Corynebacterium conjunctivae: A New Corynebacterium Species Isolated from the Ocular Surface of Healthy Horses
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care
2.2. Sample Collection
2.3. Bacterial Sampling
2.4. 16S rRNA Gene Sequencing
2.5. DNA G+C Content
2.6. Fatty Acid Composition and Cell Wall Analyses
2.7. Morphological, Physiological and Biochemical Characteristics
2.8. PFGE Typing
2.9. Susceptibility Testing
3. Results and Discussion
3.1. Phenotypic Characteristics
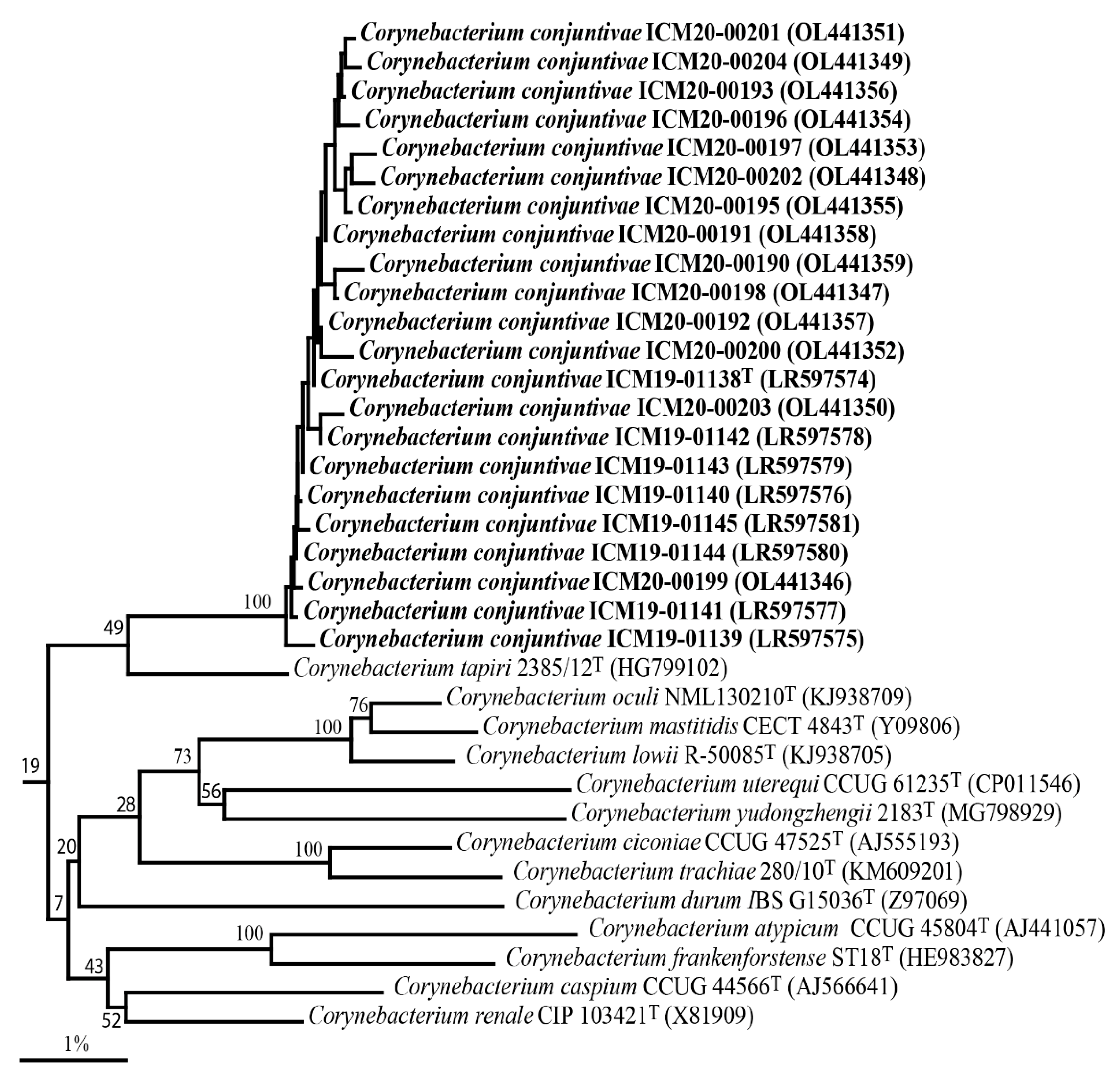
3.2. Phylogenetic Analysis
3.3. Chemotaxonomic and G+C Analysis
3.4. Antimicrobial Susceptibility and PFGE Analysis
4. Conclusions
Description of Corynebacterium conjunctivae sp. nov.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leigue, L.; Montiani-Ferreirm, F.; Moore, B.A. Antimicrobial susceptibility and minimal inhibitory concentration of Pseudomonas aeruginosa isolated from septic ocular surface disease in different animal species. Open Vet. J. 2016, 6, 215–222. [Google Scholar] [CrossRef] [Green Version]
- Willcox, M.D. Characterization of the normal microbiota of the ocular surface. Exp. Eye Res. 2013, 117, 99–105. [Google Scholar] [CrossRef]
- Kugadas, A.; Gadjeva, M. Impact of microbiome in ocular health. Ocul. Surf. 2016, 14, 342–349. [Google Scholar] [CrossRef] [Green Version]
- Andrew, S.E.; Nguyen, A.; Jones, G.L.; Brooks, D.E. Seasonal effects on the aerobic bacterial and fungal conjunctival flora of normal thoroughbred brood mares in Florida. Vet. Ophthalmol. 2003, 6, 45–50. [Google Scholar] [CrossRef]
- Gemensky-Metzler, A.J.; Wilkie, D.A.; Kowalski, J.J.; Schmall, L.M.; Willis, A.M.; Yamagata, M. Changes in bacterial and fungal ocular flora of clinically normal horses following experimental application of topical antimicrobial or antimicrobial-corticosteroid ophthalmic preparations. Am. J. Vet. Res. 2005, 66, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Hampson, E.C.G.M.; Gibson, J.S.; Barot, M.; Shapter, F.M.; Greer, R.M. Identification of bacterial and fungi samples from the conjunctival surface of normal horses in South-East Queensland, Australia. Vet. Ophthalmol. 2019, 22, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Johns, I.C.; Baxter, K.; Booler, H.; Hicks, C.; Menzies-Gow, N. Conjunctival bacterial and fungal flora in healthy horses in the UK. Vet. Ophthalmol. 2011, 14, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.P.; Heller, N.; Majors, L.J.; Whitley, R.D.; Burgess, E.C.; Weber, J. Prevalence of ocular microorganisms in hospitalized and stabled horses. Am. J. Vet. Res. 1988, 49, 773–777. [Google Scholar]
- Whitley, R.D.; Moore, C.P. Microbiology of the equine eye in health and disease. Vet. Clin. North Am. Large Anim. Pract. 1984, 6, 451–466. [Google Scholar] [CrossRef]
- Whitley, R.D.; Burgess, E.C.; Moore, C.P. Microbial isolates of the normal equine eye. Equine Vet. J. 1983, 15, 138–140. [Google Scholar] [CrossRef]
- Arsan, A.K.; Sizmaz, S.; Ozkan, S.B.; Duman, S. Corynebacterium minutissimum endophthalmitis: Management with antibiotic irrigation of the capsular bag. Int. Ophthalmol. 1995, 19, 313–316. [Google Scholar] [CrossRef]
- Bernard, K.A.; Funke, G. Corynebacterium. In Bergey’s Manual of Systematics of Archaea and Bacteria (BMSAB); Bergey’s Manual Trust: East Lansing, MI, USA, 2015; Available online: https://onlinelibrary.wiley.com/doi/pdf/10.1002/9781118960608.gbm00026 (accessed on 18 April 2022). [CrossRef] [Green Version]
- Bernard, K.A.; Pacheco, A.L.; Loomer, C.; Burdz, T.; Wiebe, D.; Huynh, C.; Kaplen, B.; Olson, A.B.; Cnockaert, M.; Eguchi, H.; et al. Corynebacterium lowii sp. nov. and Corynebacterium oculi sp. nov., derived from human clinical disease and an emended description of Corynebacterium mastitidis. Int. J. Syst. Evol. Microbiol. 2016, 66, 2803–2812. [Google Scholar] [CrossRef] [Green Version]
- Chow, S.K.; Bui, U.; Clarridge, J.E. Corynebacterium bovis eye infections, Washington, USA, 2013. Emerg. Infect. Dis. 2015, 21, 1687–1689. [Google Scholar] [CrossRef] [Green Version]
- Kojouri, G.A.; Ebrahimi, A.; Nikookhah, F. Systemic dexamethasone and its effect on normal aerobic bacterial flora of cow. Pak. J. Biol. Sci. 2007, 10, 2095–2097. [Google Scholar]
- St. Leger, A.J.; Desai, J.V.; Drummond, R.A.; Kugadas, A.; Almaghrabi, F.; Silver, P.; Raychaudhuri, K.; Gadjeva, M.; Iwakura, Y.; Lionakis, M.S.; et al. An ocular commensal protects against corneal infection by driving an Interleukin-17 response from mucosal γδ T cells. Immunity 2017, 47, 148–158. [Google Scholar] [CrossRef] [Green Version]
- LaFrentz, S.; Abarca, E.; Mohammed, H.H.; Cuming, R.; Arias, C.R. Characterization of the normal equine conjunctival bacterial community using culture-independent methods. Vet. Ophthalmol. 2020, 23, 480–488. [Google Scholar] [CrossRef]
- Vela, A.I.; Mentaberre, G.; Lavín, S.; Domínguez, L.; Fernández-Garayzábal, J.F. Streptococcus caprae sp. nov., isolated from iberian ibex (Capra pyrenaica hispanica). Int. J. Syst. Evol. Microbiol. 2016, 66, 196–200. [Google Scholar] [CrossRef]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBio-Cloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef]
- Rasmussen, S.W. SeqTools, a Program Suite for Sequence Analysis; Carlsberg Laboratory: Copenhagen, Denmark, 2002. [Google Scholar]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Page, R.D. TreeView: An application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 1996, 12, 357–358. [Google Scholar]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [Green Version]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Mesbah, M.; Premachandran, U.; Whitman, W.B. Precise measurement of the G-C content of deoxyribonucleic acid by high-performance liquid chromatography. Int. J. Syst. Bacteriol. 1989, 39, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Funke, G.; von Graevenitz, A.; Clarridge, J.E.; Bernard, K.A. Clinical microbiology of coryneform bacteria. Clin. Microbiol. Rev. 1997, 10, 125–159. [Google Scholar] [CrossRef]
- Galán-Relaño, A.; Gómez-Gascón, L.; Luque, I.; Barrero-Domínguez, B.; Casamayor, A.; Cardoso-Toset, F.l; Vela, A.I.; Fernández-Garayzábal, J.F.; Tarradas, C. Antimicrobial susceptibility and genetic characterization of Trueperella pyogenes isolates from pigs reared under intensive and extensive farming practices. Vet. Microbiol. 2019, 232, 89–95. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standard Institute. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals—Approved Standard, 2nd ed.; Document M31-A2; Clinical and Laboratory Standard Institute: Wayne, PA, USA, 2002; pp. 1–86. [Google Scholar]
- Clinical and Laboratory Standard Institute. Methods for Antimicrobial Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria Isolated from Animals—Approved Standard, 1st ed.; Document VET06; Clinical and Laboratory Standard Institute: Wayne, PA, USA, 2017; pp. 1–100. [Google Scholar]
- Dorella, F.A.; Carvalho Pacheco, L.G.; Costa Oliveira, S.; Miyoshi, A.; Azevedo, V. Corynebacterium pseudotuberculosis: Microbiology, biochemical properties, pathogenesis and molecular studies of virulence. Vet. Res. 2006, 37, 201–218. [Google Scholar] [CrossRef] [Green Version]
- Stackebrandt, E.; Goebel, B.M. Taxonomic note: A place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int. J. Syst. Bacteriol. 1994, 44, 846–849. [Google Scholar] [CrossRef] [Green Version]
- de Araújo Ferreira, A.R.; Santana, A.F.; da Veiga Rodarte de Almeida, A.C.; Sousa, R.F.; Perecmanis, S.; Galera, P.D. Bacterial culture and antibiotic sensitivity from the ocular conjunctiva of horses. Cienc. Rural 2017, 47, e20160753. [Google Scholar]
- Pors, S.E.; Hansen, M.S.; Christensen, H.; Jensen, H.E.; Petersen, A.; Bisgaard, M. Genetic diversity and associated pathology of Pasteurella multocida isolated from porcine pneumonia. Vet. Microbiol. 2011, 150, 354–361. [Google Scholar] [CrossRef] [Green Version]
- Baumgardt, S.; Loncaric, I.; Kämpfer, P.; Busse, H.J. Corynebacterium tapiri sp. nov. and Corynebacterium nasicanis sp. nov., isolated from a tapir and a dog, respectively. Int. J. Syst. Evol. Microbiol. 2015, 65, 3885–3893. [Google Scholar] [CrossRef]
| Characteristic | C. conjunctivae (22 Isolates) | C. tapiri 2385/12Tb |
|---|---|---|
| Nitrate reduction | − | + |
| Hydrolysis of: | ||
| Esculin | − | − |
| Urea | + | + |
| Production of: | ||
| Pyrazinamidase | − | + |
| Esterase C4 | + | − |
| Ester lipase C8 | + | − |
| β-glucuronidase | − | + |
| α-glucosidase | − | + |
| Alkaline phosphatase | + | + |
| Acid phosphatase | + | − |
| Acid production from: | ||
| Glucose | + | + |
| Ribose | + | + |
| Maltose | − | + |
| Saccharose | − | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Garayzábal, J.F.; LaFrentz, S.; Casamayor, A.; Abarca, E.; Mohammed, H.H.; Cuming, R.S.; Arias, C.R.; Domínguez, L.; Vela, A.I. Corynebacterium conjunctivae: A New Corynebacterium Species Isolated from the Ocular Surface of Healthy Horses. Animals 2022, 12, 1827. https://doi.org/10.3390/ani12141827
Fernández-Garayzábal JF, LaFrentz S, Casamayor A, Abarca E, Mohammed HH, Cuming RS, Arias CR, Domínguez L, Vela AI. Corynebacterium conjunctivae: A New Corynebacterium Species Isolated from the Ocular Surface of Healthy Horses. Animals. 2022; 12(14):1827. https://doi.org/10.3390/ani12141827
Chicago/Turabian StyleFernández-Garayzábal, José F., Stacey LaFrentz, Almudena Casamayor, Eva Abarca, Haitham H. Mohammed, Rosemary S. Cuming, Cova R. Arias, Lucas Domínguez, and Ana I. Vela. 2022. "Corynebacterium conjunctivae: A New Corynebacterium Species Isolated from the Ocular Surface of Healthy Horses" Animals 12, no. 14: 1827. https://doi.org/10.3390/ani12141827






