Retrospective Evaluation of Method of Treatment, Laboratory Findings, and Concurrent Diseases in Dairy Cattle Diagnosed with Left Displacement of the Abomasum during Time of Hospitalization
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Diagnosis of Left Displacement of the Abomasum
2.3. Review of Medical Records
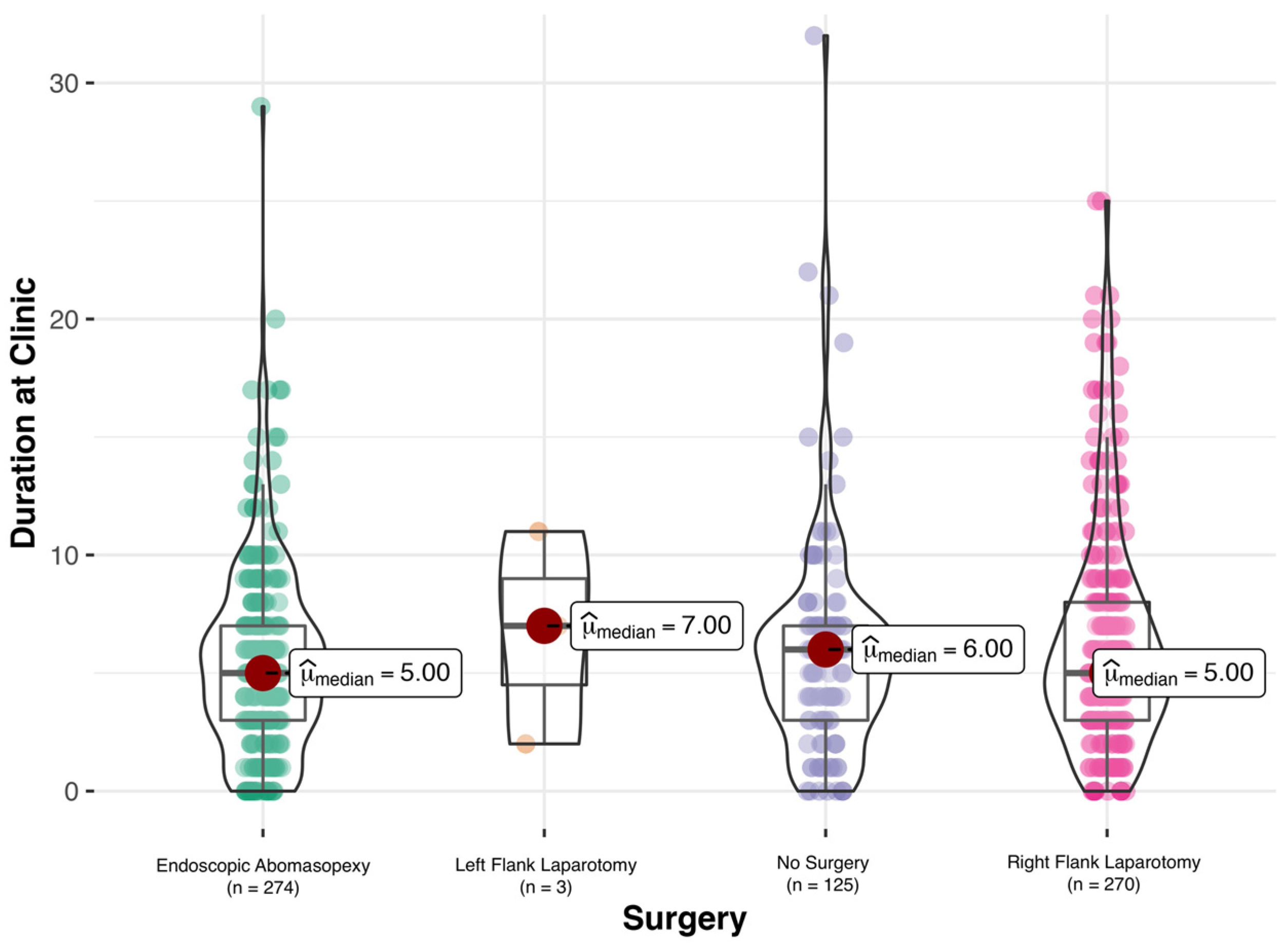
2.4. Conservative or Surgical Therapy and Outcome of Therapy
2.5. Review of Concurrent Diseases
2.6. Review of Biochemical Parameters of Blood Analysis
2.7. Statistical Analysis
3. Results
3.1. Animals
3.2. Conservative Treatment of LDA
3.3. Surgical Treatment of LDA
3.4. Outcome
3.5. Concurrent Diseases
3.6. Laboratory Findings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- LeBlanc, S.J.; Leslie, K.E.; Duffield, T.F. Metabolic predictors of displaced abomasum in dairy cattle. J. Dairy Sci. 2005, 88, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Van Winden, S.C.L.; Kuiper, R. Left displacement of the abomasum in dairy cattle: Recent developments in epidemiological and etiological aspects. Vet. Res. 2003, 34, 47–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melendez, P.; Romero, C.; Pithua, P.; Marin, M.P.; Pinedo, P.; Duchens, M. Retrospective evaluation of milk production and culling risk following either surgical, toggle-pin suture or conservative treatment of left displaced abomasum in Chilean dairy cows. N. Z. Vet. J. 2017, 65, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Trent, A.M. Surgery of the abomasum. In Farm Animal Surgery; Fubini, D.L., Ducharme, N.G., Eds.; Elsevier: St. Louis, MO, USA, 2017; Volume 2, pp. 260–280. [Google Scholar]
- Constable, P.D.; Hinchcliff, K.W.; Stanley, H.D.; Grünberg, W. Diseases of the Alimentary Tract-Ruminant. In Veterinary Medicine, 11th ed.; Constable, P.D., Hinchcliff, K.W., Stanley, H.D., Grünberg, W., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 1, pp. 436–621. [Google Scholar]
- Mueller, K. Diagnosis, treatment and control of left displaced abomasum in cattle. Practice 2011, 33, 470–481. [Google Scholar] [CrossRef]
- Niehaus, A.J. Surgery of the abomasum. Vet. Clin. N. Am.-Food Anim. Pract. 2008, 24, 349–358. [Google Scholar] [CrossRef]
- Dirksen, G.; Doll, K.; Braun, U. Krankheiten des Labmagens. In Innere Medizin und Chirurgie des Rindes; Dirksen, G., Gründer, H.-D., Stöber, M., Eds.; Parey: Stuttgart, Germany, 2006; pp. 473–513. [Google Scholar]
- Zadnik, T.; Lombar, R. Our Experience with Left-Sided Abomasal Displacement Correction via the Roll-and-Toggle-Pin Suture Procedure according to Grymer/Sterner Model. Int. Sch. Res. Not. 2011, 2011, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Mulon, P.-Y.; Babkine, M.; Desrochers, A. Ventral laparoscopic abomasopexy in 18 cattle with displaced abomasum. Vet. Surg. 2006, 35, 347–355. [Google Scholar] [CrossRef]
- Newman, K.D.; Harvey, D.; Roy, J.-P. Minimally invasive field abomasopexy techniques for correction and fixation of left displacement of the abomasum in dairy cows. Vet. Clin. N. Am.-Food Anim. Pract. 2008, 24, 359–382. [Google Scholar] [CrossRef]
- Pentecost, R.L.; Niehaus, A.J.; Anderson, D.E.; Miesner, M.D.; Rings, D.M. Outcome following surgical correction of abomasal displacement in lactating dairy cattle: A retrospective study of 127 cases (1999–2010). J. Vet. Sci. Anim. Husb. 2014, 2, 102. [Google Scholar] [CrossRef]
- Tschoner, T.; Zablotski, Y.; Feist, M. Retrospective Evaluation of Claw Lesions, Inflammatory Markers, and Outcome After Abomasal Rolling in Cattle with Left Displacement of the Abomasum. Animals 2021, 11, 1648. [Google Scholar] [CrossRef]
- Dirksen, G.; Gründer, H.-D.; Stöber, M. Die Klinische Untersuchung des Rindes; Verlag Paul Parey: Berlin und Hamburg, Germany, 1979; p. 718. [Google Scholar]
- Dirksen, G. Gegenwärtiger Stand der Diagnostik, Therapie und Prophylaxe der Dislocatio abomasi sinistra des Rindes. Dtsch. Tierarztl. Wschr. 1967, 74, 625–628. [Google Scholar]
- Janowitz, H. Laparoskopische Reposition und Fixation des nach links verlagerten Labmagens beim Rind. Tierarztl. Prax. 1998, 26, 308–313. [Google Scholar]
- Niehaus, A.J. Surgical management of abomasal disease. Vet. Clin. N. Am.-Food Anim. Pract. 2016, 32, 629–644. [Google Scholar] [CrossRef] [PubMed]
- Oetzel, G.R. Monitoring and testing dairy herds for metabolic disease. Vet. Clin. N. Am.-Food Anim. Pract. 2004, 20, 651–674. [Google Scholar] [CrossRef] [PubMed]
- Fiore, F.; Musina, D.; Cocco, R.; Di Cerbo, A.; Spissu, N. Association between left-displaced abomasum corrected with 2-step laparoscopic abomasopexy and milk production in a commercial dairy farm in Italy. Ir. Vet. J. 2018, 71, 20. [Google Scholar] [CrossRef] [PubMed]
- Jorritsma, R.; Westerlaan, B.; Bierma, M.P.R.; Frankena, K. Milk yield and survival of Holstein-Friesian dairy cattle after laparoscopic correction of left-displaced abomasum. Vet. Rec. 2008, 162, 743–746. [Google Scholar] [CrossRef]
- González-Martín, J.V.; Pérez-Villalobos, N.; Baumgartner, W.; Astiz, S. An investigation into the development of right displaced abomasum by rolling 268 dairy cows with left displaced abomasum. J. Dairy Sci. 2019, 102, 11268–11279. [Google Scholar] [CrossRef]
- Lombar, R.; Zadnik, T. Conservative treament of left-side displacement of the abomasum (LDA) wih rolling technique and oral electrolyte therapy. In Proceedings of the XXVI World Buiatrics Conference, Santiago, Chile, 14–18 November 2010; p. 371. [Google Scholar]
- Rohn, M.; Tenhagen, B.A.; Hofmann, W. Survival of dairy cows after surgery to correct abomasal displacement: 1. Clinical and laboratory parameters and overall survival. J. Vet. Med. Series 2004, 51, 294–299. [Google Scholar] [CrossRef]
- Reynen, J.L.; Kelton, D.F.; LeBlanc, S.J.; Newby, N.C.; Duffield, T.F. Factors associated with survival in the herd for dairy cows following surgery to correct left displaced abomasum. J. Dairy Sci. 2015, 98, 3806–3813. [Google Scholar] [CrossRef] [Green Version]
- Hudson, C.; Whay, H.; Huxley, J. Recognition and managment of pain in cattle. Practice 2008, 30, 126–134. [Google Scholar] [CrossRef] [Green Version]
- Tschoner, T.; Behrendt-Wipperman, M.; Rieger, A.; Metzner, M.; Knubben-Schweizer, G.; Reichmann, F.; Feist, M. Course of plasma substance P concentrations during umbilical surgery in calves. Berl. Munch. Tierarztl. Wochenschr. 2018, 11–12, 522–528. [Google Scholar]
- Interlandi, C.; Nastasi, B.; Morici, M.; Calabrò, P.; Costa, G.L. Effects of the combination romifidine/tramadol drug administration on several physiological and behavioral variables in calves. Large Anim. Rev. 2017, 23, 51–54. [Google Scholar]
- Shaver, R.D. Nutritional risk factors in the etiology of left displaced abomasum in dairy cows: A review. J. Dairy Sci. 1997, 80, 2449–2453. [Google Scholar] [CrossRef]
- Sexton, M.F.; Buckley, W.; Ryan, E. A study of 54 cases of left displacement of the abomasum: February to July 2005. Ir. Vet. J. 2007, 60, 605–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterner, K.E.; Grymer, J.; Bartlett, P.C.; Miekstyn, M.J. Factors influencing the survival of dairy cows after correction of left displaced abomasum. JAVMA 2008, 232, 1521–1529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zurr, L.; Leonhard-Marek, S. Effects of β-hydroxybutyrate and different calcium and potassium concentrations on the membrane potential and motility of abomasal smooth muscle cells in cattle. J. Dairy Sci. 2012, 95, 5750–5759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, B.K.; Cramer, M.C.; Fowler, H.N.; Varnell, H.L.; Dietsch, A.M.; Proudfoot, K.L.; Shearer, J.; Correa, M.; Pairis-Garcia, M.D. Determination of dairy cattle euthanasia criteria and analysis of barriers to humane euthanasia in the United States: The veterinarian perspective. Animals 2020, 10, 1051. [Google Scholar] [CrossRef] [PubMed]
- Dezfouli, M.M.; Eftekhari, Z.; Sadeghian, S.; Bahounar, A.; Jeloudari, M. Evaluation of hematological and biochemical profiles in dairy cows with left displacement of the abomasum. Comp. Clin. Path. 2013, 22, 175–179. [Google Scholar] [CrossRef] [Green Version]
- Constable, P.D.; Hinchcliff, K.W.; Stanley, H.D.; Grünberg, W. Clinical Examination and Making a Diagnosis. In Veterinary Medicine; Constable, P.D., Hinchcliff, K.W., Stanley, H.D., Grünberg, W., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 1, pp. 1–28. [Google Scholar]
- Rehage, J.; Mertens, M.; Stockhofe-Zurwieden, N.; Kaske, M.; Scholz, H. Post surgical convalescence of dairy cows with left abomasal displacement in relation to fatty liver. Schweiz. Arch. Tierheilkd. 1996, 138, 361–368. [Google Scholar]
- Kalaitzakis, E.; Panousis, N.; Roubies, N.; Giadinis, N.; Kaldrymidou, E.; Georgiadis, M.; Karatzias, H. Clinicopathological evaluation of downer dairy cows with fatty liver. Can. Vet. J. 2010, 51, 615. [Google Scholar]
- Rajala-Schultz, P.J.; Gröhn, Y.T.; McCulloch, C.E.; Guard, C.L. Effects of clinical mastitis on milk yield in dairy cows. J. Dairy Sci. 1999, 82, 1213–1220. [Google Scholar] [CrossRef]
- Giuliodori, M.J.; Magnasco, R.P.; Becu-Villalobos, D.; Lacau-Mengido, I.M.; Risco, C.A.; de la Sota, R.L. Metritis in dairy cows: Risk factors and reproductive performance. J. Dairy Sci. 2013, 96, 3621–3631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antanaitis, R.; Žilaitis, V.; Kučinskas, A.; Juozaitienė, V.; Leonauskaitė, K. Changes in cow activity, milk yield, and milk conductivity before clinical diagnosis of ketosis, and acidosis. Vet. Med. Zoot. 2015, 70, 3–9. [Google Scholar]
- Green, L.E.; Hedges, V.J.; Schukken, Y.H.; Blowey, R.W.; Packington, A.J. The impact of clinical lameness on the milk yield of dairy cows. J. Dairy Sci. 2002, 85, 2250–2256. [Google Scholar] [CrossRef] [Green Version]
| Treatment | Number of Cows |
|---|---|
| Analgesic Treatment | |
| Dexamethason | 1.4% (n = 3) |
| Flunixine meglumine | 48.8% (n = 102) |
| Ketoprofen | 21.1% (n = 44) |
| Meloxicam | 13.9% (n = 29) |
| Metamizole | 10.0% (n = 21) |
| Oral Treatment | |
| Calcium | 22.5% (n = 47) |
| KCl | 39.7% (n = 83) |
| MgO | 0.5% (n = 1) |
| NaBic | (n = 8) |
| NaCl | 2.4% (n = 5) |
| NadPh | 2.9% (n = 6) |
| Propylene glycole | 30.6% (n = 64) |
| Sodium sulfate | 12.9% (n = 27) |
| Vitamin E/Selenium | 37.3% (n = 78) |
| Concurrent Disease | Diagnosis |
|---|---|
| Metritis/Endometritis |
|
| Mastitis |
|
| Septicemia |
|
| Subclinical Ketosis |
|
| Clinical Ketosis |
|
| Hypocalcemia |
|
| Claw Disease |
|
| Results of Abomasal Rolling in 209 cows with LDA | Number of Cows |
| LDA solved | 92.8% (n = 194) |
| LDA not solved | 7.2% (n = 15) |
| Relapse of LDA following Abomasal Rolling in 194 cows | Number of Cows |
| Relapse: yes | 56.7% (n = 110) |
| Relapse: no | 43.3% (n = 84) |
| Surgery following Abomasal Rolling in 150 cows | Number of Cows |
| Endoscopic abomasopexy | 40.0% (n = 60) |
| Right flank laparotomy | 60.0% (n = 90) |
| Method of Surgery | Outcome | ||
|---|---|---|---|
| Recovery | Death | Abortion of Surgery | |
| Right flank laparotomy | 87% (n = 235) | 13% (n = 35) | 2.9% (n = 16) |
| Left flank laparotomy | 100% (n = 3) | 0% (n = 0) | nA 1 |
| Endoscopic abomasopexy | 93% (n = 255) | 7% (n = 19) | 0.2% (n = 1) |
| No surgery | 37% (n = 46) | 63% (n = 79) | nA |
| Concurrent Disease | Presence | Absence | Influence on Survival Rate |
|---|---|---|---|
| Metritis/Endometritis | 38.4% (n = 255) | 61.6% (n = 409) | p = 0.03 |
| Mastitis | 19.6% (n = 130) | 80.4% (n = 534) | p = 0.74 |
| Septicemia | 12.7% (n = 84) | 87.3% (n = 580) | p = 0.48 |
| Subclinical ketosis | 28.0% (n = 186) | 72.0% (n = 478) | p = 0.02 |
| Clinical ketosis | 22.4% (n = 149) | 77.6% (n = 515) | p = 0.42 |
| Hypocalcemia | 15.1% (n = 100) | 84.9% (n = 564) | p = 0.84 |
| Claw disease | 16.7% (n = 111) | 83.3% (n = 553) | p = 0.42 |
| Outcome | Statistical Model | |||
|---|---|---|---|---|
| Recovery | Death | Univariate | Multivariate | |
| Laboratory Parameter | p | p | ||
| pH 3 (7.35–7.45) | 7.4 ± 0.1 (7.22–7.64) | 7.4 ± 0.1 (7.02–7.58) | p < 0.0006 | p = 0.06 |
| Base excess 4 (−2.5–2.5 mmol/L) | 3.0 ± 5.8 (−15.1–21.5) | 1.7 ± 8.6 (-24.9–23.8) | p < 0.04 | nA |
| Packed cell volume 5 (30–36%) | 35.2 ± 5.4 (14.3–54) | 35.3 ± 6.4 (22–51.5) | p = 0.81 | nA |
| Leucocyte count (4–10 G */L) | 7.2 ± 3.5 (1.2–24.4) | 7.5 ± 3.9 (2.1–23.2) | p = 0.34 | nA |
| Thrombocyte count (200–800 G */L) | 467.9 ± 191.7 (19–1261) | 468.7 ± 283.8 (32–2631) | p = 0.97 | nA |
| Anion gap 6 (14–26 mEqu/L) | 16.1 ± 6.2 (2.7–106) | 16.9 ± 6.7 (2.3–41.3) | p = 0.21 | nA |
| Glucose 7 (2.5–3.3 mmol/L) | 5.0 ± 2.1 (1.4–16.7) | 5.5 ± 3.2 (1.8–33.6) | p < 0.03 | nA |
| L-lactate 8 (≤2.2 mmol/L) | 2.3 ± 2.1 (0.26–14.22) | 3.1 ± 2.8 (0.15–14.29) | p < 0.001 | p = 0.27 |
| Total protein (60–80 g/L) | 75.3 ± 9.5 (34.9–116.1) | 77.9 ± 12.7 (48.3–126.3) | p < 0.009 | nA |
| Aspartate aminotransferase 1 (≤80 U/L) | 286.2 ± 305.3 (29.1–5395.2) | 401.0 ± 510.1 (10.1–2918.8) | p < 0.003 | p < 0.01 |
| Gamma glutamyl transferase 2 (≤36 U/L) | 52.0 ± 67.8 (6.8–624.7) | 61.1 ± 91.9 (5.4–697.5) | p = 0.02 | nA |
| Glutamate dehydrogenase 3 (≤16 U/L) | 83.4 ± 136.7 (2.32–1261.26) | 123.3 ± 348.9 (1.92–3329.48) | p = 0.06 | nA |
| Betahydroxybutyrate 9 (≤1.0 mmol/L) | 2.0 ± 1.8 (0.08–10.14) | 2.0 ± 2.9 (0.1–14.96) | p = 0.83 | nA |
| Glutaraldehyde test 10 (<15 min) | 10.1 ± 5.8 (0.5–16) | 7.3 ± 6.2 (0.5–16) | p = 1.383 × 10−6 | p < 0.01 |
| Sodium 11 (135–150 mmol/L) | 138.1 ± 3.8 (117–157.4) | 135.6 ± 4.7 (119–145.9) | p = 6.077 × 10−10 | p < 0.01 |
| Potassium 12 (4–5 mmol/L) | 3.3 ± 0.5 (1.71–4.91) | 3.1 ± 0.7 (1.86–4.86) | p < 0.002 | nA |
| Ionized calcium 13 (1.0–1.3 mmol/L) | 1.1 ± 0.6 (0.64–1.53) | 1.1 ± 0.1 (0.71–1.99) | p = 0.11 | p = 0.08 |
| Chloride 14 (90–105 mmol/L) | 98.1 ± 6.3 (65–115) | 95.5 ± 8.2 (67–110) | p < 0.0001 | p = 0.24 |
| Phosphorus (1.5–2.1 mmol/L) | 1.5 ± 0.6 (0.2–4.1) | 1.7 ± 0.7 (0.3–3.9) | p < 0.002 | nA |
| Magnesium 15 (0.74–1.44 mmol/L) | 0.8 ± 0.2 (0.34–1.7) | 0.9 ± 0.3 (0.42–3.28) | p = 0.52 | nA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tschoner, T.; Zablotski, Y.; Feist, M. Retrospective Evaluation of Method of Treatment, Laboratory Findings, and Concurrent Diseases in Dairy Cattle Diagnosed with Left Displacement of the Abomasum during Time of Hospitalization. Animals 2022, 12, 1649. https://doi.org/10.3390/ani12131649
Tschoner T, Zablotski Y, Feist M. Retrospective Evaluation of Method of Treatment, Laboratory Findings, and Concurrent Diseases in Dairy Cattle Diagnosed with Left Displacement of the Abomasum during Time of Hospitalization. Animals. 2022; 12(13):1649. https://doi.org/10.3390/ani12131649
Chicago/Turabian StyleTschoner, Theresa, Yury Zablotski, and Melanie Feist. 2022. "Retrospective Evaluation of Method of Treatment, Laboratory Findings, and Concurrent Diseases in Dairy Cattle Diagnosed with Left Displacement of the Abomasum during Time of Hospitalization" Animals 12, no. 13: 1649. https://doi.org/10.3390/ani12131649






