J.; Sill, H.; Aguiar, R. Idh mutation, competitive inhibition of fto, and rna methylation. Cancer Cell 2017, 31, 619. [Google Scholar] [CrossRef]
- Li, Z.; Weng, H.; Su, R.; Weng, X.; Zuo, Z.; Li, C.; Huang, H.; Nachtergaele, S.; Dong, L.; Hu, C. Fto plays an oncogenic role in acute myeloid leukemia as a n6-methyladenosine rna demethylase. Cancer Cell 2016, 31, 127–141. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Kong, B.; Song, C.; Cong, J.; Hou, J.; Wang, S. Reduced m 6 a mrna methylation is correlated with the progression of human cervical cancer. Oncotarget 2017, 8, 98918–98930. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Liu, S.; Chen, D.; Zhao, Z.; Zhou, J. The role of the fat mass and obesity-associated protein in the proliferation of pancreatic cancer cells. Oncol. Lett. 2018, 17, 2473–2478. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, D.; Wang, F.; Xu, Y.; Yu, H.; Zhang, H. Expression of demethylase genes, fto and alkbh1, is associated with prognosis of gastric cancer. Dig. Dis. Sci. 2019, 64, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Shao, W.; Jiang, Y.; Wang, X.; Liu, Y.; Liu, X. Fto expression is associated with the occurrence of gastric cancer and prognosis. Oncol. Rep. 2017, 38, 2285. [Google Scholar] [CrossRef]
- Wang, D.; Qu, X.; Lu, W.; Wang, Y.; Jin, Y.; Hou, K.; Yang, B.; Li, C.; Qi, J.; Xiao, J.; et al. N6-Methyladenosine RNA Demethylase FTO Promotes Gastric Cancer Metastasis by Down-Regulating m6A Methylation Level of ITGB1. Front. Oncol. 2021, 11, 681280. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, Q.; Wang, S. Kinase gsk3β functions as a suppressor in colorectal carcinoma through the fto-mediated mzf1/c-myc axis. J. Cell. Mol. Med. 2021, 25, 2655–2665. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Q.; Yang, J.; Gao, X.; Luo, R.; Yan, Z.; Huang, X.; Wang, P.; Wang, W.; Xie, K. Comprehensive analysis of transcriptome-wide m6a methylome upon clostridium perfringens beta2 toxin exposure in porcine intestinal ep-ithelial cells by m6a sequencing. Front. Genet. 2021, 12, 689748. [Google Scholar] [CrossRef]
- Sebastian-delaCruz, M.; Olazagoitia-Garmendia, A.; Gonzalez-Moro, I.; Santin, I.; Garcia-Etxebarria, K.; Castellanos-Rubio, A. Implication of m6a mrna methylation in susceptibility to inflammatory bowel disease. Epigenomes 2020, 4, 16. [Google Scholar] [CrossRef]
- Zong, X.; Wang, H.; Xiao, X.; Zhang, Y.; Hu, Y.; Wang, F.; Wang, Y.; Lu, Z. Enterotoxigenic escherichia coli infection promotes enteric defensin expression via foxo6-mettl3-m6a-gpr161 signaling axis. RNA Biol. 2020, 21, 1820193. [Google Scholar]
- Chen, J.; Jin, L.; Wang, Z.; Wang, L.; Liu, G. N6-methyladenosine regulates pedv replication and host gene expression. Virology 2020, 548, 59–72. [Google Scholar] [CrossRef]
- Huang, X.; Sun, W.; Yan, Z.; Shi, H.; Yang, Q.; Wang, P.; Li, S.; Liu, L.; Zhao, S.; Gun, S. Integrative analyses of long non-coding rna and mrna involved in piglet ileum immune response to clostridium perfringens type c infection. Front. Cell. Infect. Microbiol. 2019, 9, 130. [Google Scholar] [CrossRef] [PubMed]
- Kenneth, J.L.; Thomas, D.S. Analysis of relative gene expression data using real-time quantitative pcr and the 2-δδct method. Methods 2002, 25, 402–408. [Google Scholar]
- Zhao, W.; Cui, Y.; Liu, L.; Qi, X.; Liu, J.; Ma, S.; Hu, X.; Zhang, Z.; Wang, Y.; Li, H. Correction to: Splicing factor derived circular rna circuhrf1 accelerates oral squamous cell carcinoma tumorigenesis via feedback loop. Cell Death Differ. 2020, 27, 2033–2034. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.J.; Zhou, H.C.; Feng, Z.Y.; Xu, Z.H.; Tang, Y.; Wu, M.H. Epigenetics in neurodevelopment: Emerging role of circular rna. Front. Cell. Neurosci. 2019, 13, 327. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Cao, G.; Zhang, T.; Sefik, E.; Amezcua Vesely, M.C.; Broughton, J.P.; Zhu, S.; Li, H.; Li, B.; Chen, L. M6a mrna methylation sustains treg suppressive functions. Cell Res. 2018, 28, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hu, X.; Huang, M.; Liu, J.; Gu, Y.; Ma, L.; Zhou, Q.; Cao, X. Mettl3-mediated mrna m6a methylation promotes dendritic cell activation. Nat. Commun. 2019, 10, 1898. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, J.; Zhao, S.; Tian, F. N6-methyladenosine mettl3 modulates the proliferation and apoptosis of lens epithelial cells in diabetic cataract. Mol. Ther.—Nucleic Acids 2020, 20, 111–116. [Google Scholar] [CrossRef]
- Lu, N.; Li, X.; Yu, J.; Li, Y.; Wang, C.; Zhang, L.; Wang, T.; Zhong, X. Curcumin attenuates lipopolysaccharide-induced hepatic lipid metabolism disorder by modification of m6a rna methylation in piglets. Lipids 2018, 53, 53–63. [Google Scholar] [CrossRef]
- Luo, R.; Huang, X.; Yan, Z.; Gao, X.; Gun, S. Identification and characterization of mapk signaling pathway genes and associated lncrnas in the ileum of piglets infected by clostridium perfringens type c. BioMed Res. Int. 2020, 2020, 8496872. [Google Scholar] [CrossRef]
- Meyer, K.D.; Saletore, Y.; Zumbo, P.; Elemento, O.; Mason, C.E.; Jaffrey, S.R. Comprehensive analysis of mrna methylation reveals enrichment in 3′ utrs and near stop codons. Cell 2012, 149, 1635–1646. [Google Scholar] [CrossRef]
- Wei, M.; Bai, J.-W.; Niu, L.; Zhang, Y.-Q.; Chen, H.-Y.; Zhang, G.-J. The complex roles and therapeutic implications of m(6)a modifications in breast cancer. Front. Cell Dev. Biol. 2021, 8, 615071. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Su, R.; Sheng, Y.; Dong, L.; Dong, Z.; Xu, H.J.; Ni, T.F.; Zhang, Z.S.; Zhang, T.; Li, C.Y.; et al. Small-molecule targeting of oncogenic fto demethylase in acute myeloid leukemia. Cancer Cell 2019, 35, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Tang, F.; Zhou, M.; Shan, D.; Chen, X.; Cao, K. Identification and validation of n6-methyladenosine-related biomarkers for bladder cancer: Implications for immunotherapy. Front. Oncol. 2022, 12, 820242. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Su, Q.; Guo, Z.; Zhou, J.; Zheng, F.; Yu, G.; Shao, W.; Hu, H.; Wu, S.; Li, H. N6-methyladenosine (m6a) demethylase fto regulates cellular apoptosis following cobalt-induced oxidative stress. Environ. Pollut. 2022, 297, 118749. [Google Scholar] [CrossRef]
- Hatok, J.; Racay, P. Bcl-2 family proteins: Master regulators of cell survival. Biomol. Concepts 2016, 7, 259–270. [Google Scholar] [CrossRef]
- Reed, J.C. Bcl-2 and the regulation of programmed cell death. J. Cell Biol. 1994, 1, 1–6. [Google Scholar] [CrossRef]
- Ashkenazi, A.; Fairbrother, W.J.; Leverson, J.D.; Souers, A.J. From basic apoptosis discoveries to advanced selective bcl-2 family inhibitors. Nat. Rev. Drug Discov. 2017, 16, 273–284. [Google Scholar] [CrossRef]
- Li, S.; Monroig, Ó.; Wang, T.; Yuan, Y.; Navarro, J.C.; Hontoria, F.; Liao, K.; Tocher, D.R.; Mai, K.; Xu, W. Functional characterization and differential nutritional regulation of putative elovl5 and elovl4 elongases in large yellow croaker (Larimichthys crocea). Sci. Rep. 2017, 7, 2303. [Google Scholar] [CrossRef]
- Thomas, S.; Quinn, B.A.; Das, S.K.; Dash, R.; Emdad, L.; Dasgupta, S.; Wang, X.Y.; Dent, P.; Reed, J.C.; Pellecchia, M. Targeting the bcl-2 family for cancer therapy. Expert Opin. Ther. Targets 2013, 17, 61–75. [Google Scholar] [CrossRef]
- Kelly, P.N.; Strasser, A. The role of bcl-2 and its pro-survival relatives in tumourigenesis and cancer therapy. Cell Death Differ. 2011, 18, 1414–1424. [Google Scholar] [CrossRef]
- Gross, A.; Jockel, J.; Wei, M.C.; Korsmeyer, S.J. Enforced dimerization of bax results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J. 2014, 17, 3878–3885. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ye, D.; Na, Y.; Wild, C.; Chen, H.; Jia, Z. Direct activation of bax protein for cancer therapy. Med. Res. Rev. 2016, 36, 313–341. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Yin, Z.; Gao, S.; Chen, M.; Xu, D.; Mai, K.; Ai, Q. Characterization of caspase8 and its role in the regulation of apoptosis-related genes in large yellow croaker (Larimichthys crocea). Aquaculture 2021, 539, 736595. [Google Scholar] [CrossRef]
- Sun, S.; Ge, X.; Zhu, J.; Zhang, W.; Zhang, Q. Molecular cloning, immunohistochemical localization, characterization and expression analysis of caspase-8 from the blunt snout bream (Megalobrama amblycephala) exposed to ammonia. Fish Shellfish Immunol. 2015, 47, 645–654. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Y.; Zhang, J.; Guan, C.; Ren, L. Cantharidin-induced acute hepatotoxicity: The role of TNF-α, IKK-α, Bcl-2, Bax and caspase3. J. Appl. Toxicol. 2020, 40, 1526–1533. [Google Scholar] [CrossRef]
- Li, Y.; Wu, K.; Quan, W.; Yu, L.; Chen, S.; Cheng, C.; Wu, Q.; Zhao, S.; Zhang, Y.; Zhou, L. The dynamics of fto binding and demethylation from the m6a motifs. RNA Biol. 2019, 16, 1179–1189. [Google Scholar] [CrossRef]
- Scholten, B.V.; Andresen, E.N.; S Rensen, T.I.; Jess, T. Aetiological factors behind adipose tissue inflammation: An unexplored research area. Public Health Nutr. 2013, 16, 27–35. [Google Scholar] [CrossRef][Green Version]
- Terra, X.; Auguet, T.; Porras, J.A.; Quintero, Y.; Aguilar, C.; Luna, A.M.; HernáNdez, M.; Sabench, F.; del Castillo, D.; Richart, C. Anti-inflammatory profile of fto gene expression in adipose tissues from morbidly obese women. Cell. Physiol. Biochem. 2010, 26, 1041–1050. [Google Scholar] [CrossRef]
- Ke, W.-L.; Huang, Z.-W.; Peng, C.-L.; Ke, Y.-P. M6a demethylase fto regulates the apoptosis and inflammation of cardiomyocytes via yap1 in ischemia-reperfusion injury. Bioengineered 2022, 13, 5443–5452. [Google Scholar] [CrossRef]
- Yu, J.-T.; Hu, X.-W.; Chen, H.-Y.; Yang, Q.; Li, H.-D.; Dong, Y.-H.; Zhang, Y.; Wang, J.-N.; Jin, J.; Wu, Y.-G. DNA methylation of fto promotes renal inflammation by enhancing m6a of ppar-α in alcohol-induced kidney injury. Pharmacol. Res. 2021, 163, 105286. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Zhang, J.; Gao, X.; Luo, R.; Xie, K.; Wang, W.; Li, J.; Yang, Q.; Huang, X.; Yan, Z.; et al. FTO Regulates Apoptosis in CPB2-Treated IPEC-J2 Cells by Targeting Caspase 3 Apoptotic Protein. Animals 2022, 12, 1644. https://doi.org/10.3390/ani12131644
Yang J, Zhang J, Gao X, Luo R, Xie K, Wang W, Li J, Yang Q, Huang X, Yan Z, et al. FTO Regulates Apoptosis in CPB2-Treated IPEC-J2 Cells by Targeting Caspase 3 Apoptotic Protein. Animals. 2022; 12(13):1644. https://doi.org/10.3390/ani12131644
Chicago/Turabian StyleYang, Jiaojiao, Juanli Zhang, Xiaoli Gao, Ruirui Luo, Kaihui Xie, Wei Wang, Jie Li, Qiaoli Yang, Xiaoyu Huang, Zunqiang Yan, and et al. 2022. "FTO Regulates Apoptosis in CPB2-Treated IPEC-J2 Cells by Targeting Caspase 3 Apoptotic Protein" Animals 12, no. 13: 1644. https://doi.org/10.3390/ani12131644
APA StyleYang, J., Zhang, J., Gao, X., Luo, R., Xie, K., Wang, W., Li, J., Yang, Q., Huang, X., Yan, Z., Wang, P., & Gun, S. (2022). FTO Regulates Apoptosis in CPB2-Treated IPEC-J2 Cells by Targeting Caspase 3 Apoptotic Protein. Animals, 12(13), 1644. https://doi.org/10.3390/ani12131644





