Effects of Probiotics on Growth and Immunity of Piglets
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Specimen Acquisition
2.2. Lymphocyte Subsets Analysis
2.3. Phagocytosis Assay
2.4. Statistical Analysis
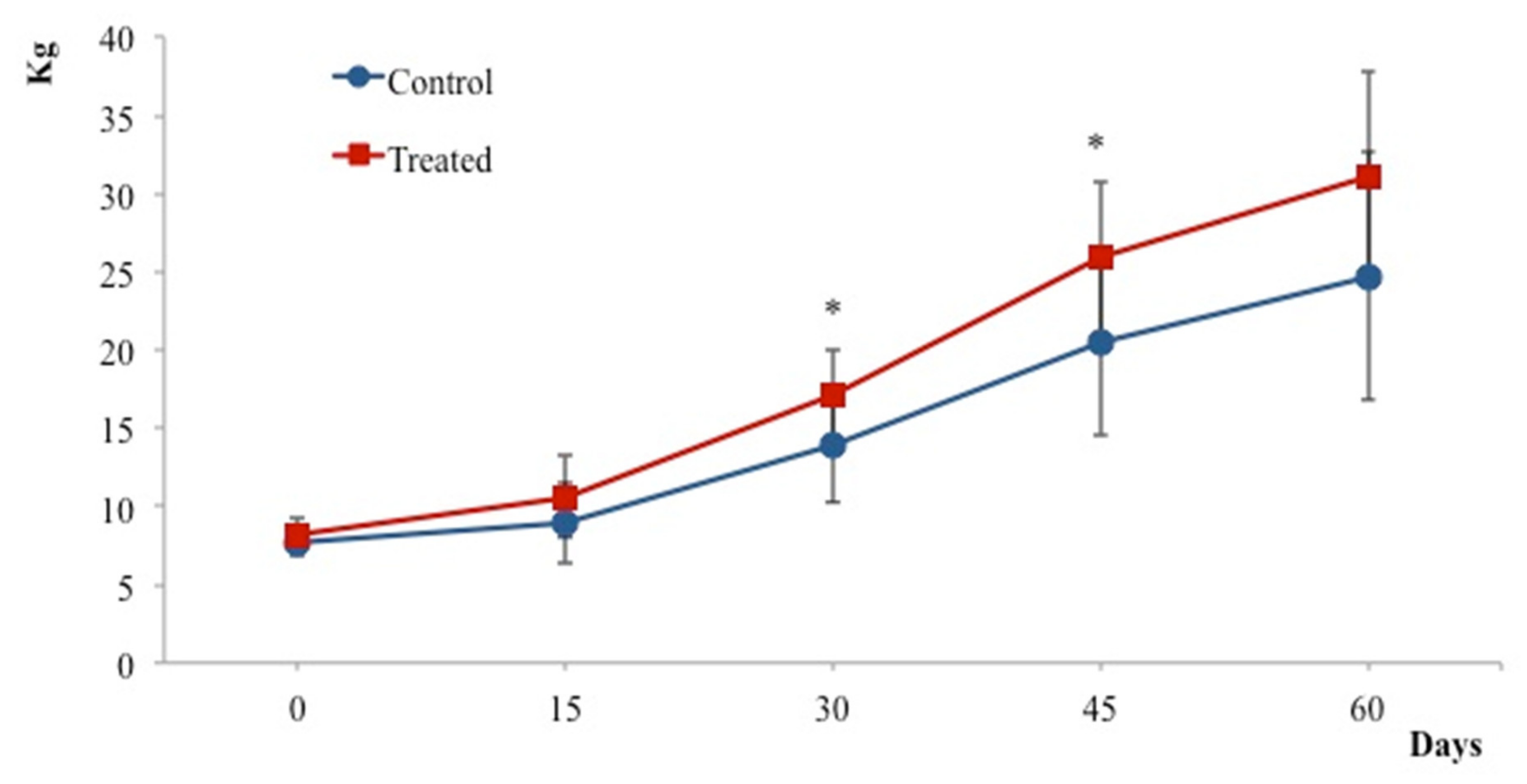
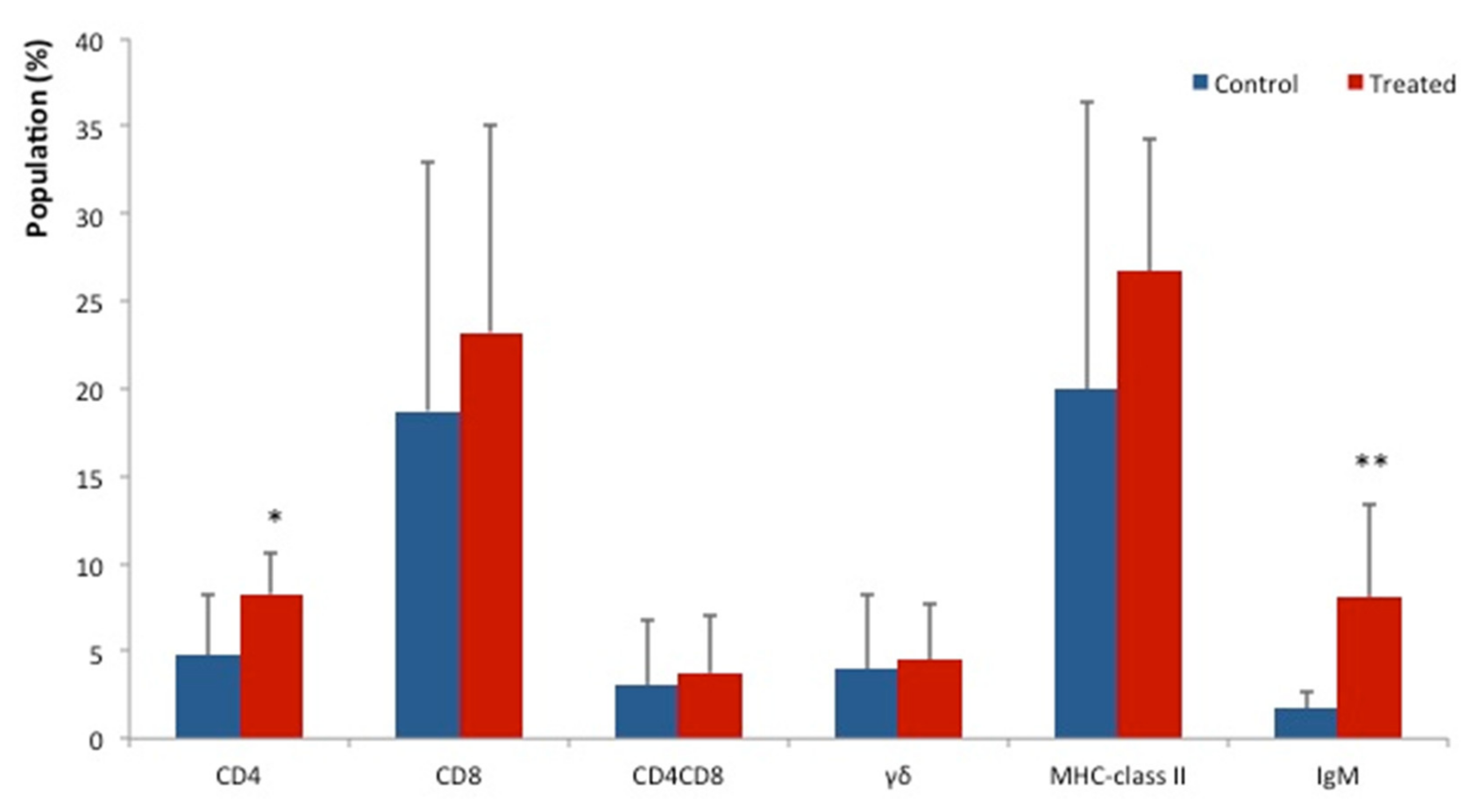
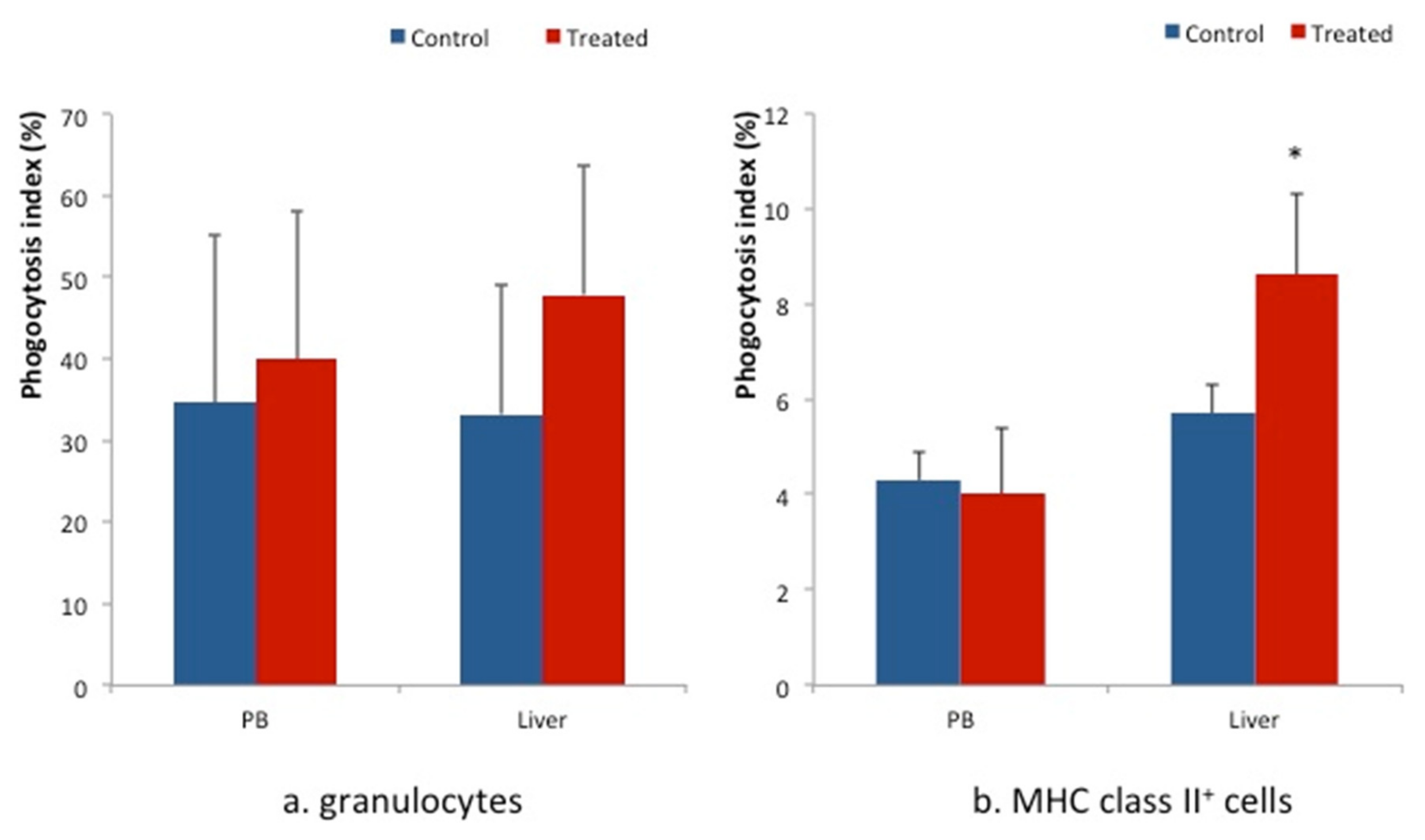
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dewey, C.E.; Cox, B.D.; Straw, B.E.; Bush, E.J.; Hurd, S. Use of antimicrobials in swine feeds in the United States. Swine Health Prod. 1999, 7, 19–25. [Google Scholar]
- Chattopadhyay, M.K. Use of antibiotics as feed additives: A burning question. Front. Microbiol. 2014, 5, 334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohanski, M.; De Pristo, M.; Collins, J. Sub-lethal antibiotic treatment leads to multidrug resistance via radical induced mutagenesis. Mol. Cell 2010, 37, 311–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gullberg, E.; Cao, S.; Berg, O.G.; Ilbäck, C.; Sandegren, L.; Hughes, D.; Andersson, D.I. Selection of resistant bacteria at very low antibiotic concentrations. PLoS Pathog. 2011, 7, e1002158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuller, R. Probiotics in man and animals. J. Appl. Microbiol. 1989, 66, 365–378. [Google Scholar]
- Isolauri, E.; Sütas, Y.; Kankaanpää, P.; Arvilommi, H.; Salminen, S. Probiotics: Effects on immunity. Am. J. Clin. Nutr. 2001, 73, 444–450. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, R.; Vasiljevic, T.; Day, S.L.; Smith, S.C.; Donkor, O.N. Lactic acid bacteria and probiotic organisms induce different cytokine profile and regulatory T cells mechanisms. J. Funct. Foods 2014, 6, 395–409. [Google Scholar] [CrossRef]
- Galdeano, C.M.; de Leblanc, A.D.M.; Vinderola, G.; Bonet, M.E.B.; Perdigón, G. Proposed model: Mechanisms of immunomodulation induced by probiotic bacteria. Clin. Vaccine Immunol. 2007, 14, 485–492. [Google Scholar] [CrossRef] [Green Version]
- Galdeano, C.M.; de Leblanc, A.D.M.; Carmuega, E.; Weill, R.; Perdigón, G. Mechanisms involved in the immunostimulation by probiotic fermented milk. J. Dairy Res. 2009, 76, 446–454. [Google Scholar] [CrossRef]
- Ishibashi, N.; Yamazaki, S. Probiotics and safety. Am. J. Clin. Nutr. 2001, 73, 465s–470s. [Google Scholar] [CrossRef]
- Gao, Q.; Qi, L.; Wu, T.; Wang, J. An important role of interleukin-10 in counteracting excessive immune response in HT-29 cells exposed to Clostridium butyricum. BMC Microbiol. 2012, 12, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López, P.; González-Rodríguez, I.; Gueimonde, M.; Margolles, A.; Suárez, A. Immune response to Bifidobacterium bifidum strains support Treg/Th17 plasticity. PLoS ONE 2011, 6, e24776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanders, M.E.; Akkermans, L.M.A.; Haller, D.; Hammerman, C.; Heimbach, J.; Hörmannsperger, G.; Huys, G.; Levy, D.D.; Lutgendorff, F.; Mack, D.; et al. Safety assessment of probiotics for human use. Gut Microbes 2010, 1, 164–185. [Google Scholar] [CrossRef] [PubMed]
- Ramah, A.; Yasuda, M.; Ohashi, Y.; Imatake, S.; Imaizumi, N.; Kida, T.; Yanagita, T.; Uemura, R.; Baakhtari, M.; Bakry, H.H.; et al. Protective effect of probiotics against tannin-induced immunosuppression in broiler chickens. Biosci. Microbiota Food Health 2022, 2021-058. [Google Scholar] [CrossRef]
- Galdeano, C.M.; Perdigo, G. The probiotic bacterium Lactobacillus casei induces activation of the gut mucosal immune system through innate immunity. Clin. Vaccine Immunol. 2006, 13, 219–226. [Google Scholar] [CrossRef] [Green Version]
- Qadis, A.Q.; Goya, S.; Yatsu, M.; Yoshida, Y.U.; Ichijo, T.; Sato, S. Immune-stimulatory effects of a bacteria-based probiotic on subpopulations of peripheral leukocytes and their cytokine mRNA expression in calves. J. Vet. Med. Sci. 2014, 76, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Nakamoto, N.; Kanai, T. Role of Toll-like receptors in immune activation and tolerance in the liver. Front. Immunol. 2014, 5, 221. [Google Scholar] [CrossRef] [Green Version]
- Doherty, D.G. Immunity, tolerance and autoimmunity in the liver: A comprehensive review. J. Autoimmun. 2016, 66, 60–75. [Google Scholar] [CrossRef]
- Bu, H.F.; Wang, X.; Zhu, Y.Q.; Williams, R.Y.; Hsueh, W.; Zheng, X.; Rozenfeld, R.A.; Zuo, X.L.; Tan, X.D. Lysozyme-modified probiotic components protect rats against polymicrobialsepsis: Role of macrophages and cathelicidin-related innate immunity. J. Immunol. 2006, 177, 8767–8776. [Google Scholar] [CrossRef] [Green Version]
- Azizi, A.F.N.; Miyazaki, R.; Yumito, T.; Ohashi, Y.; Uno, S.; Miyajima, U.; Kumamoto, M. Effect of maternal supplementation with seaweed powder on immune status of liver and lymphoid organs of piglets. J. Vet. Med. Sci. 2018, 80, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Baakhtari, M.; Imaizumi, N.; Kida, T.; Yanagita, T.; Ramah, A.; Ahmadi, P.; Takebe, N.; Iwamoto, Y.; Korosue, K.; Tsuzuki, N.; et al. Effects of Branched-chain Amino Acids on Immune Status of Young Racing Horses. J. Vet. Med. Sci. 2022, 84, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Berman, S.H.; Eichelsdoerfer, P.; Yim, D.; Elmer, G.W.; Wenner, C.A. Daily ingestion of a nutritional probiotic supplement enhances innate immune function in healthy adults. Nutr. Res. 2006, 26, 454–459. [Google Scholar] [CrossRef]
- Higgins, S.E.; Erf, G.F.; Higgins, J.P.; Henderson, S.N.; Wolfenden, A.D.; Gaona-Ramirez, G.; Hargis, B.M. Effect of probiotic treatment in broiler chicks on intestinal macrophage numbers and phagocytosis of Salmonella enteritidis by abdominal exudate cells. Poult. Sci. 2007, 86, 2315–2321. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, H.R.; Gong, J.; Gyles, C.L.; Hayes, M.A.; Zhou, H.; Sanei, B.; Chambers, J.R.; Sharif, S. Probiotics stimulate production of natural antibodies in chickens. Clin. Vaccine Immunol. 2006, 13, 975–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, Q.; Qu, F.; Gill, H.S. Probiotic treatment using Bifidobacterium lactis HN019 reduces weanling diarrhea associated with rotavirus and Escherichia coli infection in a piglet model. J. Pediatr. Gastroenterol. Nutr. 2001, 33, 171–177. [Google Scholar] [CrossRef]
- Tortuero, F.; Rioperez, J.; Fernandez, E.; Rodriguez, M.L. Response of piglets to oral administration of Lactic acid bacteria. J. Food Prot. 1995, 58, 1369–1374. [Google Scholar] [CrossRef]
- Kmieć, Z. Cooperation of Liver Cells in Health and Disease; Springer: Berlin/Heidelberg, Germany, 2001; pp. 1–151. [Google Scholar]
- Pescovitz, M.D.; Sakopoulos, A.G.; Gaddy, J.A.; Husmann, R.J.; Zuckermann, F.A. Porcine peripheral blood CD4+/CD8+ dual expressing T-cells. Vet. Immunol. Immunopathol. 1994, 43, 53–62. [Google Scholar] [CrossRef]
- Zuckermann, F.A. Extrathymic CD4/CD8 double positive T cells. Vet. Immunol. Immunopathol. 1999, 72, 55–66. [Google Scholar] [CrossRef]
- Parel, Y.; Chizzolini, C. CD4+CD8+ double positive (DP) T cells in health and disease. Autoimmun. Rev. 2004, 3, 215–220. [Google Scholar] [CrossRef]
| Subsets | Groups | MLN | JPP | SPT | |||
|---|---|---|---|---|---|---|---|
| Mean ± SD | p-Value | Mean ± SD | p-Value | Mean ± SD | p-Value | ||
| CD4 | Control | 27.00 ± 5.45 | 0.29 | 11.00 ± 3.76 | 0.5 | 11.40 ± 2.66 | 0.28 |
| Treated | 30.00 ± 6.28 | 12.01 ± 3.21 | 14.00 ± 2.62 | ||||
| CD8 | Control | 16.41 ± 3.65 | 0.72 | 13.00 ± 7.31 | 0.95 | 4.23 ± 0.76 | 0.57 |
| Treated | 20.00 ± 3.44 | 13.00 ± 6.89 | 5.00 ± 1.16 | ||||
| CD4CD8 | Control | 4.07 ± 1.60 | 0.94 | 7.20 ± 3.77 | 0.01 | 6.60 ± 1.28 | 0.5 |
| Treated | 4.02 ± 1.54 | 4.30 ± 1.95 | 7.36 ± 1.75 | ||||
| γδ | Control | 10.34 ± 11.00 | 0.73 | 9.00 ± 6.14 | 0.5 | 18.13 ± 9.62 | 0.82 |
| Treated | 9.00 ± 8.00 | 6.00 ± 10.4 | 17.00 ± 5.61 | ||||
| MHC class II | Control | 31.01 ± 6.14 | 0.09 | 41.00 ± 13.25 | 0.96 | 55.00 ± 2.22 | 0.15 |
| Treated | 25.00 ± 8.52 | 41.00 ± 9.13 | 50.00 ± 6.14 | ||||
| IgM | Control | 6.81 ± 3.44 | 0.54 | 5.00 ± 4.8 | 0.54 | 8.07 ± 2.00 | 0.85 |
| Treated | 11.63 ± 8.45 | 6.56 ± 6.54 | 8.00 ± 1.32 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azizi, A.F.N.; Uemura, R.; Omori, M.; Sueyoshi, M.; Yasuda, M. Effects of Probiotics on Growth and Immunity of Piglets. Animals 2022, 12, 1786. https://doi.org/10.3390/ani12141786
Azizi AFN, Uemura R, Omori M, Sueyoshi M, Yasuda M. Effects of Probiotics on Growth and Immunity of Piglets. Animals. 2022; 12(14):1786. https://doi.org/10.3390/ani12141786
Chicago/Turabian StyleAzizi, Ahmad Farid Nikmal, Ryoko Uemura, Mariko Omori, Masuo Sueyoshi, and Masahiro Yasuda. 2022. "Effects of Probiotics on Growth and Immunity of Piglets" Animals 12, no. 14: 1786. https://doi.org/10.3390/ani12141786
APA StyleAzizi, A. F. N., Uemura, R., Omori, M., Sueyoshi, M., & Yasuda, M. (2022). Effects of Probiotics on Growth and Immunity of Piglets. Animals, 12(14), 1786. https://doi.org/10.3390/ani12141786






