Alternative Practices in Organic Dairy Production and Effects on Animal Behavior, Health, and Welfare
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Organic Livestock Production
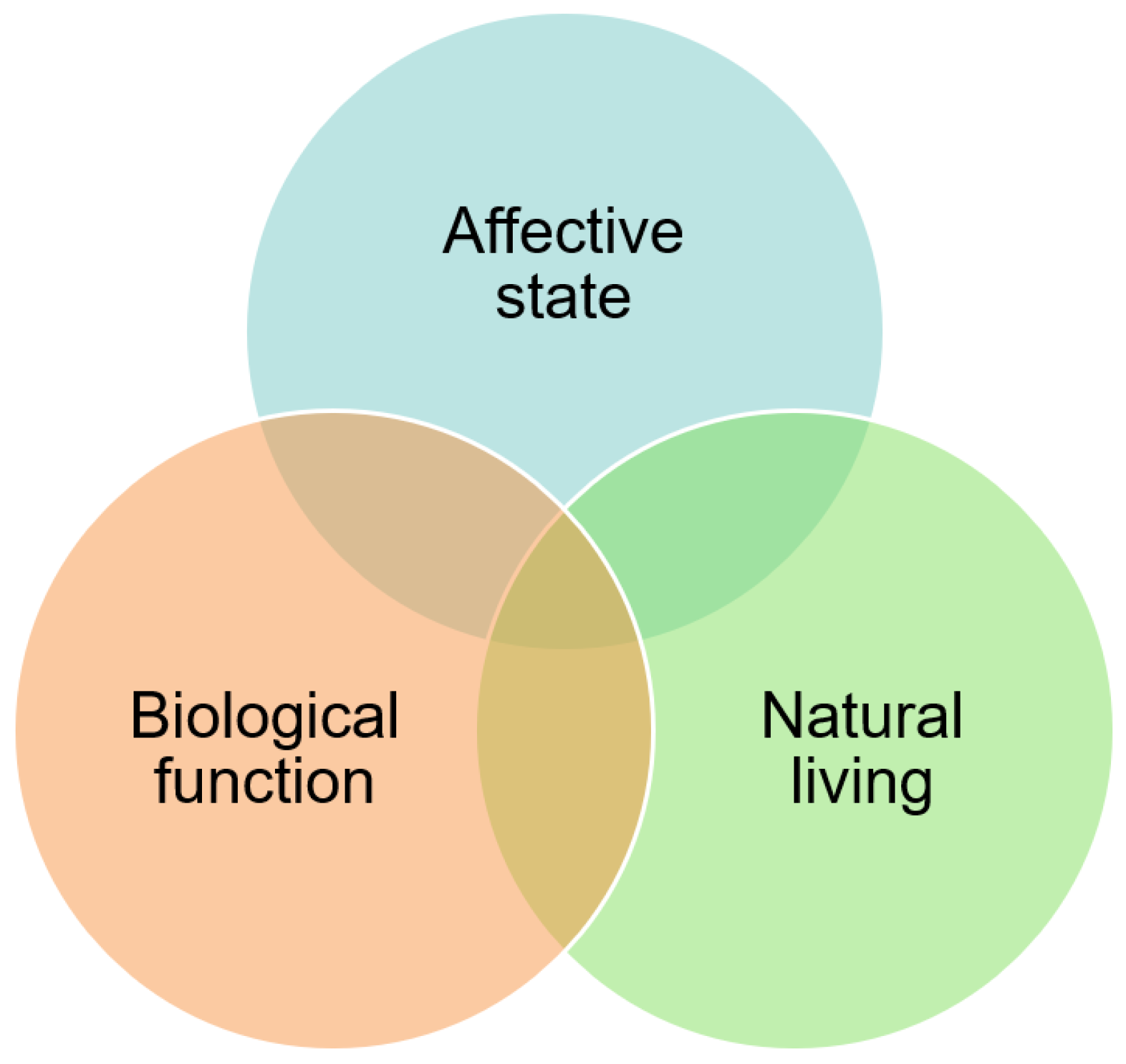
1.2. Animal Welfare
2. Pain Management for Disbudding
2.1. Horn Removal
2.2. Pain Management
2.3. Alternative Non-Steroidal Anti-Inflammatory Drugs—Synthetic Salicylates
2.4. Alternative Non-Steroidal Anti-Inflammatory Drugs—White Willow Bark
3. Managing Transition Organic Dairy Heifer Behaviors and Udder Health
3.1. Challenges of Mastitis for Organic Dairy Farms
3.2. Challenges for Early-Lactation Heifers
3.3. Methods to Modulate Aversive Behaviors and Mastitis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heckman, J. A History of Organic Farming: Transitions from Sir Albert Howard’s War in the Soil to USDA National Organic Program. Renew. Agric. Food Syst. 2006, 21, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Electronic Code of Federal Regulations. Available online: https://www.ecfr.gov/ (accessed on 4 July 2022).
- Reganold, J.P.; Wachter, J.M. Organic Agriculture in the Twenty-First Century. Nat. Plants 2016, 2, 15221. [Google Scholar] [CrossRef]
- Willer, E.H.; Lernoud, J. The World of Organic Agriculture Statistics and Emerging Trends 2019. In Proceedings of the BIOFACH, Nirnberg, Germany, 13–16 February 2019; Volume 351. [Google Scholar]
- Sorge, U.S.; Moon, R.; Wolff, L.J.; Michels, L.; Schroth, S.; Kelton, D.F.; Heins, B. Management Practices on Organic and Conventional Dairy Herds in Minnesota. J. Dairy Sci. 2016, 99, 3183–3192. [Google Scholar] [CrossRef]
- Botreau, R.; Bracke, M.B.M.; Perny, P.; Butterworth, A.; Capdeville, J.; Reenen, C.G.V.; Veissier, I. Aggregation of Measures to Produce an Overall Assessment of Animal Welfare. Part 2: Analysis of Constraints. Animal 2007, 1, 1188–1197. [Google Scholar] [CrossRef] [Green Version]
- Broom, D.M. Indicators of Poor Welfare. Br. Vet. J. 1986, 142, 524–526. [Google Scholar] [CrossRef]
- Farm Animal Welfare Council. Five Freedoms. 1979. Available online: https://webarchive.nationalarchives.gov.uk/20121010012427/http://www.fawc.org.uk/freedoms.htm (accessed on 23 June 2021).
- Brambell, F.W.R. Technical Committee to Enquire into the Welfare of Animals Kept under Intensive Livestock Husbandry Systems Report of the Technical Committee to Enquire into the Welfare of Animals Kept under Intensive Livestock Husbandry Systems; Her Majesty’s Stationery Office: London, UK, 1965. [Google Scholar]
- Korte, S.M.; Olivier, B.; Koolhaas, J.M. A New Animal Welfare Concept Based on Allostasis. Physiol. Behav. 2007, 92, 422–428. [Google Scholar] [CrossRef] [Green Version]
- Fraser, D.; Matthews, L. Preference and Motivation Testing. In Validation of Animal Experimentation Collection; Appleby, M.C., Hughes, B.O., Eds.; CAB International: New York, NY, USA, 1997; pp. 159–173. [Google Scholar]
- Lund, V. Natural Living—A Precondition for Animal Welfare in Organic Farming. Livest. Sci. 2006, 100, 71–83. [Google Scholar] [CrossRef] [Green Version]
- Hovi, M.; Sundrum, A.; Thamsborg, S.M. Animal Health and Welfare in Organic Livestock Production in Europe: Current State and Future Challenges. Livest. Prod. Sci. 2003, 80, 41–53. [Google Scholar] [CrossRef] [Green Version]
- Charlton, G.L.; Rutter, S.M.; East, M.; Sinclair, L.A. Preference of Dairy Cows: Indoor Cubicle Housing with Access to a Total Mixed Ration vs. Access to Pasture. Appl. Anim. Behav. Sci. 2011, 130, 1–9. [Google Scholar] [CrossRef]
- Perttu, R.K.; Heins, B.J.; Phillips, H.N.; Endres, M.I.; Moon, R.D.; Sorge, U.S. Short Communication: Effects of Mesh Leggings on Fly Pressure and Fly Avoidance Behaviors of Pastured Dairy Cows. J. Dairy Sci. 2020, 103, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Basiel, B.L.; Hardie, L.C.; Heins, B.J.; Dechow, C.D. Genetic Parameters and Genomic Regions Associated with Horn Fly Resistance in Organic Holstein Cattle. J. Dairy Sci. 2021, 104, 12724–12740. [Google Scholar] [CrossRef] [PubMed]
- Veissier, I.; Palme, R.; Moons, C.P.H.; Ampe, B.; Sonck, B.; Andanson, S.; Tuyttens, F.A.M. Heat Stress in Cows at Pasture and Benefit of Shade in a Temperate Climate Region. Int. J. Biometeorol. 2018, 62, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, K.T.; Heins, B.J.; Buchanan, E.S.; Reese, M.H. Evaluation of Solar Photovoltaic Systems to Shade Cows in a Pasture-Based Dairy Herd. J. Dairy Sci. 2021, 104, 2794–2806. [Google Scholar] [CrossRef] [PubMed]
- Sorge, U.S.; Moon, R.D.; Stromberg, B.E.; Schroth, S.L.; Michels, L.; Wolff, L.J.; Kelton, D.F.; Heins, B.J. Parasites and Parasite Management Practices of Organic and Conventional Dairy Herds in Minnesota. J. Dairy Sci. 2015, 98, 3143–3151. [Google Scholar] [CrossRef] [Green Version]
- Neave, H.W.; Zobel, G.; Thoday, H.; Saunders, K.; Edwards, J.P.; Webster, J. Toward On-Farm Measurement of Personality Traits and Their Relationships to Behavior and Productivity of Grazing Dairy Cattle. J. Dairy Sci. 2022, 105, 6055–6069. [Google Scholar] [CrossRef]
- Titterington, F.M.; Knox, R.; Buijs, S.; Lowe, D.E.; Morrison, S.J.; Lively, F.O.; Shirali, M. Human–Animal Interactions with Bos Taurus Cattle and Their Impacts on On-Farm Safety: A Systematic Review. Animals 2022, 12, 776. [Google Scholar] [CrossRef]
- Sutherland, M.A.; Webster, J.; Sutherland, I. Animal Health and Welfare Issues Facing Organic Production Systems. Animals 2013, 3, 1021–1035. [Google Scholar] [CrossRef] [Green Version]
- Bergman, M.A.; Richert, R.M.; Cicconi-Hogan, K.M.; Gamroth, M.J.; Schukken, Y.H.; Stiglbauer, K.E.; Ruegg, P.L. Comparison of Selected Animal Observations and Management Practices Used to Assess Welfare of Calves and Adult Dairy Cows on Organic and Conventional Dairy Farms. J. Dairy Sci. 2014, 97, 4269–4280. [Google Scholar] [CrossRef]
- Sorge, U.S.; Yamashita, S.; Pieper, L. Bovine Veterinarians’ Perspective on Organic Livestock Production in the USA. Vet. Rec. 2019, 184, 384. [Google Scholar] [CrossRef]
- Legrand, A.L.; von Keyserlingk, M.A.G.; Weary, D.M. Preference and Usage of Pasture versus Free-Stall Housing by Lactating Dairy Cattle. J. Dairy Sci. 2009, 92, 3651–3658. [Google Scholar] [CrossRef] [Green Version]
- Charlton, G.L.; Rutter, S.M.; East, M.; Sinclair, L.A. The Motivation of Dairy Cows for Access to Pasture. J. Dairy Sci. 2013, 96, 4387–4396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duncan, I.J.H. Science-Based Assessment of Animal Welfare: Farm Animals. Rev. Sci. Tech. 2005, 24, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Dawkins, M.S. Behavioural Deprivation: A Central Problem in Animal Welfare. Appl. Anim. Behav. Sci. 1988, 20, 209–225. [Google Scholar] [CrossRef] [Green Version]
- von Keyserlingk, M.A.G.; Amorim Cestari, A.; Franks, B.; Fregonesi, J.A.; Weary, D.M. Dairy Cows Value Access to Pasture as Highly as Fresh Feed. Sci. Rep. 2017, 7, 44953. [Google Scholar] [CrossRef]
- Åkerfeldt, M.P.; Gunnarsson, S.; Bernes, G.; Blanco-Penedo, I. Health and Welfare in Organic Livestock Production Systems—A Systematic Mapping of Current Knowledge. Org. Agric. 2021, 11, 105–132. [Google Scholar] [CrossRef]
- Sutherland, M.A.; Huddart, F.J. The Effect of Training First-Lactation Heifers to the Milking Parlor on the Behavioral Reactivity to Humans and the Physiological and Behavioral Responses to Milking and Productivity. J. Dairy Sci. 2012, 95, 6983–6993. [Google Scholar] [CrossRef]
- Pol, M.; Ruegg, P.L. Treatment Practices and Quantification of Antimicrobial Drug Usage in Conventional and Organic Dairy Farms in Wisconsin. J. Dairy Sci. 2007, 90, 249–261. [Google Scholar] [CrossRef]
- Ruegg, P.L. Management of Mastitis on Organic and Conventional Dairy Farms. J. Anim. Sci. 2009, 87, 43–55. [Google Scholar] [CrossRef]
- Robbins, J.; Weary, D.; Schuppli, C.; von Keyserlingk, M. Stakeholder Views on Treating Pain Due to Dehorning Dairy Calves. Anim. Welf. 2015, 24, 399–406. [Google Scholar] [CrossRef]
- Ventura, B.A.; von Keyserlingk, M.A.G.; Weary, D.M. Animal Welfare Concerns and Values of Stakeholders Within the Dairy Industry. J. Agric. Environ. Ethics 2015, 28, 109–126. [Google Scholar] [CrossRef]
- USDA. Dairy 2014. In Health and Management Practices on U.S. Dairy Operations, 2014; USDA–APHIS–VS–CEAH–NAHMS: Fort Collins, CO, USA, 2014. [Google Scholar]
- Wikman, I.; Hokkanen, A.-H.; Pastell, M.; Kauppinen, T.; Valros, A.; Hänninen, L. Dairy Producer Attitudes to Pain in Cattle in Relation to Disbudding Calves. J. Dairy Sci. 2013, 96, 6894–6903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knierim, U.; Irrgang, N.; Roth, B.A. To Be or Not to Be Horned—Consequences in Cattle. Livest. Sci. 2015, 179, 29–37. [Google Scholar] [CrossRef]
- Gottardo, F.; Nalon, E.; Contiero, B.; Normando, S.; Dalvit, P.; Cozzi, G. The Dehorning of Dairy Calves: Practices and Opinions of 639 Farmers. J. Dairy Sci. 2011, 94, 5724–5734. [Google Scholar] [CrossRef] [PubMed]
- Kling-Eveillard, F.; Knierim, U.; Irrgang, N.; Gottardo, F.; Ricci, R.; Dockès, A.C. Attitudes of Farmers towards Cattle Dehorning. Livest. Sci. 2015, 179, 12–21. [Google Scholar] [CrossRef]
- Cozzi, G.; Gottardo, F.; Brscic, M.; Contiero, B.; Irrgang, N.; Knierim, U.; Pentelescu, O.; Windig, J.J.; Mirabito, L.; Kling Eveillard, F.; et al. Dehorning of Cattle in the EU Member States: A Quantitative Survey of the Current Practices. Livest. Sci. 2015, 179, 4–11. [Google Scholar] [CrossRef]
- Phillips, H.N.; Sharpe, K.T.; Endres, M.I.; Heins, B.J. Effects of Oral White Willow Bark (Salix Alba) and Intravenous Flunixin Meglumine on Prostaglandin E2 in Healthy Dairy Calves. JDS Commun. 2022, 3, 49–54. [Google Scholar] [CrossRef]
- Stock, M.L.; Baldridge, S.L.; Griffin, D.; Coetzee, J.F. Bovine Dehorning: Assessing Pain and Providing Analgesic Management. Vet. Clin. N. Am. Food Anim. Pract. 2013, 29, 103–133. [Google Scholar] [CrossRef]
- Farmers Assuring Responsible Management. Animal Care Reference Manual; Version 4.0; National Dairy FARM Program: Arlington, VA, USA; Available online: https://nationaldairyfarm.com/farm-animal-care-version-4–0 (accessed on 30 June 2021).
- Vickers, K.J.; Niel, L.; Kiehlbauch, L.M.; Weary, D.M. Calf Response to Caustic Paste and Hot-Iron Dehorning Using Sedation with and Without Local Anesthetic. J. Dairy Sci. 2005, 88, 1454–1459. [Google Scholar] [CrossRef] [Green Version]
- Morisse, J.P.; Cotte, J.P.; Huonnic, D. Effect of Dehorning on Behaviour and Plasma Cortisol Responses in Young Calves. Appl. Anim. Behav. Sci. 1995, 43, 239–247. [Google Scholar] [CrossRef]
- Costa, J.H.C.; von Keyserlingk, M.A.G.; Weary, D.M. Invited Review: Effects of Group Housing of Dairy Calves on Behavior, Cognition, Performance, and Health. J. Dairy Sci. 2016, 99, 2453–2467. [Google Scholar] [CrossRef]
- Grøndahl-Nielsen, C.; Simonsen, H.B.; Lund, D.J.; Hesselholt, M. Behavioural, Endocrine and Cardiac Responses in Young Calves Undergoing Dehorning without and with Use of Sedation and Analgesia. Vet. J. 1999, 158, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Stafford, K.J.; Mellor, D.J. Dehorning and Disbudding Distress and Its Alleviation in Calves. Vet. J. 2005, 169, 337–349. [Google Scholar] [CrossRef]
- Stafford, K.J.; Mellor, D.J. Addressing the Pain Associated with Disbudding and Dehorning in Cattle. Appl. Anim. Behav. Sci. 2011, 135, 226–231. [Google Scholar] [CrossRef]
- Stewart, M.; Stafford, K.J.; Dowling, S.K.; Schaefer, A.L.; Webster, J.R. Eye Temperature and Heart Rate Variability of Calves Disbudded with or without Local Anaesthetic. Physiol. Behav. 2008, 93, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Neave, H.W.; Daros, R.R.; Costa, J.H.C.; von Keyserlingk, M.A.G.; Weary, D.M. Pain and Pessimism: Dairy Calves Exhibit Negative Judgement Bias Following Hot-Iron Disbudding. PLoS ONE 2013, 8, e80556. [Google Scholar] [CrossRef] [Green Version]
- Adcock, S.J.J.; Tucker, C.B. The Effect of Disbudding Age on Healing and Pain Sensitivity in Dairy Calves. J. Dairy Sci. 2018, 101, 10361–10373. [Google Scholar] [CrossRef]
- Casoni, D.; Mirra, A.; Suter, M.R.; Gutzwiller, A.; Spadavecchia, C. Can Disbudding of Calves (One versus Four Weeks of Age) Induce Chronic Pain? Physiol. Behav. 2019, 199, 47–55. [Google Scholar] [CrossRef]
- Winder, C.B.; Miltenburg, C.L.; Sargeant, J.M.; LeBlanc, S.J.; Haley, D.B.; Lissemore, K.D.; Godkin, M.A.; Duffield, T.F. Effects of Local Anesthetic or Systemic Analgesia on Pain Associated with Cautery Disbudding in Calves: A Systematic Review and Meta-Analysis. J. Dairy Sci. 2018, 101, 5411–5427. [Google Scholar] [CrossRef] [Green Version]
- Julius, D.; Basbaum, A.I. Molecular Mechanisms of Nociception. Nature 2001, 413, 203–210. [Google Scholar] [CrossRef]
- Stock, M.L.; Coetzee, J.F. Clinical Pharmacology of Analgesic Drugs in Cattle. Vet. Clin. N. Am. Food Anim. Pract. 2015, 31, 113–138. [Google Scholar] [CrossRef]
- Vasseur, E.; Borderas, F.; Cue, R.I.; Lefebvre, D.; Pellerin, D.; Rushen, J.; Wade, K.M.; Passillé, A.M. de A Survey of Dairy Calf Management Practices in Canada That Affect Animal Welfare. J. Dairy Sci. 2010, 93, 1307–1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Code of Federal Regulations, Code of Federal Regulations, CFR 7§205.603: Synthetic Substances Allowed for Use in Organic Livestock Production. Available online: https://www.ecfr.gov/current/title-7/subtitle-B/chapter-I/subchapter-M/part-205/subpart-G (accessed on 10 November 2021).
- Weary, D.M.; Niel, L.; Flower, F.C.; Fraser, D. Identifying and Preventing Pain in Animals. Appl. Anim. Behav. Sci. 2006, 100, 64–76. [Google Scholar] [CrossRef] [Green Version]
- Adam, M.; Salla, K.; Aho, R.; Hänninen, L.; Taponen, S.; Norring, M.; Raekallio, M.; Hokkanen, A.-H. A Comparison of Sedative Effects of Xylazine Alone or Combined with Levomethadone or Ketamine in Calves Prior to Disbudding. Vet. Anaesth. Analg. 2021, 48, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Vasseur, E.; Rushen, J.; de Passillé, A.M. Short Communication: Calf Body Temperature Following Chemical Disbudding with Sedation: Effects of Milk Allowance and Supplemental Heat. J. Dairy Sci. 2014, 97, 5185–5190. [Google Scholar] [CrossRef] [PubMed]
- Reedman, C.N.; Duffield, T.F.; DeVries, T.J.; Lissemore, K.D.; Duncan, I.J.; Winder, C.B. Randomized Controlled Trial Assessing the Effects of Xylazine Sedation in 2- to 6-Week-Old Dairy Calves Disbudded with a Cautery Iron. J. Dairy Sci. 2021, 104, 5881–5897. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.B.D.; Brito, A.F.; Townson, L.L.; Townson, D.H. Assessing the Research and Education Needs of the Organic Dairy Industry in the Northeastern United States. J. Dairy Sci. 2013, 96, 7340–7348. [Google Scholar] [CrossRef] [PubMed]
- Phillips, H.N.; Heins, B.J. Evaluation of an Herbal Therapy to Alleviate Acute Pain and Stress of Disbudded Dairy Calves under Organic Management. Transl. Anim. Sci. 2021, 5. [Google Scholar] [CrossRef]
- Barkema, H.W.; von Keyserlingk, M.A.G.; Kastelic, J.P.; Lam, T.J.G.M.; Luby, C.; Roy, J.-P.; LeBlanc, S.J.; Keefe, G.P.; Kelton, D.F. Invited Review: Changes in the Dairy Industry Affecting Dairy Cattle Health and Welfare. J. Dairy Sci. 2015, 98, 7426–7445. [Google Scholar] [CrossRef] [Green Version]
- Minton, J.E. Function of the Hypothalamic-Pituitary-Adrenal Axis and the Sympathetic Nervous System in Models of Acute Stress in Domestic Farm Animals. J. Anim. Sci. 1994, 72, 1891–1898. [Google Scholar] [CrossRef]
- Mormède, P.; Andanson, S.; Aupérin, B.; Beerda, B.; Guémené, D.; Malmkvist, J.; Manteca, X.; Manteuffel, G.; Prunet, P.; van Reenen, C.G.; et al. Exploration of the Hypothalamic–Pituitary–Adrenal Function as a Tool to Evaluate Animal Welfare. Physiol. Behav. 2007, 92, 317–339. [Google Scholar] [CrossRef]
- Faulkner, P.M.; Weary, D.M. Reducing Pain After Dehorning in Dairy Calves. J. Dairy Sci. 2000, 83, 2037–2041. [Google Scholar] [CrossRef]
- Coetzee, J.F.; Gehring, R.; Bettenhausen, A.C.; Lubbers, B.V.; Toerber, S.E.; Thomson, D.U.; Kukanich, B.; Apley, M.D. Attenuation of Acute Plasma Cortisol Response in Calves Following Intravenous Sodium Salicylate Administration Prior to Castration. J. Vet. Pharmacol. Ther. 2007, 30, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Baldridge, S.L.; Coetzee, J.F.; Dritz, S.S.; Reinbold, J.B.; Gehring, R.; Havel, J.; Kukanich, B. Pharmacokinetics and Physiologic Effects of Intramuscularly Administered Xylazine Hydrochloride-Ketamine Hydrochloride-Butorphanol Tartrate Alone or in Combination with Orally Administered Sodium Salicylate on Biomarkers of Pain in Holstein Calves Following Castration and Dehorning. Am. J. Vet. Res. 2011, 72, 1305–1317. [Google Scholar] [CrossRef] [PubMed]
- Maroon, J.C.; Bost, J.W.; Maroon, A. Natural Anti-Inflammatory Agents for Pain Relief. Surg. Neurol. Int. 2010, 1, 80. [Google Scholar] [CrossRef] [Green Version]
- Kammerer, B.; Kahlich, R.; Biegert, C.; Gleiter, C.H.; Heide, L. HPLC-MS/MS Analysis of Willow Bark Extracts Contained in Pharmaceutical Preparations. Phytochem. Anal. 2005, 16, 470–478. [Google Scholar] [CrossRef]
- Mahdi, J.G. Biosynthesis and Metabolism of β-d-Salicin: A Novel Molecule That Exerts Biological Function in Humans and Plants. Biotechnol. Rep. 2014, 4, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Drummond, E.M.; Harbourne, N.; Marete, E.; Martyn, D.; Jacquier, J.; O’Riordan, D.; Gibney, E.R. Inhibition of Proinflammatory Biomarkers in THP1 Macrophages by Polyphenols Derived from Chamomile, Meadowsweet and Willow Bark. Phytother. Res. 2013, 27, 588–594. [Google Scholar] [CrossRef]
- Schmid, B.; Kötter, I.; Heide, L. Pharmacokinetics of Salicin after Oral Administration of a Standardised Willow Bark Extract. Eur. J. Clin. Pharmacol. 2001, 57, 387–391. [Google Scholar] [CrossRef]
- Uehleke, B.; Müller, J.; Stange, R.; Kelber, O.; Melzer, J. Willow Bark Extract STW 33-I in the Long-Term Treatment of Outpatients with Rheumatic Pain Mainly Osteoarthritis or Back Pain. Phytomedicine 2013, 20, 980–984. [Google Scholar] [CrossRef]
- Moore, K.M.; Barry, T.N.; Cameron, P.N.; Lopez-Villalobos, N.; Cameron, D.J. Willow (Salix Sp.) as a Supplement for Grazing Cattle under Drought Conditions. Anim. Feed. Sci. Technol. 2003, 104, 1–11. [Google Scholar] [CrossRef]
- Pitta, D.W.; Barry, T.N.; Lopez-Villalobos, N.; Kemp, P.D. Effects on Ewe Reproduction of Grazing Willow Fodder Blocks during Drought. Anim. Feed. Sci. Technol. 2005, 120, 217–234. [Google Scholar] [CrossRef]
- McWilliam, E.L.; Barry, T.N.; Lopez-Villalobos, N.; Cameron, P.N.; Kemp, P.D. Effects of Willow (Salix) versus Poplar (Populus) Supplementation on the Reproductive Performance of Ewes Grazing Low Quality Drought Pasture during Mating. Anim. Feed. Sci. Technol. 2005, 119, 69–86. [Google Scholar] [CrossRef]
- Vandermeulen, S.; Ramírez-Restrepo, C.A.; Beckers, Y.; Claessens, H.; Bindelle, J. Agroforestry for Ruminants: A Review of Trees and Shrubs as Fodder in Silvopastoral Temperate and Tropical Production Systems. Anim. Prod. Sci. 2018, 58, 767–777. [Google Scholar] [CrossRef]
- Tyśkiewicz, K.; Konkol, M.; Kowalski, R.; Rój, E.; Warmiński, K.; Krzyżaniak, M.; Gil, Ł.; Stolarski, M.J. Characterization of Bioactive Compounds in the Biomass of Black Locust, Poplar and Willow. Trees 2019, 33, 1235–1263. [Google Scholar] [CrossRef] [Green Version]
- Kenstavičienė, P.; Nenortienė, P.; Kiliuvienė, G.; Ževžikovas, A.; Lukošius, A.; Kazlauskienė, D. Application of High-Performance Liquid Chromatography for Research of Salicin in Bark of Different Varieties of Salix. Medicina 2009, 45, 644–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmid, B.; Lüdtke, R.; Selbmann, H.-K.; Kötter, I.; Tschirdewahn, B.; Schaffner, W.; Heide, L. Efficacy and Tolerability of a Standardized Willow Bark Extract in Patients with Osteoarthritis: Randomized Placebo-Controlled, Double Blind Clinical Trial. Phytother. Res. 2001, 15, 344–350. [Google Scholar] [CrossRef]
- Gingerich, D.A.; Baggot, J.D.; Yeary, R.A. Pharmacokinetics and Dosage of Aspirin in Cattle. J. Am. Vet. Med. Assoc. 1975, 167, 945–948. [Google Scholar]
- Kawabata, A. Prostaglandin E2 and Pain—An Update. Biol. Pharm. Bull. 2011, 34, 1170–1173. [Google Scholar] [CrossRef] [Green Version]
- Takada, M.; Fukusaki, M.; Terao, Y.; Yamashita, K.; Inadomi, C.; Takada, M.; Sumikawa, K. Preadministration of Flurbiprofen Suppresses Prostaglandin Production and Postoperative Pain in Orthopedic Patients Undergoing Tourniquet Inflation. J. Clin. Anesth. 2007, 19, 97–100. [Google Scholar] [CrossRef]
- Morita, I. Distinct Functions of COX-1 and COX-2. Prostaglandins Other Lipid Mediat. 2002, 68–69, 165–175. [Google Scholar] [CrossRef]
- Fiebich, B.L.; Chrubasik, S. Effects of an Ethanolic Salix Extract on the Release of Selected Inflammatory Mediators in Vitro. Phytomedicine 2004, 11, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Higgs, G.A.; Salmon, J.A.; Henderson, B.; Vane, J.R. Pharmacokinetics of Aspirin and Salicylate in Relation to Inhibition of Arachidonate Cyclooxygenase and Antiinflammatory Activity. Proc. Natl. Acad. Sci. USA 1987, 84, 1417–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraccaro, E.; Coetzee, J.F.; Odore, R.; Edwards-Callaway, L.N.; KuKanich, B.; Badino, P.; Bertolotti, L.; Glynn, H.; Dockweiler, J.; Allen, K.; et al. A Study to Compare Circulating Flunixin, Meloxicam and Gabapentin Concentrations with Prostaglandin E2 Levels in Calves Undergoing Dehorning. Res. Vet. Sci. 2013, 95, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.A.; Coetzee, J.F.; Edwards-Callaway, L.N.; Glynn, H.; Dockweiler, J.; KuKanich, B.; Lin, H.; Wang, C.; Fraccaro, E.; Jones, M.; et al. The Effect of Timing of Oral Meloxicam Administration on Physiological Responses in Calves after Cautery Dehorning with Local Anesthesia. J. Dairy Sci. 2013, 96, 5194–5205. [Google Scholar] [CrossRef]
- Hardie, L.C.; Haagen, I.W.; Heins, B.J.; Dechow, C.D. Genetic Parameters and Association of National Evaluations with Breeding Values for Health Traits in US Organic Holstein Cows. J. Dairy Sci. 2022, 105, 495–508. [Google Scholar] [CrossRef]
- Sundberg, T.; Berglund, B.; Rydhmer, L.; Strandberg, E. Fertility, Somatic Cell Count and Milk Production in Swedish Organic and Conventional Dairy Herds. Livest. Sci. 2009, 126, 176–182. [Google Scholar] [CrossRef]
- Tikofsky, L.L.; Barlow, J.W.; Santisteban, C.; Schukken, Y.H. A Comparison of Antimicrobial Susceptibility Patterns for Staphylococcus Aureus in Organic and Conventional Dairy Herds. Microb. Drug Resist. 2003, 9, 39–45. [Google Scholar] [CrossRef]
- Zwald, A.G.; Ruegg, P.L.; Kaneene, J.B.; Warnick, L.D.; Wells, S.J.; Fossler, C.; Halbert, L.W. Management Practices and Reported Antimicrobial Usage on Conventional and Organic Dairy Farms. J. Dairy Sci. 2004, 87, 191–201. [Google Scholar] [CrossRef] [Green Version]
- Ahlman, T.; Berglund, B.; Rydhmer, L.; Strandberg, E. Culling Reasons in Organic and Conventional Dairy Herds and Genotype by Environment Interaction for Longevity. J. Dairy Sci. 2011, 94, 1568–1575. [Google Scholar] [CrossRef] [Green Version]
- Gruet, P.; Maincent, P.; Berthelot, X.; Kaltsatos, V. Bovine Mastitis and Intramammary Drug Delivery: Review and Perspectives. Adv. Drug Deliv. Rev. 2001, 50, 245–259. [Google Scholar] [CrossRef]
- Fernandes, L.; Guimaraes, I.; Noyes, N.R.; Caixeta, L.S.; Machado, V.S. Effect of Subclinical Mastitis Detected in the First Month of Lactation on Somatic Cell Count Linear Scores, Milk Yield, Fertility, and Culling of Dairy Cows in Certified Organic Herds. J. Dairy Sci. 2021, 104, 2140–2150. [Google Scholar] [CrossRef] [PubMed]
- Brock, C.C.; Pempek, J.A.; Jackson-Smith, D.; Weaver, K.; da Costa, L.; Habing, G.G. Organic Dairy Producer Experiences and Decisions Related to Disease Prevention and Treatment. J. Dairy Sci. 2021, 104, 5867–5880. [Google Scholar] [CrossRef] [PubMed]
- Mullen, K.A.E.; Lyman, R.L.; Washburn, S.P.; Baynes, R.E.; Anderson, K.L. Short Communication: Effect of 3 Phytoceutical Products on Elimination of Bacteria in Experimentally Induced Streptococcus Uberis Clinical Mastitis. J. Dairy Sci. 2018, 101, 10409–10413. [Google Scholar] [CrossRef] [PubMed]
- Mullen, K.A.E.; Anderson, K.L.; Washburn, S.P. Effect of 2 Herbal Intramammary Products on Milk Quantity and Quality Compared with Conventional and No Dry Cow Therapy. J. Dairy Sci. 2014, 97, 3509–3522. [Google Scholar] [CrossRef] [PubMed]
- Rajala-Schultz, P.J.; Hogan, J.S.; Smith, K.L. Short Communication: Association Between Milk Yield at Dry-Off and Probability of Intramammary Infections at Calving. J. Dairy Sci. 2005, 88, 577–579. [Google Scholar] [CrossRef]
- Tucker, C.B.; Lacy-Hulbert, S.J.; Webster, J.R. Effect of Milking Frequency and Feeding Level before and after Dry off on Dairy Cattle Behavior and Udder Characteristics. J. Dairy Sci. 2009, 92, 3194–3203. [Google Scholar] [CrossRef]
- Reenen, C.G.V.; der Werf, J.T.N.V.; Bruckmaier, R.M.; Hopster, H.; Engel, B.; Noordhuizen, J.P.T.M.; Blokhuis, H.J. Individual Differences in Behavioral and Physiological Responsiveness of Primiparous Dairy Cows to Machine Milking. J. Dairy Sci. 2002, 85, 2551–2561. [Google Scholar] [CrossRef]
- Watts, M.; Meisel, E.M. Cattle Associated Trauma—A One Year Prospective Study of All Injuries. Injury 2011, 42, 1084–1087. [Google Scholar] [CrossRef]
- Nitz, J.; Krömker, V.; Klocke, D.; Wente, N.; Zhang, Y.; tho Seeth, M. Intramammary Infections in Heifers—Time of Onset and Associated Risk Factors. Animals 2020, 10, 1053. [Google Scholar] [CrossRef]
- Ivemeyer, S.; Knierim, U.; Waiblinger, S. Effect of Human-Animal Relationship and Management on Udder Health in Swiss Dairy Herds. J. Dairy Sci. 2011, 94, 5890–5902. [Google Scholar] [CrossRef]
- Bludau, M.J.; Maeschli, A.; Leiber, F.; Klocke, P.; Berezowski, J.A.; Bodmer, M.; Vidondo, B. The Influence of the Rearing Period on Intramammary Infections in Swiss Dairy Heifers: A Cross-Sectional Study. Prev. Vet. Med. 2016, 129, 23–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, K.I.; Compton, C.; Anniss, F.M.; Weir, A.; Heuer, C.; McDougall, S. Subclinical and Clinical Mastitis in Heifers Following the Use of a Teat Sealant Precalving. J. Dairy Sci. 2007, 90, 207–218. [Google Scholar] [CrossRef]
- Persson Waller, K.; Bengtsson, B.; Lindberg, A.; Nyman, A.; Ericsson Unnerstad, H. Incidence of Mastitis and Bacterial Findings at Clinical Mastitis in Swedish Primiparous Cows—Influence of Breed and Stage of Lactation. Vet. Microbiol. 2009, 134, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Huijps, K.; De Vliegher, S.; Lam, T.; Hogeveen, H. Cost Estimation of Heifer Mastitis in Early Lactation by Stochastic Modelling. Vet. Microbiol. 2009, 134, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Cha, E.; Kristensen, A.R.; Hertl, J.A.; Schukken, Y.H.; Tauer, L.W.; Welcome, F.L.; Gröhn, Y.T. Optimal Insemination and Replacement Decisions to Minimize the Cost of Pathogen-Specific Clinical Mastitis in Dairy Cows. J. Dairy Sci. 2014, 97, 2101–2117. [Google Scholar] [CrossRef] [Green Version]
- Zadoks, R.N.; Allore, H.G.; Barkema, H.W.; Sampimon, O.C.; Wellenberg, G.J.; Gröhn, Y.T.; Schukken, Y.H. Cow- and Quarter-Level Risk Factors for Streptococcus Uberis and Staphylococcus Aureus Mastitis. J. Dairy Sci. 2001, 84, 2649–2663. [Google Scholar] [CrossRef]
- Breuer, K.; Hemsworth, P.H.; Barnett, J.L.; Matthews, L.R.; Coleman, G.J. Behavioural Response to Humans and the Productivity of Commercial Dairy Cows. Appl. Anim. Behav. Sci. 2000, 66, 273–288. [Google Scholar] [CrossRef]
- Hemsworth, P.H.; Barnett, J.L.; Tilbrook, A.J.; Hansen, C. The Effects of Handling by Humans at Calving and during Milking on the Behaviour and Milk Cortisol Concentrations of Primiparous Dairy Cows. Appl. Anim. Behav. Sci. 1989, 22, 313–326. [Google Scholar] [CrossRef]
- Hardie, L.C.; Heins, B.J.; Dechow, C.D. Genetic Parameters for Stayability of Holsteins in US Organic Herds. J. Dairy Sci. 2021, 104, 4507–4515. [Google Scholar] [CrossRef]
- Eicher, S.D.; Schutz, M.; Kearney, F.; Willard, S.; Bowers, S.; Gandy, S.; Graves, K. Prepartum Milking Effects on Parlour Behaviour, Endocrine and Immune Responses in Holstein Heifers. J. Dairy Res. 2007, 74, 417–424. [Google Scholar] [CrossRef]
- Kutzer, T.; Steilen, M.; Gygax, L.; Wechsler, B. Habituation of Dairy Heifers to Milking Routine—Effects on Human Avoidance Distance, Behavior, and Cardiac Activity during Milking. J. Dairy Sci. 2015, 98, 5241–5251. [Google Scholar] [CrossRef] [PubMed]
- Bertenshaw, C.; Rowlinson, P.; Edge, H.; Douglas, S.; Shiel, R. The Effect of Different Degrees of ‘Positive’ Human–Animal Interaction during Rearing on the Welfare and Subsequent Production of Commercial Dairy Heifers. Appl. Anim. Behav. Sci. 2008, 114, 65–75. [Google Scholar] [CrossRef]
- Das, K.S.; Das, N. Pre-Partum Udder Massaging as a Means for Reduction of Fear in Primiparous Cows at Milking. Appl. Anim. Behav. Sci. 2004, 89, 17–26. [Google Scholar] [CrossRef]
- Parker, K.I.; Compton, C.W.R.; Anniss, F.M.; Heuer, C.; McDougall, S. Quarter-Level Analysis of Subclinical and Clinical Mastitis in Primiparous Heifers Following the Use of a Teat Sealant or an Injectable Antibiotic, or Both, Precalving. J. Dairy Sci. 2008, 91, 169–181. [Google Scholar] [CrossRef]
- Nickerson, S.C. Control of Heifer Mastitis: Antimicrobial Treatment—An Overview. Vet. Microbiol. 2009, 134, 128–135. [Google Scholar] [CrossRef]
- Daniels, K.J.; Donkin, S.S.; Eicher, S.D.; Pajor, E.A.; Schutz, M.M. Prepartum Milking of Heifers Influences Future Production and Health. J. Dairy Sci. 2007, 90, 2293–2301. [Google Scholar] [CrossRef] [Green Version]
- Santos, J.E.P.; Cerri, R.L.A.; Kirk, J.H.; Juchem, S.O.; Villaseňor, M. Effect of Prepartum Milking of Primigravid Cows on Mammary Gland Health and Lactation Performance. Livest. Prod. Sci. 2004, 86, 105–116. [Google Scholar] [CrossRef]
- Lopez-Benavides, M.G.; Williamson, J.H.; Lacy-Hulbert, S.J.; Cursons, R.T. Heifer Teats Sprayed in the Dry Period with an Iodine Teat Sanitizer Have Reduced Streptococcus Uberis Teat-End Contamination and Less Streptococcus Uberis Intra-Mammary Infections at Calving. Vet. Microbiol. 2009, 134, 186–191. [Google Scholar] [CrossRef]
- Chang, Y.; Brito, L.F.; Alvarenga, A.B.; Wang, Y. Incorporating Temperament Traits in Dairy Cattle Breeding Programs: Challenges and Opportunities in the Phenomics Era. Anim. Front. 2020, 10, 29–36. [Google Scholar] [CrossRef]
- Fogsgaard, K.K.; Bennedsgaard, T.W.; Herskin, M.S. Behavioral Changes in Freestall-Housed Dairy Cows with Naturally Occurring Clinical Mastitis. J. Dairy Sci. 2015, 98, 1730–1738. [Google Scholar] [CrossRef] [Green Version]
- Phillips, H.N.; Sorge, U.S.; Heins, B.J. Effects of Pre-Parturient Iodine Teat Dip Applications on Modulating Aversive Behaviors and Mastitis in Primiparous Cows. Animals 2021, 11, 1623. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phillips, H.N.; Heins, B.J. Alternative Practices in Organic Dairy Production and Effects on Animal Behavior, Health, and Welfare. Animals 2022, 12, 1785. https://doi.org/10.3390/ani12141785
Phillips HN, Heins BJ. Alternative Practices in Organic Dairy Production and Effects on Animal Behavior, Health, and Welfare. Animals. 2022; 12(14):1785. https://doi.org/10.3390/ani12141785
Chicago/Turabian StylePhillips, Hannah N., and Bradley J. Heins. 2022. "Alternative Practices in Organic Dairy Production and Effects on Animal Behavior, Health, and Welfare" Animals 12, no. 14: 1785. https://doi.org/10.3390/ani12141785
APA StylePhillips, H. N., & Heins, B. J. (2022). Alternative Practices in Organic Dairy Production and Effects on Animal Behavior, Health, and Welfare. Animals, 12(14), 1785. https://doi.org/10.3390/ani12141785






