Prediction of Acute and Chronic Mastitis in Dairy Cows Based on Somatic Cell Score and Mid-Infrared Spectroscopy of Milk
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data and Data Preparation
2.2. Pre-Treatment of MIR Spectra
2.3. Prediction Models
3. Results
3.1. Descriptive Statistics
3.2. Prediction of Mastitis Based on SCS and MIR Spectral Data
3.3. Prediction of Acute and Chronic Cases
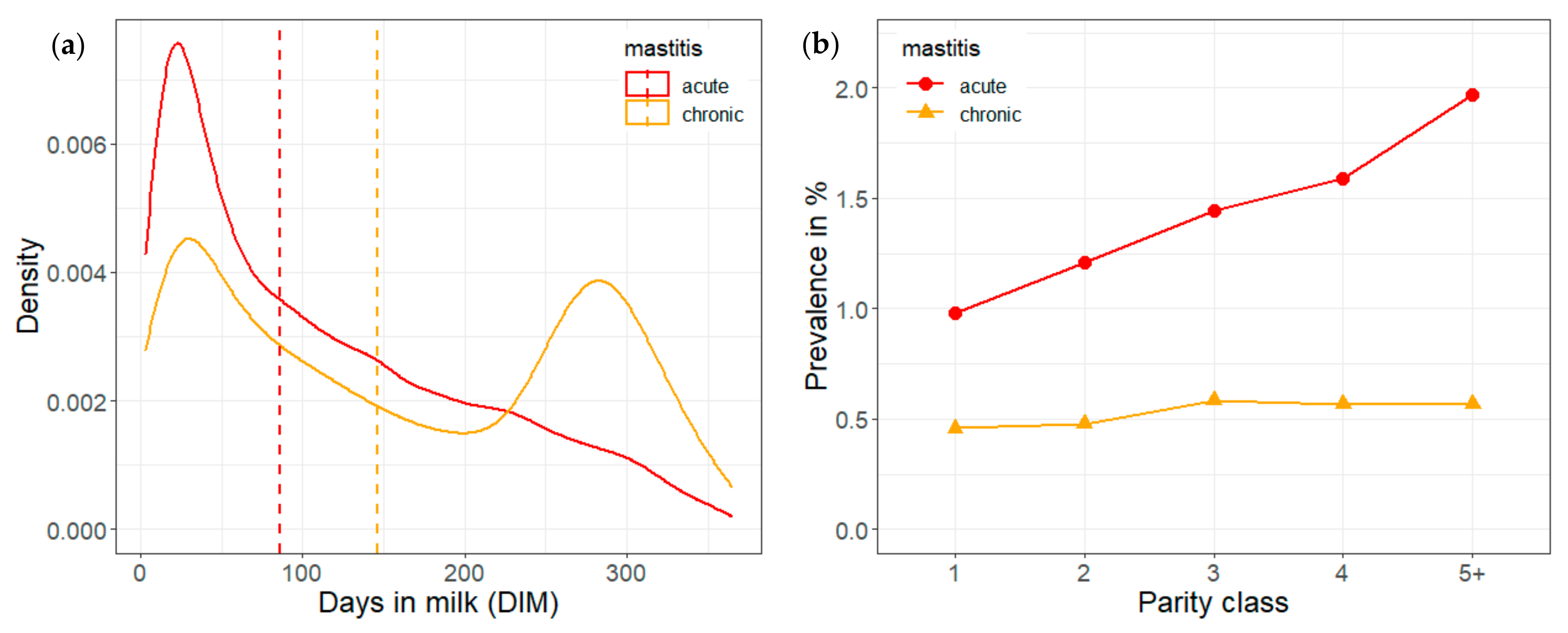
3.4. Predicted Probabilities of Acute and Chronic Mastitis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Halasa, T.; Huijps, K.; Østerås, O.; Hogeveen, H. Economic Effects of Bovine Mastitis and Mastitis Management: A Review. Vet. Q. 2007, 29, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Heikkilä, A.-M.; Nousiainen, J.I.; Pyörälä, S. Costs of Clinical Mastitis with Special Reference to Premature Culling. J. Dairy Sci. 2011, 95, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Seegers, H.; Fourichon, C.; Beaudeau, F. Production Effects Related to Mastitis and Mastitis Economics in Dairy Cattle Herds Henri. Vet. Res. 2003, 34, 475–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egger-Danner, C.; Fuerst-Waltl, B.; Obritzhauser, W.; Fuerst, C.; Schwarzenbacher, H.; Grassauer, B.; Mayerhofer, M.; Koeck, A. Recording of Direct Health Traits in Austria-Experience Report with Emphasis on Aspects of Availability for Breeding Purposes. J. Dairy Sci. 2012, 95, 2765–2777. [Google Scholar] [CrossRef] [Green Version]
- Winter, P.; Burvenich, C.; Hogeveen, H.; Neijenhuis, F.; Rasmussen, M.D.; Schweigert, F.J.; de Spiegeleer, B.; Zehle, H.-H. Klinik Der Mastitisformen. In Praktischer Leitfaden Mastitis; Petra Winter: Vienna, Austria, 2009; pp. 95–101. [Google Scholar]
- Blowey, R.W.; Edmondson, P. Mastitis Control in Dairy Herds, 2nd ed.; CAB International: Wallington, UK, 2010; ISBN 9780080453705. [Google Scholar]
- Sharma, N.; Singh, N.K.; Bhadwal, M.S. Relationship of Somatic Cell Count and Mastitis: An Overview. Asian-Australasian J. Anim. Sci. 2011, 24, 429–438. [Google Scholar] [CrossRef]
- International Dairy Federation. Guidelines for the Use and Interpretation of Bovine Milk Somatic Cell Count; Bulletin no 466/2013; International Dairy Federation: Brussels, Belgium, 2013. [Google Scholar]
- Harmon, R.J. Physiology of Mastitis and Factors Affecting Somatic Cell Counts. J. Dairy Sci. 1994, 77, 2103–2112. [Google Scholar] [CrossRef]
- Grelet, C.; Bastin, C.; Gelé, M.; Davière, J.B.; Johan, M.; Werner, A.; Reding, R.; Fernandez Pierna, J.A.; Colinet, F.G.; Dardenne, P.; et al. Development of Fourier Transform Mid-Infrared Calibrations to Predict Acetone, β-Hydroxybutyrate, and Citrate Contents in Bovine Milk through a European Dairy Network. J. Dairy Sci. 2016, 99, 4816–4825. [Google Scholar] [CrossRef] [Green Version]
- Soyeurt, H.; Dehareng, F.; Gengler, N.; McParland, S.; Wall, E.; Berry, D.P.; Coffey, M.; Dardenne, P. Mid-Infrared Prediction of Bovine Milk Fatty Acids across Multiple Breeds, Production Systems, and Countries. J. Dairy Sci. 2011, 94, 1657–1667. [Google Scholar] [CrossRef] [Green Version]
- Bonfatti, V.; Di Martino, G.; Carnier, P. Effectiveness of Mid-Infrared Spectroscopy for the Prediction of Detailed Protein Composition and Contents of Protein Genetic Variants of Individual Milk of Simmental Cows. J. Dairy Sci. 2011, 94, 5776–5785. [Google Scholar] [CrossRef]
- Soyeurt, H.; Bruwier, D.; Romnee, J.M.; Gengler, N.; Bertozzi, C.; Veselko, D.; Dardenne, P. Potential Estimation of Major Mineral Contents in Cow Milk Using Mid-Infrared Spectrometry. J. Dairy Sci. 2009, 92, 2444–2454. [Google Scholar] [CrossRef] [Green Version]
- De Marchi, M.; Toffanin, V.; Cassandro, M.; Penasa, M. Invited Review: Mid-Infrared Spectroscopy as Phenotyping Tool for Milk Traits. J. Dairy Sci. 2014, 97, 1171–1186. [Google Scholar] [CrossRef] [PubMed]
- Tiplady, K.M.; Lopdell, T.J.; Littlejohn, M.D.; Garrick, D.J. The Evolving Role of Fourier-Transform Mid-Infrared Spectroscopy in Genetic Improvement of Dairy Cattle. J. Anim. Sci. Biotechnol. 2020, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- McParland, S.; Lewis, E.; Kennedy, E.; Moore, S.G.; McCarthy, B.; O’Donovan, M.; Butler, S.T.; Pryce, J.E.; Berry, D.P. Mid-Infrared Spectrometry of Milk as a Predictor of Energy Intake and Efficiency in Lactating Dairy Cows. J. Dairy Sci. 2014, 97, 5863–5871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Knegsel, A.T.M.; van der Drift, S.G.A.; Horneman, M.; de Roos, A.P.W.; Kemp, B.; Graat, E.A.M. Short Communication: Ketone Body Concentration in Milk Determined by Fourier Transform Infrared Spectroscopy: Value for the Detection of Hyperketonemia in Dairy Cows. J. Dairy Sci. 2010, 93, 3065–3069. [Google Scholar] [CrossRef] [PubMed]
- Mineur, A.; Hammami, H.; Grelet, C.; Egger-Danner, C.; Sölkner, J.; Gengler, N. Short Communication: Investigation of the Temporal Relationships between Milk Mid-Infrared Predicted Biomarkers and Lameness Events in Later Lactation. J. Dairy Sci. 2020, 103, 4475–4482. [Google Scholar] [CrossRef] [Green Version]
- Ho, P.N.; Bonfatti, V.; Luke, T.D.W.; Pryce, J.E. Classifying the Fertility of Dairy Cows Using Milk Mid-Infrared Spectroscopy. J. Dairy Sci. 2019, 102, 10460–10470. [Google Scholar] [CrossRef] [Green Version]
- Delhez, P.; Ho, P.N.; Gengler, N.; Soyeurt, H.; Pryce, J.E. Diagnosing the Pregnancy Status of Dairy Cows: How Useful Is Milk Mid-Infrared Spectroscopy? J. Dairy Sci. 2020, 103, 3264–3274. [Google Scholar] [CrossRef] [Green Version]
- Rienesl, L.; Pfeiffer, P.; Khayatzadeh, N.; Köck, A.; Dale, L.; Werner, A.; Grelet, C.; Gengler, N.; Auer, F.J.; Egger-Danner, C.; et al. Prediction of Pregnancy State from Milk Mid-Infrared (MIR) Spectroscopy in Dairy Cows. Acta Fytotech. Zootech. 2020, 23, 224–232. [Google Scholar] [CrossRef]
- Soyeurt, H.; Bastin, C.; Colinet, F.G.; Arnould, V.M.-R.; Berry, D.P.; Wall, E.; Dehareng, F.; Nguyen, H.N.; Dardenne, P.; Schefers, J.; et al. Mid-Infrared Prediction of Lactoferrin Content in Bovine Milk: Potential Indicator of Mastitis. Animal 2012, 6, 1830–1838. [Google Scholar] [CrossRef] [Green Version]
- Dale, L.; Werner, A. “MastiMIR”-Ein Mastitis-Frühwarnsystem Basierend Auf MIR-Spektren. In Proceedings of the Vortragstagung der DCfZ und GfT, Stuttgart, Germany, 20–21 September 2017. [Google Scholar]
- Rienesl, L.; Khayatzadeh, N.; Köck, A.; Dale, L.; Werner, A.; Grelet, C.; Gengler, N.; Auer, F.J.; Egger-Danner, C.; Massart, X.; et al. Mastitis Detection from Milk Mid-Infrared (MIR) Spectroscopy in Dairy Cows. Acta Univ. Agric. Silvic. Mendelianae Brun. 2019, 67, 1221–1226. [Google Scholar] [CrossRef] [Green Version]
- Suntinger, M.; Fuerst-Waltl, B.; Obritzhauser, W.; Firth, C.L.; Köck, A.; Egger-Danner, C. Usability of Bacteriological Milk Analyses for Genetic Improvement of Udder Health in Austrian Fleckvieh Cows. J. Dairy Sci. 2022, 105, 5167–5177. [Google Scholar] [CrossRef] [PubMed]
- The R Development Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Grelet, C.; Fernández Pierna, J.A.; Dardenne, P.; Baeten, V.; Dehareng, F. Standardization of Milk Mid-Infrared Spectra from a European Dairy Network. J. Dairy Sci. 2015, 98, 2150–2160. [Google Scholar] [CrossRef]
- Grelet, C.; Pierna, J.A.F.; Dardenne, P.; Soyeurt, H.; Vanlierde, A.; Colinet, F.; Bastin, C.; Gengler, N.; Baeten, V.; Dehareng, F. Standardization of Milk Mid-Infrared Spectrometers for the Transfer and Use of Multiple Models. J. Dairy Sci. 2017, 100, 7910–7921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grelet, C.; Dardenne, P.; Soyeurt, H.; Fernandez, J.A.; Vanlierde, A.; Stevens, F.; Gengler, N.; Dehareng, F. Large-Scale Phenotyping in Dairy Sector Using Milk MIR Spectra: Key Factors Affecting the Quality of Predictions. Methods 2021, 186, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Vanlierde, A.; Vanrobays, M.-L.; Dehareng, F.; Froidmont, E.; Soyeurt, H.; McParland, S.; Lewis, E.; Deighton, M.H.; Grandl, F.; Kreuzer, M.; et al. Hot Topic: Innovative Lactation-Stage-Dependent Prediction of Methane Emissions from Milk Mid-Infrared Spectra. J. Dairy Sci. 2015, 98, 5740–5747. [Google Scholar] [CrossRef] [Green Version]
- Gengler, N.; Tijani, A.; Wiggans, G.R.; Misztal, I. Estimation of (Co)Variance Function Coefficients for Test Day Yield with a Expectation-Maximization Restricted Maximum Likelihood Algorithm. J. Dairy Sci. 1999, 82, 1849.e1–1849.e23. [Google Scholar] [CrossRef]
- Ali, A.K.A.; Shook, G.E. An Optimum Transformation for Somatic Cell Concentration in Milk. J. Dairy Sci. 1980, 63, 487–490. [Google Scholar] [CrossRef]
- Kuhn, M. Building Predictive Models in R Using the Caret Package. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Simundic, A.M. Measures of Diagnostic Accuracy: Basic Definitions. Ejifcc 2009, 19, 203–211. [Google Scholar]
- Lantz, B. Machine Learning with R, 2nd ed.; Packt Publishing Ltd.: Birmingham, UK, 2015; ISBN 9781784393908. [Google Scholar]
- De Haas, Y.; Barkema, H.W.; Veerkamp, R.F. The Effect of Pathogen-Specific Clinical Mastitis on the Lactation Curve for Somatic Cell Count. J. Dairy Sci. 2002, 85, 1314–1323. [Google Scholar] [CrossRef]
- Svensson, C.; Nyman, A.K.; Waller, K.P.; Emanuelson, U. Effects of Housing, Management, and Health of Dairy Heifers on First-Lactation Udder Health in Southwest Sweden. J. Dairy Sci. 2006, 89, 1990–1999. [Google Scholar] [CrossRef]
- Steeneveld, W.; Hogeveen, H.; Barkema, H.W.; Van Den Broek, J.; Huirne, R.B.M. The Influence of Cow Factors on the Incidence of Clinical Mastitis in Dairy Cows. J. Dairy Sci. 2008, 91, 1391–1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehareng, F.; Delfosse, C.; Froidmont, E.; Soyeurt, H.; Martin, C.; Gengler, N.; Vanlierde, A.; Dardenne, P. Potential Use of Milk Mid-Infrared Spectra to Predict Individual Methane Emission of Dairy Cows. Animal 2012, 6, 1694–1701. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Unit | Records (n) |
|---|---|
| Farms | 2621 |
| Cows | 59,002 |
| Fleckvieh (Dual purpose Simmental) | 46,042 |
| Holstein Friesian | 7645 |
| Brown Swiss | 5315 |
| Test-day records | 764,542 |
| Mastitis records 1 | 12,656 |
| Acute | 8917 |
| Chronic | 3739 |
| Dataset | Time Window 1 (days) | Records (n) * | |
|---|---|---|---|
| Healthy | Mastitis | ||
| Calibration | −21 to +21 (overall) | 8846 | 8846 |
| Validation | −21 to +21 (overall) | 224,202 | 3810 |
| −21 to −15 | 224,202 | 586 | |
| −14 to −8 | 224,202 | 629 | |
| −7 to 0 | 224,202 | 738 | |
| 0 to +7 | 224,202 | 651 | |
| +8 to +14 | 224,202 | 647 | |
| +15 to +21 | 224,202 | 643 | |
| Dataset | Predictor Variables | Sensitivity | Specificity | Balanced Accuracy | AUC |
|---|---|---|---|---|---|
| calibration | MIR | 0.620 a (0.005) | 0.697 a (0.005) | 0.658 a (0.004) | - |
| SCS | 0.610 b (0.003) | 0.725 b (0.005) | 0.668 b (0.003) | - | |
| MIR + SCS | 0.657 c (0.003) | 0.763 c (0.005) | 0.710 c (0.003) | - | |
| validation | MIR | 0.605 a (0.010) | 0.686 a (0.005) | 0.645 a (0.004) | 0.696 a (0.005) |
| SCS | 0.610 a (0.010) | 0.722 b (0.008) | 0.666 b (0.004) | 0.722 b (0.004) | |
| MIR + SCS | 0.645 b (0.007) | 0.751 c (0.006) | 0.698 c (0.003) | 0.760 c (0.005) |
| Time Window | Predictor Variables | Sensitivity | Balanced Accuracy | AUC |
|---|---|---|---|---|
| −21 to −15 | MIR | 0.529 a (0.017) | 0.607 a (0.007) | 0.644 a (0.009) |
| SCS | 0.524 a (0.018) | 0.623 b (0.008) | 0.660 b (0.008) | |
| MIR + SCS | 0.544 b (0.015) | 0.647 c (0.006) | 0.696 c (0.008) | |
| −14 to −8 | MIR | 0.526 a (0.022) | 0.606 a (0.010) | 0.650 a (0.010) |
| SCS | 0.581 b (0.017) | 0.652 b (0.008) | 0.695 b (0.012) | |
| MIR + SCS | 0.587 b (0.017) | 0.669 c (0.006) | 0.722 c (0.009) | |
| −7 to 0 | MIR | 0.617 a (0.021) | 0.651 a (0.010) | 0.708 a (0.012) |
| SCS | 0.686 b (0.013) | 0.704 b (0.006) | 0.770 b (0.007) | |
| MIR + SCS | 0.691 b (0.015) | 0.721 c (0.009) | 0.787 c (0.011) | |
| 0 to +7 | MIR | 0.709 a (0.014) | 0.697 a (0.007) | 0.767 a (0.009) |
| SCS | 0.769 b (0.021) | 0.746 b (0.010) | 0.828 b (0.012) | |
| MIR + SCS | 0.790 c (0.014) | 0.771 c (0.007) | 0.849 c (0.008) | |
| +8 to +14 | MIR | 0.664 a (0.014) | 0.675 a (0.007) | 0.727 a (0.010) |
| SCS | 0.594 b (0.012) | 0.650 b (0.007) | 0.714 b (0.005) | |
| MIR + SCS | 0.666 a (0.015) | 0.708 c (0.006) | 0.772 c (0.007) | |
| +15 to +21 | MIR | 0.587 a (0.019) | 0.637 a (0.010) | 0.681 a (0.011) |
| SCS | 0.505 b (0.028) | 0.614 b (0.014) | 0.662 b (0.014) | |
| MIR + SCS | 0.588 a (0.026) | 0.670 c (0.013) | 0.732 c (0.015) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rienesl, L.; Khayatzdadeh, N.; Köck, A.; Egger-Danner, C.; Gengler, N.; Grelet, C.; Dale, L.M.; Werner, A.; Auer, F.-J.; Leblois, J.; et al. Prediction of Acute and Chronic Mastitis in Dairy Cows Based on Somatic Cell Score and Mid-Infrared Spectroscopy of Milk. Animals 2022, 12, 1830. https://doi.org/10.3390/ani12141830
Rienesl L, Khayatzdadeh N, Köck A, Egger-Danner C, Gengler N, Grelet C, Dale LM, Werner A, Auer F-J, Leblois J, et al. Prediction of Acute and Chronic Mastitis in Dairy Cows Based on Somatic Cell Score and Mid-Infrared Spectroscopy of Milk. Animals. 2022; 12(14):1830. https://doi.org/10.3390/ani12141830
Chicago/Turabian StyleRienesl, Lisa, Negar Khayatzdadeh, Astrid Köck, Christa Egger-Danner, Nicolas Gengler, Clément Grelet, Laura Monica Dale, Andreas Werner, Franz-Josef Auer, Julie Leblois, and et al. 2022. "Prediction of Acute and Chronic Mastitis in Dairy Cows Based on Somatic Cell Score and Mid-Infrared Spectroscopy of Milk" Animals 12, no. 14: 1830. https://doi.org/10.3390/ani12141830
APA StyleRienesl, L., Khayatzdadeh, N., Köck, A., Egger-Danner, C., Gengler, N., Grelet, C., Dale, L. M., Werner, A., Auer, F.-J., Leblois, J., & Sölkner, J. (2022). Prediction of Acute and Chronic Mastitis in Dairy Cows Based on Somatic Cell Score and Mid-Infrared Spectroscopy of Milk. Animals, 12(14), 1830. https://doi.org/10.3390/ani12141830






