Comparative Analyses of Production Performance, Meat Quality, and Gut Microbial Composition between Two Chinese Goose Breeds
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experiments
2.2. Sample Collection
2.3. Morphologic Examination of Ileum and Muscle Fiber Tissue
2.4. Meat Quality Trait Analysis
2.4.1. Physical Properties
2.4.2. Proximate Composition
2.5. DNA Extraction, Microbiota Analysis, and Functional Prediction
2.6. Short-Chain Fatty Acid Analysis
2.7. Statistical Analysis
3. Results
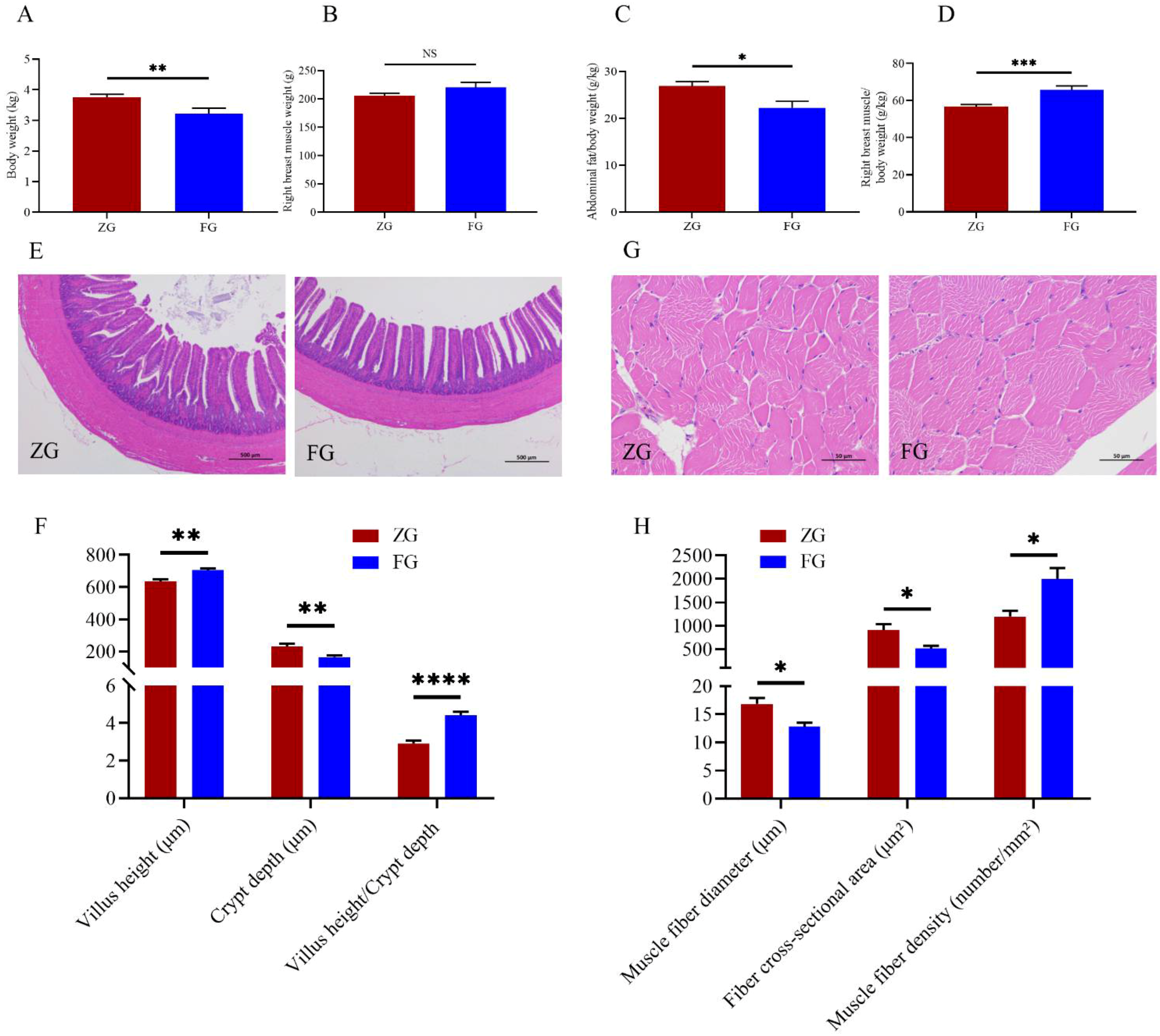
3.1. Comparisons of Production Performance and Ileum Epithelial Histological Characteristics between the Two Goose Breeds
3.2. Comparison of Breast Muscle Quality Traits between the Two Goose Breeds
3.2.1. Physical Properties and Muscle Fiber Characteristics
3.2.2. Proximate Composition
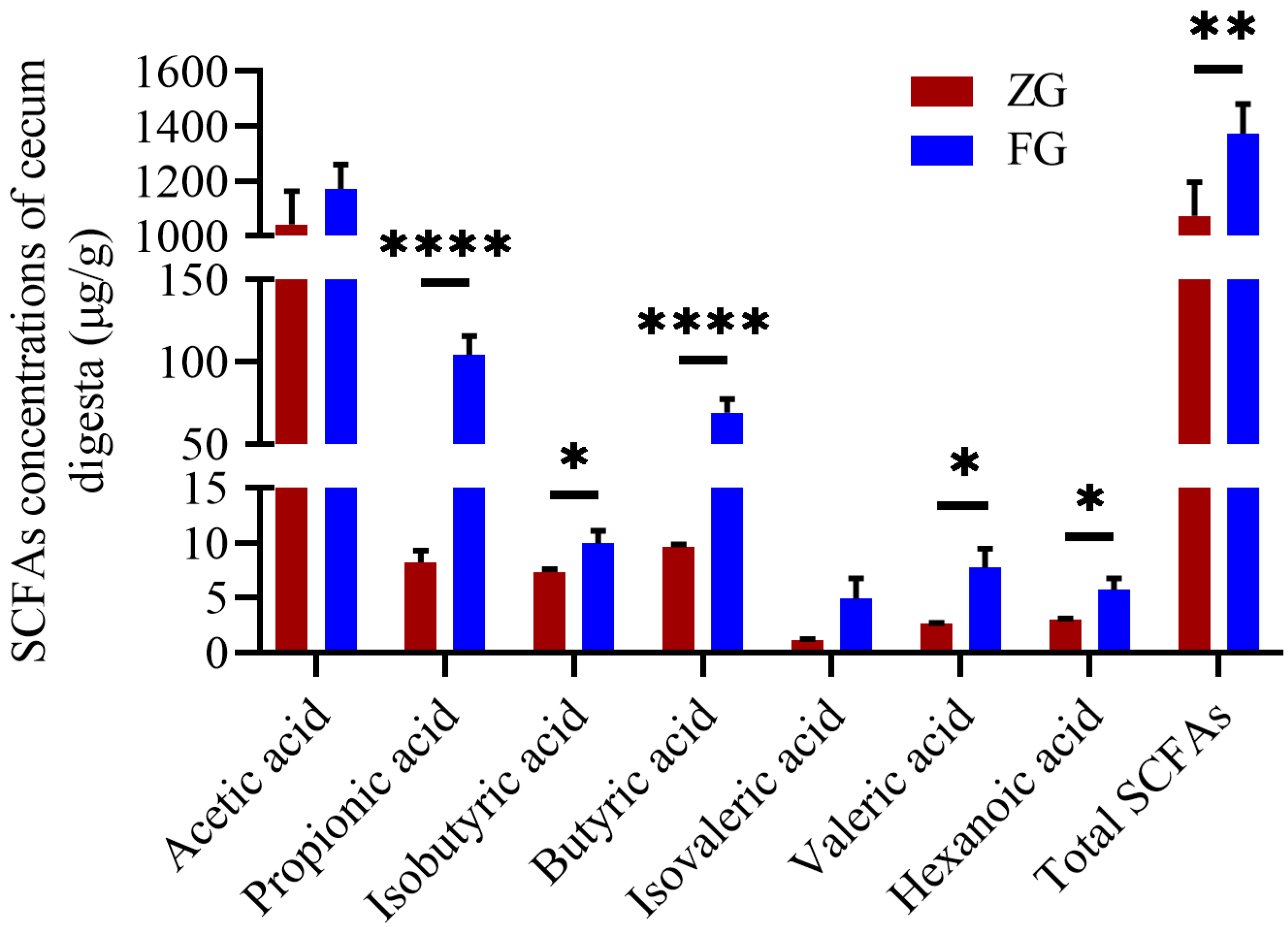
3.3. Comparison of Cecum Microbiota Composition, Functional Prediction, and SCFA Concentrations between the Two Goose Breeds
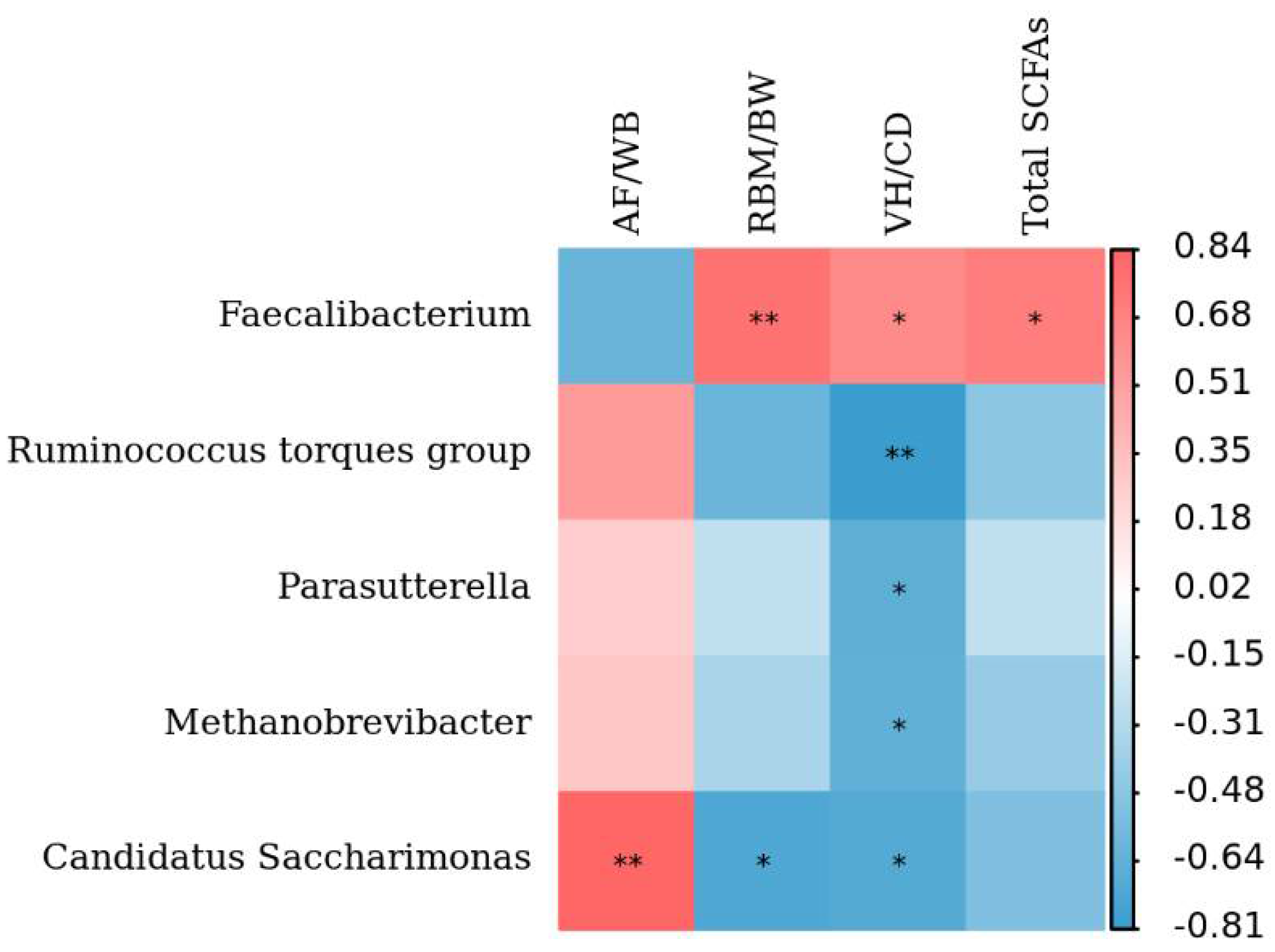
3.4. Associations of the Cecum Microbiota Composition with Production Performance Indexes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Niu, J.L.; Wei, L.Q.; Luo, Y.Q.; Yang, W.T.; Lu, Q.C.; Zheng, X.X.; Niu, Y.J.; Sheng, W.; Cheng, H.; Zhang, W.J.; et al. Fermented cottonseed meal improves production performance and reduces fat deposition in broiler chickens. Anim. Biosci. 2021, 34, 680–691. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.Q.; Zhang, X.Y.; Wang, S.Z.; Jiang, X.F.; Zhang, K.; Ma, G.W.; Wu, M.Q.; Li, H.; Zhang, H. Construction of multiple linear regression models using blood biomarkers for selecting against abdominal fat traits in broilers. Poult. Sci. 2018, 97, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Lamas, L.; Barron, L.J.R.; Farmer, L.; Aldai, N. Fatty acid composition of intramuscular fat and odour-active compounds of lamb commercialized in northern Spain. Meat Sci. 2018, 139, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.T.; Hwang, Y.H.; Frank, D. Characteristics of Hanwoo cattle and health implications of consuming highly marbled Hanwoo beef. Meat Sci. 2017, 132, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Velasco, S.; Cañeque, V.; Pérez, C.; Lauzurica, S.; Díaz, M.T.; Huidobro, F.; Manzanares, C.; González, J. Fatty acid composition of adipose depots of suckling lambs raised under different production systems. Meat Sci. 2001, 59, 325–333. [Google Scholar] [CrossRef]
- Mushi, D.E.; Thomassen, M.S.; Kifaro, G.C.; Eik, L.O. Fatty acid composition of minced meat, longissimus muscle and omental fat from Small East African goats finished on different levels of concentrate supplementation. Meat Sci. 2010, 86, 337–342. [Google Scholar] [CrossRef]
- Chung, H.; Pamp, S.J.; Hill, J.A.; Surana, N.K.; Edelman, S.M.; Troy, E.B.; Reading, N.C.; Villablanca, E.J.; Wang, S.; Mora, J.R.; et al. Gut immune maturation depends on colonization with a host-specific microbiota. Cell 2012, 149, 1578–1593. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Zhou, H.; Pan, X.; Xu, T.; Zhang, Z.; Zi, X.; Jiang, Y. Cassava foliage affects the microbial diversity of Chinese indigenous geese caecum using 16S rRNA sequencing. Sci. Rep. 2017, 7, 45697. [Google Scholar] [CrossRef]
- Pan, D.; Yu, Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes 2014, 5, 108–119. [Google Scholar] [CrossRef]
- Hou, Q.; Qian, Z.; Wu, P.; Shen, M.; Li, L.; Zhao, W. 1-Deoxynojirimycin from mulberry leaves changes gut digestion and microbiota composition in geese. Poult. Sci. 2020, 99, 5858–5866. [Google Scholar] [CrossRef]
- Li, J.; Tang, C.; Zhao, Q.; Yang, Y.; Li, F.; Qin, Y.; Liu, X.; Yue, X.; Zhang, J. Integrated lipidomics and targeted metabolomics analyses reveal changes in flavor precursors in psoas major muscle of castrated lambs. Food Chem. 2020, 333, 127451. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, J.; Yang, Y.; Zhu, J.; He, W.; Zhao, Q.; Tang, C.; Qin, Y.; Zhang, J. Comparative characterization of lipids and volatile compounds of Beijing Heiliu and Laiwu Chinese black pork as markers. Food Res. Int. 2021, 146, 110433. [Google Scholar] [CrossRef] [PubMed]
- Huo, W.; Weng, K.; Gu, T.; Zhang, Y.; Zhang, Y.; Chen, G.; Xu, Q. Effect of muscle fiber characteristics on meat quality in fast- and slow-growing ducks. Poult. Sci. 2021, 100, 101264. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.R.; Zhao, G.P.; Chen, J.L.; Zheng, M.Q.; Zhao, J.P.; Li, P.; Hu, J.; Wen, J. Effect of dietary supplemental nicotinic acid on growth performance, carcass characteristics and meat quality in three genotypes of chicken. J. Anim. Physiol. Anim. Nutr. 2011, 95, 137–145. [Google Scholar] [CrossRef]
- Agnew, M.P.; Craigie, C.R.; Weralupitiya, G.; Reis, M.M.; Johnson, P.L.; Reis, M.G. Comprehensive Evaluation of Parameters Affecting One-Step Method for Quantitative Analysis of Fatty Acids in Meat. Metabolites 2019, 9, 189. [Google Scholar] [CrossRef] [Green Version]
- Fadrosh, D.W.; Ma, B.; Gajer, P.; Sengamalay, N.; Ott, S.; Brotman, R.M.; Ravel, J. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2014, 2, 6. [Google Scholar] [CrossRef] [Green Version]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Li, M.; Shao, D.; Zhou, J.; Gu, J.; Qin, J.; Chen, W.; Wei, W. Signatures within esophageal microbiota with progression of esophageal squamous cell carcinoma. Chin. J. Cancer Res. 2020, 32, 755–767. [Google Scholar] [CrossRef]
- Fellows, R.; Denizot, J.; Stellato, C.; Cuomo, A.; Jain, P.; Stoyanova, E.; Balázsi, S.; Hajnády, Z.; Liebert, A.; Kazakevych, J.; et al. Microbiota derived short chain fatty acids promote histone crotonylation in the colon through histone deacetylases. Nat. Commun. 2018, 9, 105. [Google Scholar] [CrossRef]
- Weng, K.; Huo, W.; Li, Y.; Zhang, Y.; Zhang, Y.; Chen, G.; Xu, Q. Fiber characteristics and meat quality of different muscular tissues from slow- and fast-growing broilers. Poult. Sci. 2022, 101, 101537. [Google Scholar] [CrossRef]
- Kim, G.D.; Jeong, J.Y.; Jung, E.Y.; Yang, H.S.; Lim, H.T.; Joo, S.T. The influence of fiber size distribution of type IIB on carcass traits and meat quality in pigs. Meat Sci. 2013, 94, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Liu, Q.; Meng, Q.; Wang, L.; Yan, H.; Zhang, L.; Wang, L. TMT-based quantitative proteomic analysis of porcine muscle associated with postmortem meat quality. Food Chem. 2020, 328, 127133. [Google Scholar] [CrossRef] [PubMed]
- Gentry, J.G.; McGlone, J.J.; Blanton, J.R.; Jr Miller, M.F. Impact of spontaneous exercise on performance, meat quality, and muscle fiber characteristics of growing/finishing pigs. J. Anim. Sci. 2002, 80, 2833–2839. [Google Scholar] [CrossRef]
- Hu, F.B.; van Dam, R.M.; Liu, S. Diet and risk of Type II diabetes: The role of types of fat and carbohydrate. Diabetologia 2001, 44, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Eyres, G.T.; Reis, M.G.; Schreurs, N.M.; Silcock, P.; Agnew, M.P.; Johnson, P.L.; Maclean, P.; Realini, C.E. Fatty Acid Composition and Volatile Profile of M. longissimus thoracis from Commercial Lambs Reared in Different Forage Systems. Foods 2020, 9, 1885. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, M.C.; Saadoun, A. An overview of the nutritional value of beef and lamb meat from South America. Meat Sci. 2014, 98, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Choi, S.H.; Nogoy, K.M.; Liang, S. Review: The development of the gastrointestinal tract microbiota and intervention in neonatal ruminants. Animal 2021, 15, 100316. [Google Scholar] [CrossRef]
- Guo, B.; Li, D.; Zhou, B.; Jiang, Y.; Bai, H.; Zhang, Y.; Xu, Q.; Zhao, W.; Chen, G. Comparative characterization of bacterial communities in geese consuming of different proportions of ryegrass. PLoS ONE 2019, 14, e0223445. [Google Scholar] [CrossRef]
- Bui, T.P.; Shetty, S.A.; Lagkouvardos, I.; Ritari, J.; Chamlagain, B.; Douillard, F.P.; Paulin, L.; Piironen, V.; Clavel, T.; Plugge, C.M.; et al. Comparative genomics and physiology of the butyrate-producing bacterium Intestinimonas butyriciproducens. Environ. Microbiol. Rep. 2016, 8, 1024–1037. [Google Scholar] [CrossRef]
- Afouda, P.; Durand, G.A.; Lagier, J.C.; Labas, N.; Cadoret, F.; Armstrong, N.; Raoult, D.; Dubourg, G. Noncontiguous finished genome sequence and description of Intestinimonas massiliensis sp. nov strain GD2(T), the second Intestinimonas species cultured from the human gut. Microbiologyopen 2019, 8, e00621. [Google Scholar] [CrossRef] [Green Version]
- Qin, S.M.; Zhang, K.Y.; Ding, X.M.; Bai, S.P.; Wang, J.P.; Zeng, Q.F. Effect of dietary graded resistant potato starch levels on growth performance, plasma cytokines concentration, and intestinal health in meat ducks. Poult. Sci. 2019, 98, 3523–3532. [Google Scholar] [CrossRef] [PubMed]
- Lyu, W.; Liu, X.; Lu, L.; Dai, B.; Wang, W.; Yang, H.; Xiao, Y. Cecal Microbiota Modulates Fat Deposition in Muscovy Ducks. Front. Vet. Sci. 2021, 8, 609348. [Google Scholar] [CrossRef]
- Misiukiewicz, A.; Gao, M.; Filipiak, W.; Cieslak, A.; Patra, A.K.; Szumacher-Strabel, M. Review: Methanogens and methane production in the digestive systems of nonruminant farm animals. Animal 2021, 15, 100060. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, P.; Liu, S.; Zhang, B.; Hu, Y.; Ma, H.; Wang, S. Green tea leaf powder prevents dyslipidemia in high-fat diet-fed mice by modulating gut microbiota. Food Nutr. Res. 2020, 64, 14589. [Google Scholar] [CrossRef] [PubMed]
- Dziarski, R.; Park, S.Y.; Kashyap, D.R.; Dowd, S.E.; Gupta, D. Pglyrp-Regulated Gut Microflora Prevotella falsenii, Parabacteroides distasonis and Bacteroides eggerthii Enhance and Alistipes finegoldii Attenuates Colitis in Mice. PLoS ONE 2016, 11, e0146162. [Google Scholar] [CrossRef]
- Chen, Y.J.; Wu, H.; Wu, S.D.; Lu, N.; Wang, Y.T.; Liu, H.N.; Dong, L.; Liu, T.T.; Shen, X.Z. Parasutterella, in association with irritable bowel syndrome and intestinal chronic inflammation. J. Gastroenterol. Hepatol. 2018, 33, 1844–1852. [Google Scholar] [CrossRef]
- Wang, Y.; Nan, X.; Zhao, Y.; Jiang, L.; Wang, H.; Zhang, F.; Hua, D.; Liu, J.; Yao, J.; Yang, L.; et al. Dietary Supplementation of Inulin Ameliorates Subclinical Mastitis via Regulation of Rumen Microbial Community and Metabolites in Dairy Cows. Microbiol. Spectr. 2021, 9, e0010521. [Google Scholar] [CrossRef]


| Ingredients | Weeks | |
|---|---|---|
| 0–4 | 5–20 | |
| Apparent metabolic energy (MJ/kg) | 11.2 | 10.85 |
| Crude protein (%) | 19.25 | 17.16 |
| Crude fiber (%) | 4.75 | 5.88 |
| Crude ash (%) | 5.13 | 5.08 |
| Calcium (%) | 0.80 | 0.80 |
| Total phosphorus (%) | 0.42 | 0.37 |
| Total lysine (%) | 0.90 | 0.65 |
| Total methionine (%) | 0.42 | 0.35 |
| Item | ZG (n = 30) | FG (n = 30) | SEM 1 | p Value 2 |
|---|---|---|---|---|
| pH | 6.10 a | 5.62 b | 0.12 | 0.003 |
| Meat color | 84.23 b | 89.76 a | 1.21 | 0.001 |
| Shear force (N) | 41.31 a | 36.48 b | 2.31 | 0.047 |
| Water loss (%) | 8.95 b | 20.81 a | 2.75 | 0.002 |
| Cooking loss (%) | 22.80 | 21.74 | 1.98 | 0.603 |
| Items | ZG (n = 30) | FG (n = 30) | SEM 1 | p Value 2 |
|---|---|---|---|---|
| IMF (%) | 2.98 a | 2.61 b | 0.10 | 0.001 |
| Fatty acid proportions (% of total fatty acids) | ||||
| C14:0 | 0.25 | 0.22 | 0.01 | 0.138 |
| C16:0 | 20.95 a | 20.08 b | 0.28 | 0.002 |
| C16:1 | 2.06 | 2.24 | 0.11 | 0.53 |
| C18:0 | 9.26 b | 10.59 a | 0.32 | <0.0001 |
| C18:1n9c | 37.78 a | 32.87 b | 0.79 | <0.0001 |
| C18:2n6c | 22.82 b | 24.98 a | 0.46 | <0.0001 |
| C18:3n3 | 0.50 | 0.46 | 0.03 | 0.067 |
| C20:3n6 | 0.30 | 0.26 | 0.05 | 0.453 |
| C20:4n6 | 6.09 b | 8.23 a | 0.41 | <0.0001 |
| C22:6n3 | 0.42 b | 0.49 a | 0.04 | 0.031 |
| SFAs | 30.32 | 30.76 | 0.31 | 0.159 |
| MUFAs | 39.86 a | 35.11 b | 0.84 | <0.0001 |
| PUFAs | 29.82 b | 34.12 a | 0.76 | <0.0001 |
| Items | ZG (n = 5) | FG (n = 5) | SEM 1 | p Values 2 |
|---|---|---|---|---|
| Observed ASVs | 665 | 637 | 51.41 | >0.999 |
| Chao1 | 664.40 | 636.85 | 51.52 | >0.999 |
| Shannon | 7.82 | 7.46 | 0.26 | 0.310 |
| Simpson | 0.99 | 0.98 | 0.004 | 0.087 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, H.; Zhang, Y.; Yang, Y.; Li, Y.; Yin, Y.; Sun, X.; Xie, H.; Zheng, J.; Dong, L.; Diao, J.; et al. Comparative Analyses of Production Performance, Meat Quality, and Gut Microbial Composition between Two Chinese Goose Breeds. Animals 2022, 12, 1815. https://doi.org/10.3390/ani12141815
Ni H, Zhang Y, Yang Y, Li Y, Yin Y, Sun X, Xie H, Zheng J, Dong L, Diao J, et al. Comparative Analyses of Production Performance, Meat Quality, and Gut Microbial Composition between Two Chinese Goose Breeds. Animals. 2022; 12(14):1815. https://doi.org/10.3390/ani12141815
Chicago/Turabian StyleNi, Hongyu, Yonghong Zhang, Yuwei Yang, Yumei Li, Yijing Yin, Xueqi Sun, Hengli Xie, Jinlei Zheng, Liping Dong, Jizhe Diao, and et al. 2022. "Comparative Analyses of Production Performance, Meat Quality, and Gut Microbial Composition between Two Chinese Goose Breeds" Animals 12, no. 14: 1815. https://doi.org/10.3390/ani12141815






