Feeding Fiber-Bound Polyphenol Ingredients at Different Levels Modulates Colonic Postbiotics to Improve Gut Health in Cats
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Foods
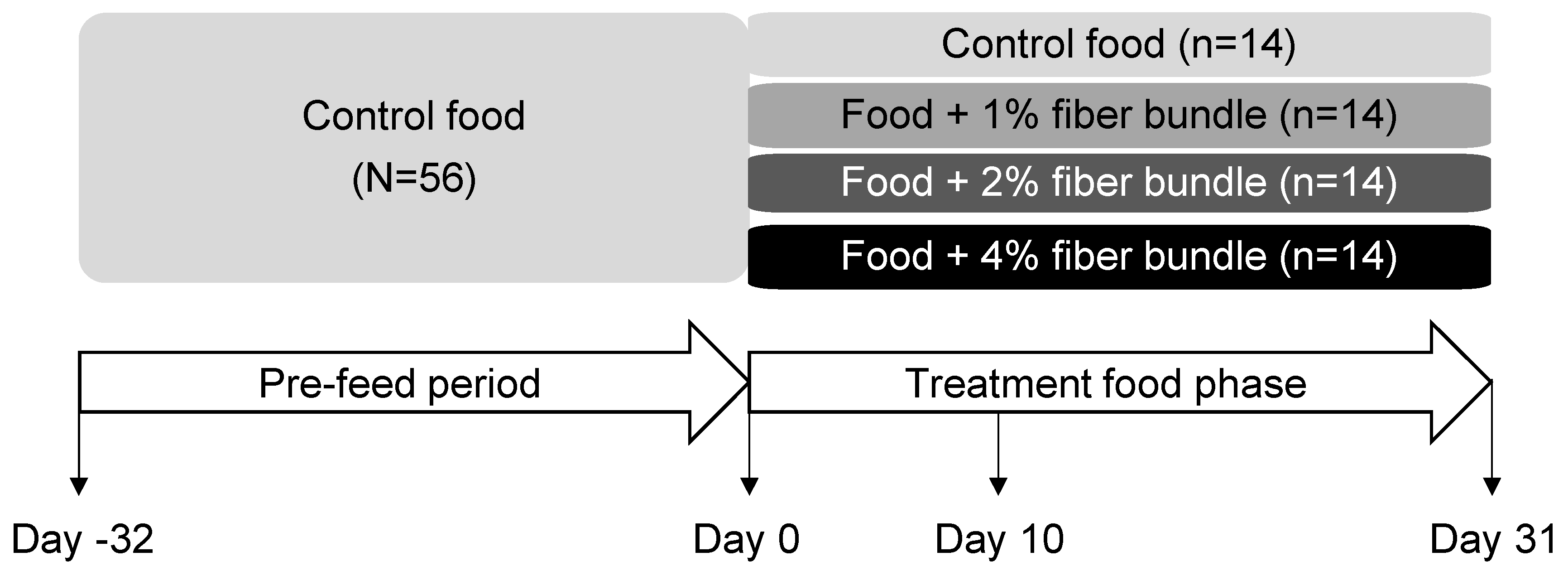
2.2. Animals and Experimental Design
2.3. Serum and Metabolite Analyses
2.4. Stool Scoring and Fecal Sample Processing
2.5. Fecal Microbiome Analysis and Bioinformatics Processing
2.6. Statistical Analysis
3. Results
3.1. Study Design, Animals, and Food
3.2. Fecal Parameters and Metabolites
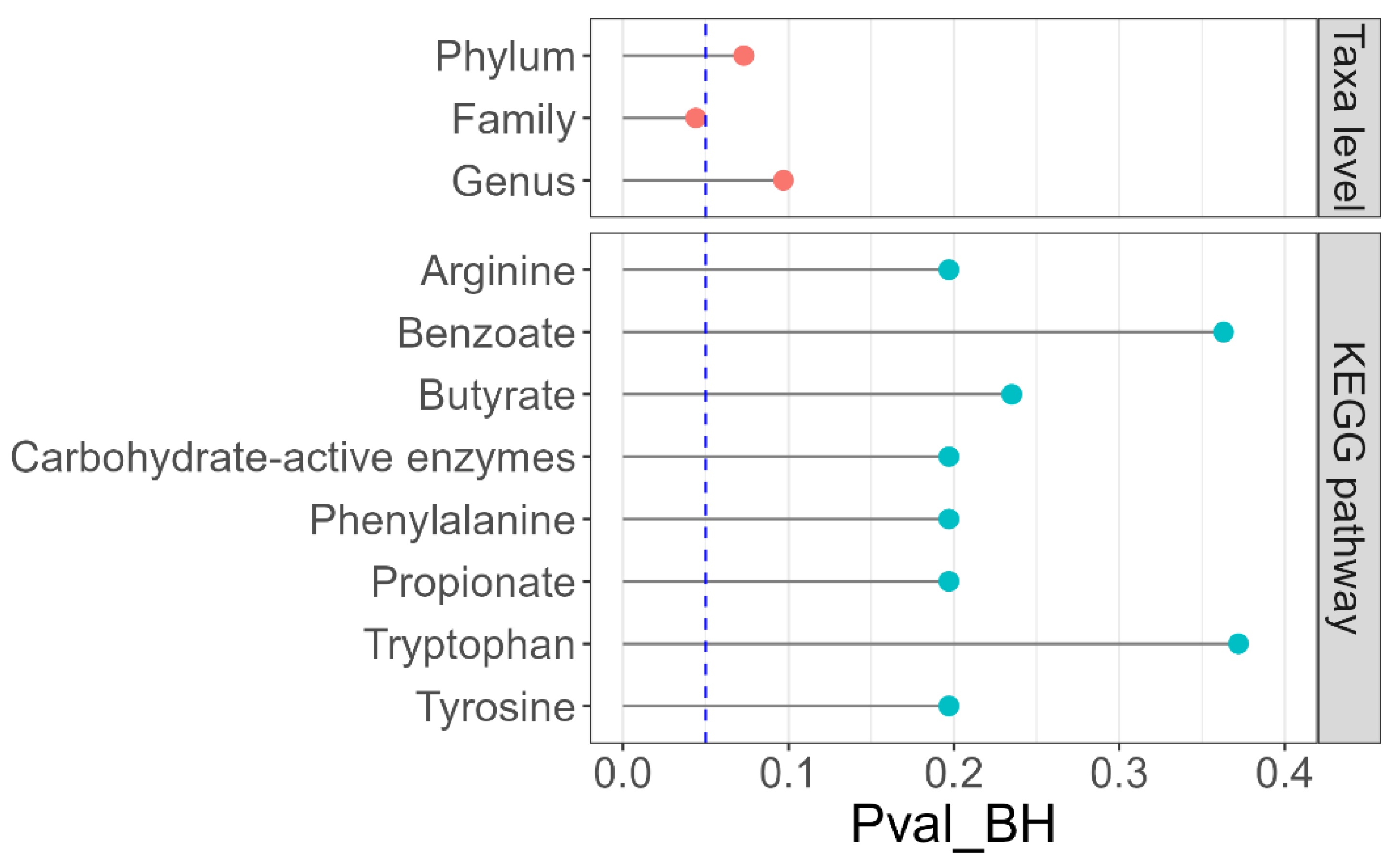
3.3. Fecal Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wernimont, S.M.; Radosevich, J.; Jackson, M.I.; Ephraim, E.; Badri, D.V.; MacLeay, J.M.; Jewell, D.E.; Suchodolski, J.S. The effects of nutrition on the gastrointestinal microbiome of cats and dogs: Impact on health and disease. Front. Microbiol. 2020, 11, 1266. [Google Scholar] [CrossRef] [PubMed]
- Barry, K.A.; Middelbos, I.S.; Vester Boler, B.M.; Dowd, S.E.; Suchodolski, J.S.; Henrissat, B.; Coutinho, P.M.; White, B.A.; Fahey, G.C., Jr.; Swanson, K.S. Effects of dietary fiber on the feline gastrointestinal metagenome. J. Proteome Res. 2012, 11, 5924–5933. [Google Scholar] [CrossRef] [PubMed]
- Rochus, K.; Janssens, G.P.; Hesta, M. Dietary fibre and the importance of the gut microbiota in feline nutrition: A review. Nutr. Res. Rev. 2014, 27, 295–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, R.W.; Scott-Moncrieff, J.C.; Feldman, E.C.; DeVries-Concannon, S.E.; Kass, P.H.; Davenport, D.J.; Kiernan, C.T.; Neal, L.A. Effect of dietary insoluble fiber on control of glycemia in cats with naturally acquired diabetes mellitus. J. Am. Vet. Med. Assoc. 2000, 216, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Verbrugghe, A.; Hesta, M.; Gommeren, K.; Daminet, S.; Wuyts, B.; Buyse, J.; Janssens, G.P. Oligofructose and inulin modulate glucose and amino acid metabolism through propionate production in normal-weight and obese cats. Br. J. Nutr. 2009, 102, 694–702. [Google Scholar] [CrossRef] [Green Version]
- Hall, J.A.; Jewell, D.E.; Ephraim, E. Changes in the fecal metabolome are associated with feeding fiber not health status in cats with chronic kidney disease. Metabolites 2020, 10, 281. [Google Scholar] [CrossRef]
- Hall, J.A.; Jackson, M.I.; Jewell, D.E.; Ephraim, E. Chronic kidney disease in cats alters response of the plasma metabolome and fecal microbiome to dietary fiber. PLoS ONE 2020, 15, e0235480. [Google Scholar] [CrossRef] [PubMed]
- Kanakupt, K.; Vester Boler, B.M.; Dunsford, B.R.; Fahey, G.C., Jr. Effects of short-chain fructooligosaccharides and galactooligosaccharides, individually and in combination, on nutrient digestibility, fecal fermentative metabolite concentrations, and large bowel microbial ecology of healthy adults cats. J. Anim. Sci. 2011, 89, 1376–1384. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Mazcorro, J.F.; Barcenas-Walls, J.R.; Suchodolski, J.S.; Steiner, J.M. Molecular assessment of the fecal microbiota in healthy cats and dogs before and during supplementation with fructo-oligosaccharides (FOS) and inulin using high-throughput 454-pyrosequencing. PeerJ 2017, 5, e3184. [Google Scholar] [CrossRef] [Green Version]
- Jackson, M.I.; Jewell, D.E. Balance of saccharolysis and proteolysis underpins improvements in stool quality induced by adding a fiber bundle containing bound polyphenols to either hydrolyzed meat or grain-rich foods. Gut Microbes 2019, 10, 298–320. [Google Scholar] [CrossRef] [Green Version]
- Jewell, D.E.; Jackson, M.I.; Cochrane, C.Y.; Badri, D.V. Feeding fiber-bound polyphenol ingredients at different levels modulates colonic postbiotics to improve gut health in dogs. Animals 2022, 12, 627. [Google Scholar] [CrossRef] [PubMed]
- Bedu-Ferrari, C.; Biscarrat, P.; Langella, P.; Cherbuy, C. Prebiotics and the human gut microbiota: From breakdown mechanisms to the impact on metabolic health. Nutrients 2022, 14, 2096. [Google Scholar] [CrossRef] [PubMed]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar]
- National Research Council Committee update of the guide for the care and use of laboratory animals. In The National Academies Collection: Reports Funded by National Institutes of Health; National Academies Press: Washington, DC, USA; National Academy of Sciences: Washington, DC, USA, 2011. [CrossRef]
- Hall, J.A.; Melendez, L.D.; Jewell, D.E. Using gross energy improves metabolizable energy predictive equations for pet foods whereas undigested protein and fiber content predict stool quality. PLoS ONE 2013, 8, e54405. [Google Scholar] [CrossRef] [Green Version]
- Ephraim, E.; Cochrane, C.Y.; Jewell, D.E. Varying protein levels influence metabolomics and the gut microbiome in healthy adult dogs. Toxins 2020, 12, 517. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [Green Version]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef] [Green Version]
- Langille, M.G.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic. Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Naran, R.; Chen, G.; Carpita, N.C. Novel rhamnogalacturonan I and arabinoxylan polysaccharides of flax seed mucilage. Plant Physiol. 2008, 148, 132–141. [Google Scholar] [CrossRef] [Green Version]
- Birkett, A.; Muir, J.; Phillips, J.; Jones, G.; O’Dea, K. Resistant starch lowers fecal concentrations of ammonia and phenols in humans. Am. J. Clin. Nutr. 1996, 63, 766–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef] [Green Version]
- Scoditti, E. Neuroinflammation and neurodegeneration: The promising protective role of the citrus flavanone hesperetin. Nutrients 2020, 12, 2336. [Google Scholar] [CrossRef] [PubMed]
- Stevens, Y.; Rymenant, E.V.; Grootaert, C.; Camp, J.V.; Possemiers, S.; Masclee, A.; Jonkers, D. The intestinal fate of citrus flavanones and their effects on gastrointestinal health. Nutrients 2019, 11, 1464. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, G.R.; Vasconcelos, A.B.S.; Wu, D.T.; Li, H.B.; Antony, P.J.; Li, H.; Geng, F.; Gurgel, R.Q.; Narain, N.; Gan, R.Y. Citrus flavonoids as promising phytochemicals targeting diabetes and related complications: A systematic review of in vitro and in vivo studies. Nutrients 2020, 12, 2907. [Google Scholar] [CrossRef]
- Leray, V.; Freuchet, B.; Le Bloc’h, J.; Jeusette, I.; Torre, C.; Nguyen, P. Effect of citrus polyphenol- and curcumin-supplemented diet on inflammatory state in obese cats. Br. J. Nutr. 2011, 106 (Suppl. S1), S198–S201. [Google Scholar] [CrossRef]
- Pla-Pagà, L.; Valls, R.M.; Pedret, A.; Calderón-Pérez, L.; Llauradó, E.; Companys, J.; Domenech-Coca, C.; Canela, N.; Del Bas, J.M.; Caimari, A.; et al. Effect of the consumption of hesperidin in orange juice on the transcriptomic profile of subjects with elevated blood pressure and stage 1 hypertension: A randomized controlled trial (CITRUS study). Clin. Nutr. 2021, 40, 5812–5822. [Google Scholar] [CrossRef]
- Valls, R.M.; Pedret, A.; Calderón-Pérez, L.; Llauradó, E.; Pla-Pagà, L.; Companys, J.; Moragas, A.; Martín-Luján, F.; Ortega, Y.; Giralt, M.; et al. Effects of hesperidin in orange juice on blood and pulse pressures in mildly hypertensive individuals: A randomized controlled trial (Citrus study). Eur. J. Nutr. 2021, 60, 1277–1288. [Google Scholar] [CrossRef]
- Pla-Pagà, L.; Pedret, A.; Valls, R.M.; Calderón-Pérez, L.; Llauradó, E.; Companys, J.; Martín-Luján, F.; Moragas, A.; Canela, N.; Puiggròs, F.; et al. Effects of hesperidin consumption on the cardiovascular system in pre- and stage 1 hypertensive subjects: Targeted and non-targeted metabolomic approaches (CITRUS Study). Mol. Nutr. Food Res. 2021, 65, e2001175. [Google Scholar] [CrossRef]
- Alharbi, M.H.; Lamport, D.J.; Dodd, G.F.; Saunders, C.; Harkness, L.; Butler, L.T.; Spencer, J.P. Flavonoid-rich orange juice is associated with acute improvements in cognitive function in healthy middle-aged males. Eur. J. Nutr. 2016, 55, 2021–2029. [Google Scholar] [CrossRef] [Green Version]
- Kean, R.J.; Lamport, D.J.; Dodd, G.F.; Freeman, J.E.; Williams, C.M.; Ellis, J.A.; Butler, L.T.; Spencer, J.P. Chronic consumption of flavanone-rich orange juice is associated with cognitive benefits: An 8-wk, randomized, double-blind, placebo-controlled trial in healthy older adults. Am. J. Clin. Nutr. 2015, 101, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guirro, M.; Gual-Grau, A.; Gibert-Ramos, A.; Alcaide-Hidalgo, J.M.; Canela, N.; Arola, L.; Mayneris-Perxachs, J. Metabolomics elucidates dose-dependent molecular beneficial effects of hesperidin supplementation in rats fed an obesogenic diet. Antioxidants 2020, 9, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mas-Capdevila, A.; Teichenne, J.; Domenech-Coca, C.; Caimari, A.; Del Bas, J.M.; Escote, X.; Crescenti, A. Effect of hesperidin on cardiovascular disease risk factors: The role of intestinal microbiota on hesperidin bioavailability. Nutrients 2020, 12, 1488. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Khan, F. A mechanistic review of the anticancer potential of hesperidin, a natural flavonoid from citrus fruits. Nutr. Res. 2021, 92, 21–31. [Google Scholar] [CrossRef]
- Kim, D.H.; Jung, E.A.; Sohng, I.S.; Han, J.A.; Kim, T.H.; Han, M.J. Intestinal bacterial metabolism of flavonoids and its relation to some biological activities. Arch. Pharm. Res. 1998, 21, 17–23. [Google Scholar] [CrossRef]
- Liu, P.; Li, J.; Liu, M.; Zhang, M.; Xue, Y.; Zhang, Y.; Han, X.; Jing, X.; Chu, L. Hesperetin modulates the Sirt1/Nrf2 signaling pathway in counteracting myocardial ischemia through suppression of oxidative stress, inflammation, and apoptosis. Biomed. Pharmacother. 2021, 139, 111552. [Google Scholar] [CrossRef]
- Khan, A.; Ikram, M.; Hahm, J.R.; Kim, M.O. Antioxidant and anti-inflammatory effects of citrus flavonoid hesperetin: Special focus on neurological disorders. Antioxidants 2020, 9, 609. [Google Scholar] [CrossRef]
- Noda, S.; Tanabe, S.; Suzuki, T. Differential effects of flavonoids on barrier integrity in human intestinal Caco-2 cells. J. Agric. Food Chem. 2012, 60, 4628–4633. [Google Scholar] [CrossRef]
- Kang, G.D.; Kim, D.H. Poncirin and its metabolite ponciretin attenuate colitis in mice by inhibiting LPS binding on TLR4 of macrophages and correcting Th17/Treg imbalance. J. Ethnopharmacol. 2016, 189, 175–185. [Google Scholar] [CrossRef]
- Kang, G.D.; Kim, D.H. Ponciretin attenuates ethanol-induced gastric damage in mice by inhibiting inflammatory responses. Int. Immunopharmacol. 2017, 43, 179–186. [Google Scholar] [CrossRef]
- Kezimana, P.; Dmitriev, A.A.; Kudryavtseva, A.V.; Romanova, E.V.; Melnikova, N.V. Secoisolariciresinol diglucoside of flaxseed and its metabolites: Biosynthesis and potential for nutraceuticals. Front. Genet. 2018, 9, 641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imran, M.; Ahmad, N.; Anjum, F.M.; Khan, M.K.; Mushtaq, Z.; Nadeem, M.; Hussain, S. Potential protective properties of flax lignan secoisolariciresinol diglucoside. Nutr. J. 2015, 14, 71. [Google Scholar] [CrossRef] [Green Version]
- Senizza, A.; Rocchetti, G.; Mosele, J.I.; Patrone, V.; Callegari, M.L.; Morelli, L.; Lucini, L. Lignans and gut microbiota: An interplay revealing potential health implications. Molecules 2020, 25, 5709. [Google Scholar] [CrossRef] [PubMed]
- Ephraim, E.; Jewell, D.E. Effect of added dietary betaine and soluble fiber on metabolites and fecal microbiome in dogs with early renal disease. Metabolites 2020, 10, 370. [Google Scholar] [CrossRef] [PubMed]
- Possemiers, S.; Bolca, S.; Eeckhaut, E.; Depypere, H.; Verstraete, W. Metabolism of isoflavones, lignans and prenylflavonoids by intestinal bacteria: Producer phenotyping and relation with intestinal community. FEMS Microbiol. Ecol. 2007, 61, 372–383. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, T.; Yang, C.; Bao, T.; Yang, X.; He, F.; Zhang, Y.; Zhu, L.; Chen, H.; Rong, S.; et al. Secoisolariciresinol diglucoside suppresses dextran sulfate sodium salt-induced colitis through inhibiting NLRP1 inflammasome. Int. Immunopharmacol. 2020, 78, 105931. [Google Scholar] [CrossRef]
- Zhang, S.; Cheng, M.; Wang, Z.; Liu, Y.; Ren, Y.; Rong, S.; Wang, X. Secoisolariciresinol diglucoside exerts anti-inflammatory and antiapoptotic effects through inhibiting the Akt/IκB/NF-κB pathway on human umbilical vein endothelial cells. Mediat. Inflamm. 2020, 2020, 3621261. [Google Scholar] [CrossRef]
- Aqeel, T.; Gurumallu, S.C.; Bhaskar, A.; Hashimi, S.M.; Javaraiah, R. Secoisolariciresinol diglucoside protects against cadmium-induced oxidative stress-mediated renal toxicity in rats. J. Trace Elem. Med. Biol. 2020, 61, 126552. [Google Scholar] [CrossRef]
- Ozgocmen, M.; Bayram, D.; Yavuz Turel, G.; Togay, V.A.; Calapoğlu, N.S. Secoisolariciresinol diglucoside induces caspase-3-mediated apoptosis in monolayer and spheroid cultures of human colon carcinoma cells. J. Food Biochem. 2021, 45, e13719. [Google Scholar] [CrossRef]
- Shin, M.K.; Jeon, Y.D.; Jin, J.S. Apoptotic effect of enterodiol, the final metabolite of edible lignans, in colorectal cancer cells. J. Sci. Food Agric. 2019, 99, 2411–2419. [Google Scholar] [CrossRef]
- Hu, Y.; Li, Y.; Sampson, L.; Wang, M.; Manson, J.E.; Rimm, E.; Sun, Q. Lignan intake and risk of coronary heart disease. J. Am. Coll. Cardiol. 2021, 78, 666–678. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.P.; Aryal, P.; Robinson, S.; Rafiu, R.; Obrenovich, M.; Perry, G. Polyphenols in Alzheimer’s Disease and in the gut-brain axis. Microorganisms 2020, 8, 199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilla, R.; Suchodolski, J.S. The gut microbiome of dogs and cats, and the influence of diet. Vet. Clin. N. Am. Small Anim. Pract. 2021, 51, 605–621. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.A.; Jewell, D.E.; Ephraim, E. Feeding cats with chronic kidney disease food supplemented with betaine and prebiotics increases total body mass and reduces uremic toxins. PLoS ONE 2022, 17, e0268624. [Google Scholar] [CrossRef] [PubMed]

| Fiber Bundle Percentage in Food | ||||
|---|---|---|---|---|
| Nutrient Parameter | Control Food | 1% | 2% | 4% |
| Moisture | 4.97 | 4.70 | 4.76 | 4.30 |
| Crude protein | 37.00 | 36.63 | 35.81 | 38.06 |
| Crude fat | 19.53 | 19.31 | 19.34 | 19.67 |
| Atwater energy 1 (kcal/kg) | 4066 | 4049 | 4031 | 4052 |
| Calories (kcal/kg) | 5225 | 5225 | 5313 | 5357 |
| Ash | 6.23 | 6.09 | 6.41 | 6.33 |
| Crude fiber | 0.6 | 1.1 | 1.3 | 1.7 |
| Nitrogen-free extract | 31.7 | 32.2 | 32.4 | 29.9 |
| Total dietary fiber | 4.3 | 5.3 | 4.6 | 6.1 |
| Total insoluble fiber | 3.2 | 4.7 | 4.2 | 5.3 |
| Total soluble fiber | 1.1 | 0.6 | 0.4 | 0.8 |
| Neutral detergent fiber | 3.70 | 5.20 | 4.20 | 4.30 |
| Calcium | 1.09 | 1.09 | 1.18 | 1.15 |
| Phosphorus | 0.87 | 0.90 | 0.92 | 0.90 |
| Sodium | 0.33 | 0.36 | 0.34 | 0.34 |
| Omega-3 sum | 0.19 | 0.25 | 0.28 | 0.36 |
| Omega-6 sum | 3.41 | 3.55 | 3.40 | 3.60 |
| Palmitic acid [16:0] | 4.23 | 4.38 | 4.43 | 4.23 |
| Palmitoleic acid [16:1] | 1.08 | 1.13 | 1.09 | 1.15 |
| Stearic acid [18:0] | 1.08 | 1.10 | 1.09 | 1.15 |
| Oleic acid [18:1] | 6.78 | 7.03 | 6.82 | 7.19 |
| Linoleic acid [18:2 (n − 6)] | 3.15 | 3.29 | 3.15 | 3.31 |
| Alpha-linolenic acid [18:3 (n − 3)] | 0.14 | 0.20 | 0.23 | 0.31 |
| Arachidonic acid [20:4 (n − 6)] | 0.12 | 0.12 | 0.13 | 0.14 |
| Lysine | 2.01 | 2.01 | 2.03 | 2.34 |
| Threonine | 1.37 | 1.39 | 1.39 | 1.44 |
| Methionine | 1.29 | 1.27 | 1.25 | 1.35 |
| Cystine | 0.51 | 0.50 | 0.47 | 0.49 |
| Tryptophan | 0.36 | 0.35 | 0.36 | 0.40 |
| Fiber Bundle Percentage in Food | ||||
|---|---|---|---|---|
| Parameter | Control Food | 1% | 2% | 4% |
| Body weight, kg | ||||
| Day 0 | 5.60 ± 0.26 | 5.60 ± 0.26 | 5.09 ± 0.26 | 5.10 ± 0.26 |
| Day 31 | 5.63 ± 0.25 | 5.65 ± 0.25 | 5.17 ± 0.25 | 5.17 ± 0.25 |
| Change | 0.03 ± 0.03 | 0.05 ± 0.03 | 0.08 ± 0.03 1 | 0.07 ± 0.03 1 |
| Food intake, kcal/(body weight in kg) 0.75 | 53.0 ± 2.5 | 55.8 ± 2.5 | 54.4 ± 2.5 | 53.5 ± 2.5 |
| Albumin, mg/dL | ||||
| Day 0 | 3.34 ± 0.08 | 3.27 ± 0.08 | 3.29 ± 0.08 | 3.30 ± 0.08 |
| Day 31 | 3.38 ± 0.08 | 3.29 ± 0.08 | 3.31 ± 0.08 | 3.38 ± 0.08 |
| Change | 0.03 ± 0.04 | 0.01 ± 0.04 | 0.01 ± 0.04 | 0.08 ± 0.04 |
| Total protein, mg/dL | ||||
| Day 0 | 6.59 ± 0.12 | 6.73 ± 0.12 | 6.72 ± 0.12 | 6.55 ± 0.12 |
| Day 31 | 6.39 ± 0.12 | 6.46 ± 0.12 | 6.57 ± 0.12 | 6.41 ± 0.12 |
| Change | −0.20 ± 0.08 1 | −0.26 ± 0.08 1 | −0.15 ± 0.08 | −0.14 ± 0.08 |
| Urea nitrogen, mg/dL | ||||
| Day 0 | 22.6 ± 0.9 | 23.0 ± 0.9 | 22.5 ± 0.9 | 22.1 ± 0.9 |
| Day 31 | 20.1 ± 0.9 | 20.5 ± 0.9 | 20.7 ± 0.9 | 20.8 ± 0.9 |
| Change | −1.4 ± 0.4 1 | −2.5 ± 0.4 1 | −1.9 ± 0.4 1 | −1.3 ± 0.4 1 |
| Creatinine, mg/dL | ||||
| Day 0 | 1.15 ± 0.05 | 1.19 ± 0.05 | 1.11 ± 0.05 | 1.12 ± 0.05 |
| Day 31 | 1.25 ± 0.05 | 1.28 ± 0.05 | 1.18 ± 0.05 | 1.22 ± 0.05 |
| Change | 0.10 ± 0.03 1 | 0.10 ± 0.03 1 | 0.07 ± 0.03 1 | 0.10 ± 0.03 1 |
| Triglycerides, mg/dL | ||||
| Day 0 | 54.4 ± 9.5 | 54.6 ± 9.5 | 54.0 ± 9.5 | 59.8 ± 9.5 |
| Day 31 | 36.1 ± 10.7 | 41.4 ± 10.7 | 41.6 ± 10.7 | 57.6 ± 10.7 |
| Change | −18.2 ± 7.5 1 | −15.1 ± 7.5 1 | −12.4 ± 7.5 | −2.2 ± 7.5 |
| Cholesterol, mg/dL | ||||
| Day 0 | 212.9 ± 15.0 | 208.0 ± 15.0 | 203.0 ± 15.0 | 220.6 ± 15.0 |
| Day 31 | 213.0 ± 15.3 | 205.9 ± 15.3 | 187.9 ± 15.3 | 227.4 ± 15.3 |
| Change | 0.1 ± 4.5 a | −2.1 ± 4.5 a | −15.1 ± 4.5 b,1 | 6.9 ± 4.5 a |
| Fiber Bundle Percentage in Food | ||||
|---|---|---|---|---|
| Parameter | Control Food | 1% | 2% | 4% |
| Moisture | ||||
| Day 0 | 60.0 ± 0.7 a | 56.9 ± 0.9 b | 62.7 ± 0.7 c | 62.9 ± 0.8 c |
| Day 10, % of Day 0 | 102 ± 2 b | 109 ± 2 a | 97 ± 2 b | 99 ± 2 b |
| Day 31, % of Day 0 | 101 ± 2 a,b | 105 ± 2 a | 98 ± 2 b | 100 ± 2 a,b |
| Ammonium, mmol/g | ||||
| Day 0 | 0.050 ± 0.002 a | 0.056 ± 0.002 b | 0.045 ± 0.002 c | 0.047 ± 0.002 a,c |
| Day 10, % of Day 0 | 113 ± 5 a | 91 ± 5 b | 107 ± 5 a | 92 ± 5 b |
| Day 31, % of Day 0 | 100 ± 5 | 93 ± 5 | 94 ± 5 | 86 ± 5 1 |
| pH | ||||
| Day 0 | 6.04 ± 0.07 | 6.04 ± 0.07 | 5.99 ± 0.07 | 5.97 ± 0.08 |
| Day 10, % of Day 0 | 94 ± 2 1 | 96 ± 2 | 93 ± 2 | 94 ± 2 1 |
| Day 31, % of Day 0 | 98 ± 2 | 98 ± 2 | 94 ± 2 | 96 ± 2 |
| Stool score | ||||
| Day 0 | 4.91 ± 0.12 | 5.0 ± 0.12 | 4.66 ± 0.12 | 5.0 ± 0.12 |
| Day 10 | 5.0 ± 0.12 | 4.91 ± 0.12 | 5.0 ± 0.12 | 4.63 ± 0.12 |
| Day 31 | 4.91 ± 0.12 | 5.0 ± 0.12 | 4.75 ± 0.12 | 4.9 ± 0.12 |
| Fiber Bundle Percentage in Food | ||||
|---|---|---|---|---|
| SCFA | Control Food | 1% | 2% | 4% |
| Acetic acid | ||||
| Day 0, μg/g | 3428 ± 374 | 3273 ± 374 | 4106 ± 360 | 3895 ± 389 |
| Day 10, % of Day 0 | 91 ± 6 | 107 ± 6 | 99 ± 6 | 111 ± 6 |
| Day 31, % of Day 0 | 100 ± 6 | 110 ± 6 | 98 ± 6 | 107 ± 6 |
| Propionic acid | ||||
| Day 0, μg/g | 1741 ± 271 | 1762 ± 282 | 2138 ± 282 | 1953 ± 282 |
| Day 10, % of Day 0 | 104 ± 7 a,b | 115 ± 8 a,b | 101 ± 8 a | 124 ± 8 b,1 |
| Day 31, % of Day 0 | 92 ± 7 | 97 ± 7 | 97 ± 7 | 111 ± 8 |
| Butyric acid | ||||
| Day 0, μg/g | 2254 ± 282 | 2216 ± 282 | 2406 ± 272 | 2204 ± 294 |
| Day 10, % of Day 0 | 139 ± 9 a,1 | 127 ± 8 a,b,1 | 109 ± 9 b,c | 99 ± 9 c |
| Day 31, % of Day 0 | 111 ± 9 a,b | 119 ± 9 a,1 | 104 ± 9 a,b | 90 ± 9 b |
| Valeric acid | ||||
| Day 0, μg/g | 1270 ± 161 | 1234 ± 167 | 1148 ± 167 | 1266 ± 167 |
| Day 10, % of initial | 147 ± 129 | 151 ± 135 | 123 ± 135 | 151 ± 135 |
| Day 31, % of initial | 119 ± 129 b | 128 ± 129 b | 436 ± 125 a,1 | 94 ± 135 b |
| Hexanoic acid | ||||
| Day 0, μg/g | 183 ± 39 | 258 ± 39 | 113 ± 38 | 197 ± 41 |
| Day 10, % of Day 0 | 199 ± 87 | 239 ± 92 | 104 ± 92 | 258 ± 91 |
| Day 31, % of Day 0 | 125 ± 87 b | 197 ± 88 a,b | 395 ± 85 a,1 | 130 ± 91 b |
| BCFA | ||||
| 2-methylpropionic acid | ||||
| Day 0, μg/g | 301 ± 21 | 314 ± 22 | 244 ± 22 | 250 ± 22 |
| Day 10, % of Day 0 | 116 ± 10 | 110 ± 11 | 95 ± 11 | 93 ± 10 |
| Day 31, % of Day 0 | 86 ± 11 a | 114 ± 10 b | 118 ± 10 b | 76 ± 11 a,1 |
| 2-methylbutyric acid | ||||
| Day 0, μg/g | 244 ± 19 | 276 ± 19 | 194 ± 19 | 199 ± 19 |
| Day 10, % of Day 0 | 124 ± 10 1 | 114 ± 10 | 110 ± 10 | 98 ± 10 |
| Day 31, % of Day 0 | 87 ± 10 a | 115 ± 10 b | 116 ± 10 b | 74 ± 10 a,1 |
| 3-methylbutyric acid | ||||
| Day 0, μg/g | 357 ± 26 | 394 ± 27 | 304 ± 27 | 313 ± 27 |
| Day 10, % of Day 0 | 125 ± 11 1 | 124 ± 12 1 | 101 ± 12 | 103 ± 12 |
| Day 31, % of Day 0 | 92 ± 11 a | 125 ± 12 b,1 | 119 ± 11 b | 77 ± 12 c,1 |
| Fiber Bundle Percentage in Food | ||||
|---|---|---|---|---|
| Metabolite | Control Food | 1% | 2% | 4% |
| Hesperidin | ||||
| Day 10 ratio | 0.04 ± 0.25 a | 2.89 ± 0.26 b,1 | 3.89 ± 0.26 c,1 | 5.02 ± 0.26 d,1 |
| Day 31 ratio | 0.05 ± 0.26 a | 2.22 ± 0.25 b,1 | 3.85 ± 0.24 c,1 | 4.91 ± 0.27 d,1 |
| Hesperetin | ||||
| Day 10 ratio | 0.62 ± 0.38 a | 4.66 ± 0.17 b,1 | 5.23 ± 0.17 c,1 | 6.06 ± 0.17 d,1 |
| Day 31 ratio | 0.67 ± 0.38 a | 4.19 ± 0.17 b,1 | 5.12 ± 0.16 c,1 | 5.94 ± 0.18 d,1 |
| Ponciretin | ||||
| Day 10 ratio | 0.25 ± 0.14 a | 3.51 ± 0.14 b,1 | 3.90 ± 0.14 c,1 | 5.06 ± 0.14 b,1 |
| Day 31 ratio | 0.12 ± 0.15 a | 3.21 ± 0.14 b,1 | 4.06 ± 0.13 c,1 | 4.86 ± 0.15 b,1 |
| Secoisolariciresinol diglucoside | ||||
| Day 10 ratio | 0.00 ± 0.15 a | 0.27 ± 0.16 a | 0.46 ± 0.16 b,1 | 1.14 ± 0.16 c,1 |
| Day 31 ratio | 0.00 ± 0.16 a | 0.20 ± 0.15 a | 0.90 ± 0.15 b,1 | 1.58 ± 0.17 c,1 |
| Secoisolariciresinol | ||||
| Day 10 ratio | 0.05 ± 0.16 a | 2.28 ± 0.17 b,1 | 2.49 ± 0.17 b,1 | 3.41 ± 0.17 c,1 |
| Day 31 ratio | 0.20 ± 0.17 a | 1.77 ± 0.16 b,1 | 2.29 ± 0.16 c,1 | 3.42 ± 0.18 d,1 |
| Enterodiol | ||||
| Day 10 ratio | 0.13 ± 0.14 a | 1.99 ± 0.14 b,1 | 2.93 ± 0.14 c,1 | 3.26 ± 0.14 c,1 |
| Day 31 ratio | 0.08 ± 0.14 a | 2.29 ± 0.14 b,1 | 3.12 ± 0.14 c,1 | 3.27 ± 0.14 c,1 |
| Arabinose | ||||
| Day 10 ratio | −0.60 ± 0.22 a,1 | −0.08 ± 0.23 b | −0.17 ± 0.23 a,b | 0.19 ± 0.23 b |
| Day 31 ratio | −0.46 ± 0.22 a,1 | −0.41 ± 0.22 a | 0.71 ± 0.21 b,1 | 0.91 ± 0.23 b,1 |
| Ribulose/xylulose | ||||
| Day 10 ratio | −0.65 ± 0.30 a,1 | −0.36 ± 0.31 a,b | −0.54 ± 0.31 a | 0.40 ± 0.31 b |
| Day 31 ratio | −0.45 ± 0.32 a | −0.28 ± 0.30 a | 0.87 ± 0.29 b,1 | 0.96 ± 0.33 b,1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jewell, D.E.; Jackson, M.I.; Cochrane, C.-Y.; Badri, D.V. Feeding Fiber-Bound Polyphenol Ingredients at Different Levels Modulates Colonic Postbiotics to Improve Gut Health in Cats. Animals 2022, 12, 1654. https://doi.org/10.3390/ani12131654
Jewell DE, Jackson MI, Cochrane C-Y, Badri DV. Feeding Fiber-Bound Polyphenol Ingredients at Different Levels Modulates Colonic Postbiotics to Improve Gut Health in Cats. Animals. 2022; 12(13):1654. https://doi.org/10.3390/ani12131654
Chicago/Turabian StyleJewell, Dennis E., Matthew I. Jackson, Chun-Yen Cochrane, and Dayakar V. Badri. 2022. "Feeding Fiber-Bound Polyphenol Ingredients at Different Levels Modulates Colonic Postbiotics to Improve Gut Health in Cats" Animals 12, no. 13: 1654. https://doi.org/10.3390/ani12131654
APA StyleJewell, D. E., Jackson, M. I., Cochrane, C.-Y., & Badri, D. V. (2022). Feeding Fiber-Bound Polyphenol Ingredients at Different Levels Modulates Colonic Postbiotics to Improve Gut Health in Cats. Animals, 12(13), 1654. https://doi.org/10.3390/ani12131654






