Computed Tomography-Guided Fine Needle Biopsies of Vertebral and Paravertebral Lesions in Small Animals
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods:
2.1. Study Population
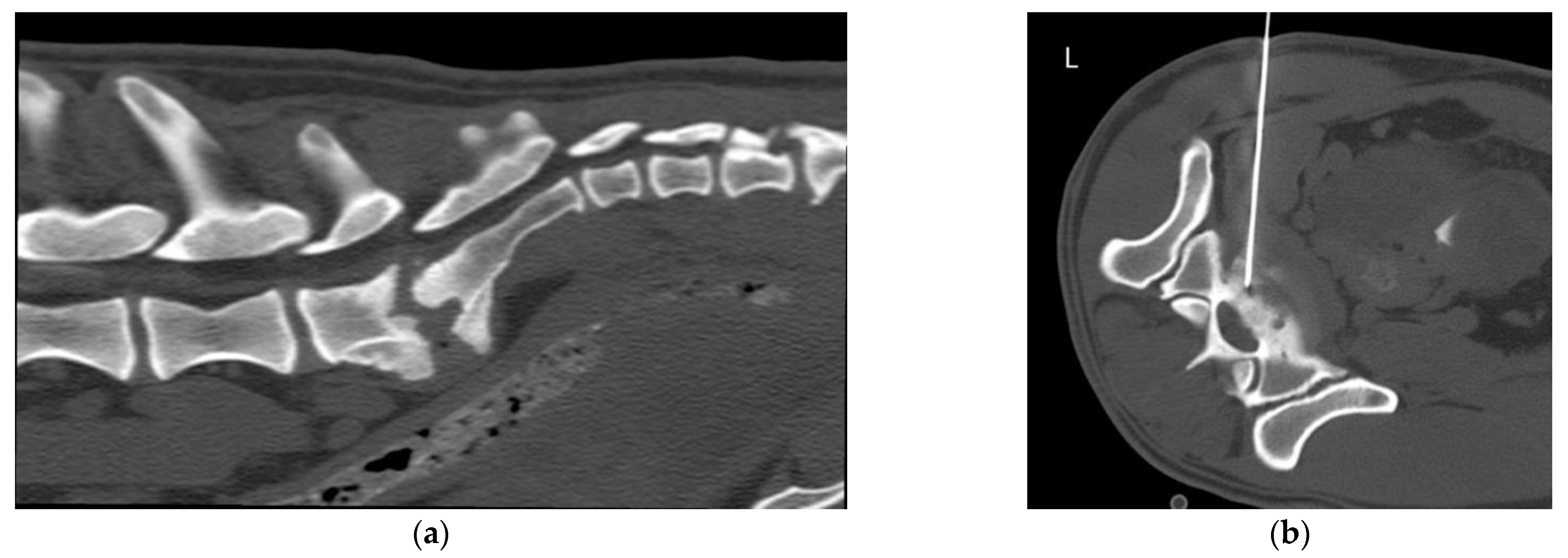
2.2. Equipment and Technique
2.3. Descriptive Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Samii, V.F.; Nyland, T.G.; Werner, L.L.; Baker, T.W. Ultrasound-Guided Fine-Needle Aspiration Biopsy of Bone Lesions: A Preliminary Report. Vet. Radiol. Ultrasound 1999, 40, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Tidwell, A.S.; Johnson, K.L. Computed Tomography-Guided Percutaneous Biopsy: Criteria for Accurate Needle Tip Identification. Vet. Radiol. Ultrasound 1994, 35, 440–444. [Google Scholar] [CrossRef]
- Tidwell, A.S.; Johnson, K.L. Computed Tomography-Guided Percutaneous Biopsy in the Dog and Cat: Description of Technique and Preliminary Evaluation in 14 Patients. Vet. Radiol. Ultrasound 1994, 35, 445–456. [Google Scholar] [CrossRef]
- Vignoli, M.; Tamburro, R.; Felici, A.; del Signore, F.; Dettori, A.; di Tommaso, M.; Ghiraldelli, A.; Terragni, R.; Simeoni, F.; Falerno, I.; et al. Clinical Value of CT-Guided Fine Needle Aspiration and Tissue-Core Biopsy of Thoracic Masses in the Dog and Cat. Animals 2021, 11, 883. [Google Scholar] [CrossRef] [PubMed]
- Zekas, L.J.; Crawford, J.T.; O’Brien, R.T. Computed Tomography-Guided Fine-Needle Aspirate and Tissue-Core Biopsy of Intrathoracic Lesions in Thirty Dogs and Cats. Vet. Radiol. Ultrasound 2005, 46, 200–204. [Google Scholar] [CrossRef]
- Vignoli, M.; Gnudi, G.; Laganga, P.; Gazzola, M.; Rossi, F.; Terragni, R.; di Giancamillo, M.; Secchiero, D.B.; Citi, S.; Cantoni, A.M.; et al. CT-Guided Fine-Needle Aspiration and Tissue-Core Biopsy of Lung Lesions in the Dog and Cat. Eur. J. Companion Anim. Pract. 2007, 17, 23–28. [Google Scholar]
- Vignoli, M.; Ohlerth, S.; Rossi, F.; Pozzi, L.; Terragni, R.; Corlazzoli, D.; Kaser-Hotz, B. Computed Tomography-Guided Fine-Needle Aspiration and Tissue-Core Biopsy of Bone Lesions in Small Animals. Vet. Radiol. Ultrasound 2004, 45, 125–130. [Google Scholar] [CrossRef]
- Giroux, A.; Jones, J.C.; Bohn, J.H.; Duncan, R.B.; Waldron, D.R.; Inzana, K.R. A New Device for Stereotactic CT-Guided Biopsy of the Canine Brain: Design, Construction, and Needle Placement Accuracy. Vet. Radiol. Ultrasound 2002, 43, 229–236. [Google Scholar] [CrossRef] [Green Version]
- Bersan, E.; Maddox, T.; Walmsley, G.; Piviani, M.; Burrow, R. CT-Guided Drainage of a Brainstem Abscess in a Cat as an Emergency Treatment Procedure. J. Feline Med. Surg. Open Rep. 2020, 6, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Añor, S.; Sturges, B.K.; Lafranco, L.; Jang, S.S.; Higgins, R.J.; Koblik, P.D.; Lecouteur, R.A. Systemic Phaeohyphomycosis (Cladophialophora Bantiana) in a Dog-Clinical Diagnosis with Stereotactic Computed Tomographic-Guided Brain Biopsy. J. Vet. Intern. Med. 2001, 15, 257–261. [Google Scholar]
- Koblik, P.D.; Lecouteur, R.A.; Higgins, R.J.; Bollen, A.W.; Vernau, K.M.; Kortz, G.D.; Ilkiw, J.E. CT-Guided Brain Biopsy Using a Modified Pelorus Mark I11 Stereotactic System: Experience with 50 Dogs. Vet. Radiol. Ultrasound 1999, 40, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Moissonnier, P.; Blot, S.; Devauchelle, P.; Delisle, F.; Beuvon, F.; Boulha, L.; Colle, M.-A.; Lefrancois, T. Stereotactic CT-Guided Brain Biopsy in the Dog. J. Small Anim. Pract. 2002, 43, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Phadke, D.M.; Lucas, D.R.; Madan, S. Fine-Needle Aspiration Biopsy of Vertebral and Intervertebral Disc Lesions. Specimen Adequacy, Diagnostic Utility, and Pitfalls. Arch. Pathol. Lab. Med. 2001, 125, 1463–1468. [Google Scholar] [CrossRef] [PubMed]
- den Heeten, G.; Oosterhuis, J.; Schraffordt Koops, H. Biopsy of Bone Tumours. J. Surg. Oncol. 1985, 28, 247–251. [Google Scholar] [CrossRef]
- Nourbakhsh, A.; Grady, J.J.; Garges, K.J. Percutaneous Spine Biopsy: A Meta-Analysis. J. Bone Jt. Surg. Ser. A 2008, 90, 1722–1725. [Google Scholar] [CrossRef]
- Garg, V.; Kosmas, C.; Young, P.C.; Togaru, U.K.; Robb, M.R. Computed Tomography-Guided Percutaneous Biopsy for Vertebral Osteomyelitis: A Department’s Experience. Neurosurg. Focus 2014, 37, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Rimondi, E.; Staals, E.L.; Errani, C.; Bianchi, G.; Casadei, R.; Alberghini, M.; Malaguti, M.C.; Rossi, G.; Durante, S.; Mercuri, M. Percutaneous CT-Guided Biopsy of the Spine: Results of 430 Biopsies. Eur. Spine J. 2008, 17, 975–981. [Google Scholar] [CrossRef] [Green Version]
- Wiesner, E.L.; Hillen, T.J.; Long, J.; Jennings, J.W. Percutaneous CT-Guided Biopsies of the Cervical Spine: Technique, Histopathologic and Microbiologic Yield, and Safety at a Single Academic Institution. Am. J. Neuroradiol. 2018, 39, 981–985. [Google Scholar] [CrossRef]
- Yapici, F.; Atici, Y.; Balioglu, M.B.; Albayrak, A.; Kargin, D.; Akman, Y.E.; Erdogan, S.; Kaygusuz, M.A. A Comparison of Two Techniques: Open and Percutaneous Biopsies of Thoracolumbar Vertebral Body Lesions. J. Craniovertebral Junction Spine 2015, 6, 36–39. [Google Scholar] [CrossRef]
- Cox, M.; Pukenas, B.; Poplawski, M.; Bress, A.; Deely, D.; Flanders, A. CT-Guided Cervical Bone Biopsy in 43 Patients: Diagnostic Yield and Safety at Two Large Tertiary Care Hospitals. Acad. Radiol. 2016, 23, 1372–1375. [Google Scholar] [CrossRef]
- Akhtar, I.; Flowers, R.; Siddiqi, A.; Heard, K.; Baliga, M. Fine Needle Aspiration Biopsy of Vertebral and Paravertebral Lesions. Retrospective Study of 124 Cases. Acta Cytol. 2006, 50, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Saad, R.S.; Liu, Y.; Clary, K.M.; Silverman, J.F.; Raab, S.A.C.S. Fine Needle Aspiration Biopsy of Vertebral Lesions Radiologically Guided. Acta Cytol. 2004, 48, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Jorda, M.; Rey, L.; Hanly, A.; Ganjei-Azar, P. Fine-Needle Aspiration Cytology of Bone: Accuracy and Pitfalls of Cytodiagnosis. Cancer 2000, 90, 47–54. [Google Scholar] [CrossRef]
- Tampieri, D.; Weill, A.; Melanson, D.; Ethier, R. Percutaneous Aspiration Biopsy in Cervical Spine Lytic Lesions Indications and Technique. Neuroradiology 1991, 33, 43–47. [Google Scholar] [CrossRef]
- Kang, M.; Gupta, S.; Khandelwal, N.; Shankar, S.; Gulati, M.; Suri, S. CT-Guided Fine-Needle Aspiration Biopsy of Spinal Lesions. Acta Radiol. 1999, 40, 474–478. [Google Scholar] [CrossRef]
- Reiter, A.M.; Schwarz, T. Computed Tomographic Appearance of Masticatory Myositis in Dogs: 7 Cases (1999–2006). J. Am. Vet. Med. Assoc. 2007, 231, 924–930. [Google Scholar] [CrossRef]
- Gala, K.B.; Shetty, N.S.; Janu, A.K.; Shetty, N.; Kulkarni, S.S. Percutaneous CT Guided Vertebral Biopsy: Anatomy and Technical Considerations. J. Clin. Interv. Radiol. ISVIR 2021, 5, 150–157. [Google Scholar] [CrossRef]
- Vignoli, M.; Saunders, J.H. Image-Guided Interventional Procedures in the Dog and Cat. Vet. J. 2011, 187, 297–303. [Google Scholar] [CrossRef]
- Uhl, M.; Theves, C.; Geiger, J.; Kersten, A.; Strohm, P.C. The Percutaneous Bone Biopsy: In Vitro Study for Comparison of Bone Biopsy Needles. Z. Orthop. Unf. 2009, 147, 327–333. [Google Scholar] [CrossRef]
- Fischer, A.; Mahaffey, M.B.; Oliver, J.E. Fluoroscopically Guided Percutaneous Disk Aspiration in 10 Dogs with Diskospondylitis. J. Vet. Intern. Med. 1997, 11, 284–287. [Google Scholar] [CrossRef]
- Finnen, A.; Blond, L.; Parent, J. Cervical Discospondylitis in 2 Great Dane Puppies Following Routine Surgery. Can. Vet. J. 2012, 53, 531–534. [Google Scholar] [PubMed]




| Case | Species Breed Gender Age | History and Clinical Signs | Neurological Examination (NE) and Neurolocalization (NLoc) | CT Findings Suspected Imaging Diagnosis | Location of Tip of the FNB Needle | Diagnosis from CT-Guided FNB (Cytology/Culture) | Diagnosis from Surgical Biopsy or Necropsy and Histopathology |
|---|---|---|---|---|---|---|---|
| 1 | Canine German shepherd dog Male intact 7 yo | Chronic progressive hindlimb weakness | NE: absence of postural reactions (PR) in both HLs, normal spinal reflexes (SR) and thoracolumbar hyperesthesia NLoc: T3-L3 myelopathy | Polyostotic aggressive bone lesion (mainly lytic) in L3 and L4 articular processes, laminae and pedicles, mostly right-sided. Non-primary bone tumor, synovial cell sarcoma | Right L4 cranial articular process | Mesenchymal tumor consistent with sarcoma | |
| 2 | Canine Staffordshire bull terrier Male intact 6 yo | Chronic progressive lumbosacral pain (currently severe pain) | NE: No neurological deficits associated NLoc: LS region | Aggressive lesion centered in the IVDS at L7-S1. Severe endplate osteolysis, moderate sclerosis, narrowing of the IVDS and marked NBF. Discospondylitis (chronic) | Intervertebral disc L7-S1 | Discospondylitis by Enterobacter cloacae | |
| 3 | Canine Bodeguero andaluz Female intact 9 yo | Chronic, progressive, intermittent RHL lameness. | NE: Currently LMN monoparesis of the RHL. Absence of PR in the RHL, decreased withdrawal reflex. NLoc: L6-S2 spinal segment, L6-S2 right nerve roots or L6-S2 right spinal nerves. | Intradural extramedullary right-sided tubular mass at the level of L5 VB and L5–L6 IVF. Peripheral nerve sheath tumor | Right IVF L5–L6 | Mesenchymal tumor consistent with sarcoma | |
| 4 | Ferret Female neutered 6 yo | Acute onset of paraplegia | NE: Absence of PR and nociception and decreased withdrawal reflex in both HLs. CTMR cut off L3. TL hyperesthesia. NLoc: T3-L3 myelopathy and associated spinal shock | Polyostotic aggressive bone lesions (mainly lytic) in vertebrae and ribs, worst on L1. Non-primary bone tumor | Right L1 VB and TP | Lymphoma | Disseminated lymphoma (necropsy) |
| 5 | Canine Labrador retriever Male intact 2 yo | Chronic progressive HLs weakness and ataxia. | NE: ambulatory paraparesis and proprioceptive ataxia of the HLs. Paresis of the tail. Decreased muscle tone of HLs and tail. Absent PR and SR in the HLs and decreased perineal reflex. NLoc: L4-Cd5 myelopathy +/− L4-Cd5 nerve roots +/− L4-Cd5 spinal nerves | Severe thickening/mass located in the epidural space in the vertebral canal along L5–L7, expanding to the L5–L6 IVF (R > L). Infiltrative neoplasia (lymphoma, sarcoma) | Right L5–L6 IVF | Lymphoma | |
| 6 | Canine Weimaraner Female intact 13 yo | Chronic progressive weakness and incoordination of the HLs and spinal pain | NE: Ambulatory paraparesis. Absence of PR and normal SR in the HLs. TL hyperesthesia. NLoc: T3-L3 myelopathy | Large mass in right hypaxial muscles infiltrating VB and TP of L3 and L4 and retroperitoneal space (CVC). Pathological fracture of L3 VB with vertebral compression. Lung nodules and periaortic lymphadenopathy. Soft tissue sarcoma | Right L3 VB/ TP and hypaxial mass | Mesenchymal tumor consistent with sarcoma | |
| 7 | Canine Cross breed large Female neutered 10 yo | Chronic and progressive history of severe cervical hyperesthesia, TL kyphosis | NE: Ambulatory tetraparesis worst in the HLs; absence of PR with normal SR in all four limbs. Severe hyperesthesia in the C- and TL- spine. NLoc: multifocal C1–C5 and T3-L3 myelopathy | Multifocal aggressive lesions centered in the IVDS of the C- and T- spine, worst at C6–C7 and T11–T12. Subluxation at C6–C7. Severe endplate osteolysis, sclerosis and narrowing of the IVDS. Marked NBF. Discospondylitis (chronic) | IVDS at T11–T12 | Discospondylitis by Pseudomona aeruginosa | - |
| 8 | Canine Fox terrier Male intact 9 yo | Chronic and progressive lameness of the RHL | NE: monoparesis of the RHL, mildly decreased muscle tone. Decreased PR and SR in RHL. Mild discomfort on palpation of the LS vertebral column. NLoc: L6–L7 spinal cord segments, L6–L7 nerve roots or L6–L7 spinal nerves (right side) | Moderate thickening of several nerve roots and spinal nerves (L5–L6 right, L6–L7 right and L7-S1 bilaterally). Moderate right-sided muscle atrophy. Peripheral nerve sheath tumor | Right L6-7 IVF | Not diagnostic | Benign peripheral nerve sheath tumor (surgical biopsy) |
| 9 | Canine West Highland white terrier Female intact 10 yo | Intermittent left-sided circling, bilateral HLs weakness, progressing to FLs weakness | NE: Proprioceptive ataxia and paraparesia in HLs. Left vestibular ataxia. Absent PR with normal SR. Slight discomfort on palpation of the C- spine. NLoc: C1–C5 myelopathy | Intradural extramedullary mass corresponding to the left ventral spinal root of C2, severely compressing and invading the spinal cord, and extending extra-axially as a large tubular mass with strong perineural enhancement. Severe left-sided paraspinal muscle atrophy. Peripheral nerve sheath tumor | Tip of the needle out of the lesion. | Not evaluable | Malignant peripheral nerve sheath tumor (necropsy) |
| 10 | Canine Galgo español Female intact 12 yo | Acute bilateral HLs weakness | NE: Non ambulatory paraparesis, absent PR with normal SR in the HLs. Present crossed extensor reflex in both HLs. NLoc: T3-L3 myelopathy | Large right-sided intradural extramedullary mass at T8, severely compressing the spinal cord and extending extra-axially through the spinal nerves from T6 to T9. Peripheral nerve sheath tumor | Right T8–T9 IVF (thickened nerve) | Mesenchymal tumor, consistent with sarcoma | - |
| 11 | Canine Crossbreed Male intact 9 yo | Chronic neck pain. Acute right FL tremors and lameness | NE: Ambulatory tetraparesis. Reduced PR with normal SP in all 4 limbs. Severe hyperesthesia in the C-spine NLoc: C1–C5 myelopathy | Monostotic aggressive bone lesion in C6 (osteolytic and osteoproductive), mainly right-sided, with moderate extradural compression of the spinal cord. Primary bone tumor | Right C6 cranial articular process. | Not diagnostic | Osteosarcoma (surgical biopsy) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laborda-Vidal, P.; Martín, M.; Orts-Porcar, M.; Vilalta, L.; Melendez-Lazo, A.; de Carellán, A.G.; Ros, C. Computed Tomography-Guided Fine Needle Biopsies of Vertebral and Paravertebral Lesions in Small Animals. Animals 2022, 12, 1688. https://doi.org/10.3390/ani12131688
Laborda-Vidal P, Martín M, Orts-Porcar M, Vilalta L, Melendez-Lazo A, de Carellán AG, Ros C. Computed Tomography-Guided Fine Needle Biopsies of Vertebral and Paravertebral Lesions in Small Animals. Animals. 2022; 12(13):1688. https://doi.org/10.3390/ani12131688
Chicago/Turabian StyleLaborda-Vidal, Patricia, Myriam Martín, Marc Orts-Porcar, Laura Vilalta, Antonio Melendez-Lazo, Alejandra García de Carellán, and Carlos Ros. 2022. "Computed Tomography-Guided Fine Needle Biopsies of Vertebral and Paravertebral Lesions in Small Animals" Animals 12, no. 13: 1688. https://doi.org/10.3390/ani12131688






