Collagen Type III as a Possible Blood Biomarker of Fibrosis in Equine Endometrium
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Animals
2.2.1. Experiment 1
2.2.2. Experiment 2
2.3. Blood Sampling and Processing
2.4. Endometrium Biopsies Processing
2.5. Collagen Determination
2.6. Hydroxyproline Determination
2.7. Statistical Analysis
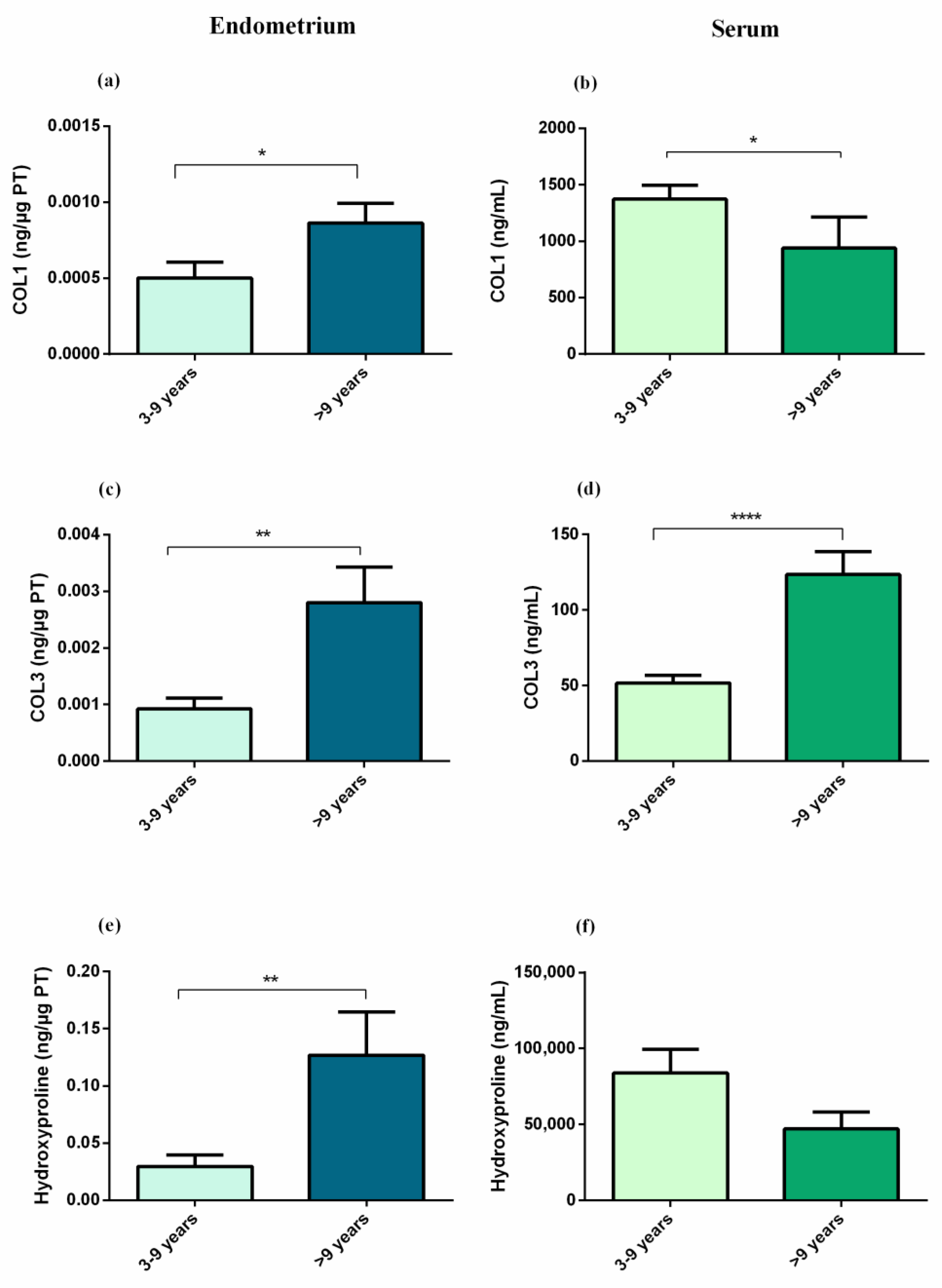
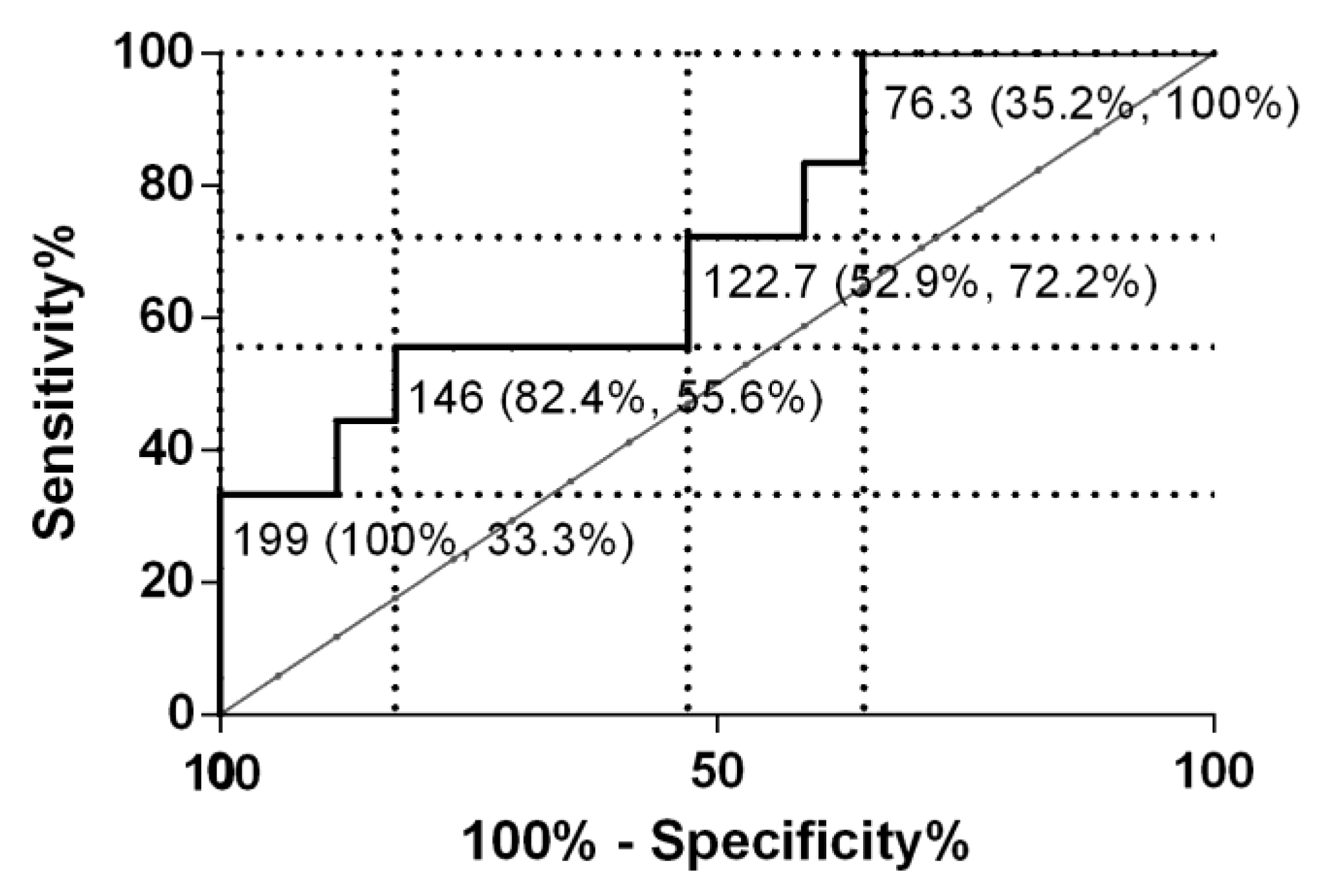
3. Results
3.1. Experiment 1
3.2. Experiment 2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kenney, R.M. The aetiology, diagnosis and classification of chronic degenerative endometritis (CDE) (endometrosis). Proceedings of the John P. Hughes International Workshop on Equine Endometritis. Davis, California, August 1992. Equine Vet. J. 1993, 25, 186. [Google Scholar]
- Schoon, H.A.; Schoon, D.; Klug, E. Uterusbiopsien als Hilfsmittel für Diagnose und Prognose von Fertilitätsstörungen der Stute. Pferdeheilkunde 1992, 8, 355–362. [Google Scholar] [CrossRef]
- Schoon, H.A.; Schoon, D.; Klug, E. Die Endometriumbiopsie bei der Stute im klinisch-gynäkologischen Kontext. Pferdeheilkun-de 1997, 13, 453–464. [Google Scholar] [CrossRef]
- Gray, C.A.; Bartol, F.F.; Tarleton, B.J.; Wiley, A.A.; Johnson, G.A.; Bazer, F.W.; Spencer, T.E. Developmental biology of uterine glands. Biol. Reprod. 2001, 65, 1311–1323. [Google Scholar] [CrossRef]
- Schoon, H.A.; Schoon, D. The category I mare (Kenney and Doig 1986): Expected foaling rate 80–90%-Fact or fiction? Pferde-heilkunde 2003, 19, 698–701. [Google Scholar] [CrossRef]
- Ebert, A.; Schoon, D.; Schoon, H.A. Age-related endometrial alterations in mares-biopsy findings of the last 20 years. In Leipziger Blaue Hefte, 7th Leipzig Veterinary Congress, 8th International Conference on Equine Reproductive Medicine; Rackwitz, R., Pees, M., Aschenbach, J.R., Gäbel, G., Eds.; Lehmanns Media GmbH: Berlin, Germany, 2014; Volume 2, pp. 230–232. [Google Scholar]
- Ricketts, S.W.; Alonso, S. The effect of age and parity on the development of equine chronic endometrial disease. Equine Vet.-J. 1991, 23, 189–192. [Google Scholar] [CrossRef]
- Hoffmann, C.; Ellenberger, C.; Mattos, R.C.; Aupperle, H.; Dhein, S.; Stief, B.; Schoon, H.-A. The equine endometrosis: New insights into the pathogenesis. Anim. Reprod. Sci. 2009, 111, 261–278. [Google Scholar] [CrossRef]
- Aresu, L.; Benali, S.; Giannuzzi, D.; Mantovani, R.; Castagnaro, M.; Falomo, M.E. The role of inflammation and matrix metallo-proteinases in equine endometriosis. J. Vet. Sci. 2012, 13, 171–177. [Google Scholar] [CrossRef]
- Raila, G. Zur Pathogenese der Endometrose der Stute. Morphologisch-Funktionelle Untersuchungen. Ph.D. Thesis, University of Leipzig, Leipzig, Germany, 2020. [Google Scholar]
- Evans, T.J.; Miller, M.A.; Ganjam, V.K.; Niswender, K.D.; Ellersieck, M.R.; Krause, W.J.; Youngquist, R.S. Morphometric analysis of endometrial periglandular fibrosis in mares. Am. J. Vet. Res. 1998, 59, 1209–1214. [Google Scholar]
- Walter, I.; Handler, J.; Reifinger, M.; Aurich, C. Association of endometriosis in horses with differentiation of periglandular myofibroblasts and changes of extracellular matrix proteins. Reproduction 2001, 121, 581–586. [Google Scholar] [CrossRef]
- Hoffmann, C.; Bazer, F.W.; Klug, J.; Aupperle, H.; Ellenberger, C.; Schoon, H.A. Immunohistochemical and histochemical identifi-cation of proteins and carbohydrates in the equine endometrium expression patterns for mares suffering from endometrosis. Theriogenology 2009, 71, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Guyot, C.; Lepreux, S.; Combe, C.; Doudnikoff, E.; Bioulac-Sage, P.; Balabaud, C.; Desmoulière, A. Hepatic fibrosis and cirrhosis: The (myo)fibroblastic cell subpopulations involved. Int. J. Biochem Cell Biol. 2006, 38, 135–151. [Google Scholar] [CrossRef] [PubMed]
- Masseno, A.P.B. Avaliação da Fibrose Endometrial e dos Miofibroblastos na Endometroses Ativa e Inativa das Éguas. Ph.D. Thesis, Faculdade de Medicina Veterinária e Zootecnia, Universidade Estadual Paulista, Botucatu, Brazil, 2012. [Google Scholar]
- Martinez-Hernandez, A. Repair, regeneration and fibrosis. In Pathology, 3rd ed.; Rubin, E., Faber, J.L., Eds.; Lippincot-Raven: Philadelphia, PA, USA, 1999. [Google Scholar]
- Bochsler, P.N.; Slauson, D.O. Inflammation and repair of tissue. In Mechanisms of Disease: A Textbook of Comparative General Pathology, 3rd ed.; Slauson, D.O., Cooper, B.J., Eds.; Mosby: St. Louis, MO, USA, 2002; pp. 140–245. [Google Scholar]
- Kenney, R.M. Cyclic and pathologic changes of the mare endometrium as detected by biopsy, with a note on early embryonic death. J. Am. Vet. Med. Assoc. 1978, 172, 241–262. [Google Scholar]
- Kenney, R.M.; Doig, P.A. Equine endometrial biopsy. In Current Therapy in Theriogenology 2: Diagnosis, Treatment, and Prevention of Reproductive Diseases in Small and Large Animals, 2nd ed.; Morrow, D.A., Ed.; Saunders WB: Philadelphia, PA, USA, 1986; pp. 723–729. [Google Scholar]
- Flores, J.M.; Rodriguez, A.; Sanchez, J.; Gomez-Cuetara, C.; Ramiro, F. Endometrosis in Mares: Incidence of Histopathological Alterations. Reprod. Dom. Anim. 1995, 30, 61–65. [Google Scholar] [CrossRef]
- Ricketts, S.W.; Barrelet, A. A retrospective review of the histopathological features seen in a series of 4241 endometrial biopsy samples collected from UK Thoroughbred mares over a 25 year period. Pferdeheilkunde 1997, 13, 525–530. [Google Scholar] [CrossRef]
- Katkiewicz, M.; Witkowski, M.; Zajac, S. Endometrial biopsy of mares: Visualization of healthy and diseased structure. Med. Weter 2007, 63, 463–466. [Google Scholar]
- Zajac, S.; Katkiewicz, M.; Witkowski, M.; Boryczko, Z.; Pawlak, M. Endometrosis in mares. Med. Weter 2008, 64, 257–261. [Google Scholar]
- Schlafer, D.H. Equine endometrial biopsy: Enhancement of clinical value by more extensive histopathology and application of new diagnostic techniques? Theriogenology 2007, 68, 413–422. [Google Scholar] [CrossRef]
- Snider, T.A.; Sepoy, C.; Holyoak, G.R. Equine endometrial biopsy reviewed: Observation, interpretation, and application of histopathologic data. Theriogenology 2011, 75, 1567–1581. [Google Scholar] [CrossRef]
- Hanada, M.; Maeda, Y.; Oikawa, M.A. Histopathological characteristics of endometrosis in thoroughbred mares in Japan: Results from 50 necropsy cases. J. Equine Sci. 2014, 25, 45–52. [Google Scholar] [CrossRef]
- Luo, Y.; Oseini, A.; Gagnon, R.; Charles, E.; Sidik, K.; Vincent, R.; Collen, R.; Idowu, M.; Contos, M.; Mirshahi, F.; et al. An evaluation of the collagen fragments related to fibrogenesis and fibrolysis in nonalcoholic steatohepatitis. Sci. Rep. 2018, 8, 12414. [Google Scholar] [CrossRef] [PubMed]
- Frisbie, D.D.; Ray, C.S.; Ionescu, M.; Poole, A.R.; Chapman, P.L.; McIlwraith, C.W. Measurement of synovial fluid and serum concentrations of the 846 epitope of chondroitin sulfate and of carboxy propeptides of type II procollagen for diagnosis of os-teochondral fragmentation in horses. Am. J. Vet. Res. 1999, 60, 306–309. [Google Scholar] [PubMed]
- Laverty, S.; Ionescu, M.; Marcoux, M.; Boure, L.; Doize, B.; Poole, A.R. Alterations in cartilage type-II procollagen and aggrecan contents in synovial fluid in equine osteochondrosis. J. Orthop. Res. 2000, 18, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Billinghurst, R.C.; Brama, P.A.J.; van Weeren, P.R.; McIlwraith, C.W. Evaluation of serum concentrations of biomarkers of skeletal metabolism and results of radiography as indicators of severity of osteochondrosis in foals. Am. J. Vet. Res. 2004, 65, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Frisbie, D.D.; Al-Sobayil, F.; Billinghurst, R.C.; Kawcak, C.E.; McIlwraith, C.W. Changes in synovial fluid and serum biomarkers with exercise and early osteoarthritis in horses. Osteoarthr. Cartil. 2008, 16, 1196–1204. [Google Scholar] [CrossRef]
- Gressner, O.A.; Weiskirchen, R.; Gressner, A.M. Biomarkers of hepatic fibrosis, fibrogenesis and genetic pre-disposition pending between fiction and reality. J. Cell Mol. Med. 2007, 11, 1031–1051. [Google Scholar] [CrossRef]
- Karsdal, M.A.; Daniels, S.J.; Holm Nielsen, S.; Bager, C.; Rasmussen, D.G.K.; Loomba, R.; Surabattula, R.; Villesen, I.F.; Luo, Y.; Shevell, D.; et al. Collagen biology and non-invasive biomarkers of liver fibrosis. Liver Int. 2020, 40, 736–750. [Google Scholar] [CrossRef]
- Bruckner, P. Suprastructures of extracellular matrices: Paradigms of functions controlled by aggregates rather than molecules. Cell Tissue Res. 2010, 339, 7–18. [Google Scholar] [CrossRef]
- Karsdal, M.A.; Woodworth, T.; Henriksen, K.; Maksymowych, W.P.; Genant, H.; Vergnaud, P.; Christiansen, C.; Schubert, T.; Qvist, P.; Schett, G.; et al. Biochemical markers of ongoing joint damage in rheumatoid arthritis--current and future applications, limitations and opportunities. Arthritis Res. Ther. 2011, 13, 215. [Google Scholar] [CrossRef][Green Version]
- Neto da Silva, A.C.; Costa, A.L.; Teixeira, A.; Alpoim-Moreira, J.; Fernandes, C.; Fradinho, M.J.; Rebordão, M.R.; Silva, E.; Ferreira da Silva, J.; Bliebernicht, M.; et al. Collagen and Microvascularization in Placentas From Young and Older Mares. Front. Vet. Sci. 2022, 8, 772658. [Google Scholar] [CrossRef]
- Rosenberg, W.M.; Voelker, M.; Thiel, R.; Becka, M.; Burt, A.; Schuppan, D.; Hubscher, S.; Roskams, T.; Pinzani, M.; Arthur, M.J. European Liver Fibrosis Group. Serum markers detect the presence of liver fibrosis: A cohort study. Gastroenterology 2004, 127, 1704–1713. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, S.; Trembling, P.M.; Hogan, B.J.; Srivastava, A.; Parkes, J.; Harris, S.; Grant, P.; Nastouli, E.; Ocker, M.; Wehr, K.; et al. Noninvasive markers of liver fibrosis: On-treatment changes of serum markers predict the outcome of antifibrotic therapy. Eur. J. Gastroenterol. Hepatol. 2017, 29, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Metschl, S.; Reutersberg, B.; Maegdefessel, L.; Eckstein, H.; Pelisek, J. Collagen Type I and III in Serum of Patients with Abdominal Aortic Aneurysm: Potential Biomarker of Risk Stratification? Arterioscler. Thromb. Vasc. Biol. 2019, 39, A255. [Google Scholar]
- Ong, K.L.; Chung, R.W.S.; Hui, N.; Festin, K.; Lundberg, A.K.; Rye, K.A.; Jonasson, L.; Kristenson, M. Usefulness of Certain Protein Biomarkers for Prediction of Coronary Heart Disease. Am. J. Cardiol. 2020, 125, 542–548. [Google Scholar] [CrossRef]
- Zannad, F.; Rossignol, P.; Iraqi, W. Extracellular matrix fibrotic markers in heart failure. Heart Fail. Rev. 2010, 15, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Ix, J.H.; Biggs, M.L.; Mukamal, K.; Djousse, L.; Siscovick, D.; Tracy, R.; Katz, R.; Delaney, J.A.; Chaves, P.; Rifkin, D.E.; et al. Urine Collagen Fragments and CKD Progres-sion-The Cardiovascular Health Study. J. Am. Soc. Nephrol. 2015, 26, 2494–2503. [Google Scholar] [CrossRef] [PubMed]
- Soylemezoglu, O.; Wild, G.; Dalley, A.J.; MacNeil, S.; Milford-Ward, A.; Brown, C.B.; el Nahas, A.M. Urinary and serum type III collagen: Markers of renal fibrosis. Nephrol. Dial. Transplant. 1997, 12, 1883–1889. [Google Scholar] [CrossRef]
- Lunelli, D.; Cirio, S.M.; Leite, S.C.; Camargo, C.E.; Kozicki, L.E. Collagen types in relation to expression of estradiol and pro-gesterone receptors in equine endometrial fibrosis. Adv. Biosci. Biotechnol. 2013, 4, 599–605. [Google Scholar] [CrossRef]
- Liu, X.; Wu, H.; Byrne, M.; Krane, S.; Jaenisch, R. Type III collagen is crucial for collagen I fibrillogenesis and for normal cardi-ovascular development. Proc. Natl. Acad. Sci. USA 1997, 94, 1852–1856. [Google Scholar] [CrossRef]
- Kuivaniemi, H.; Tromp, G. Type III collagen (COL3A1): Gene and protein structure, tissue distribution, and associated diseases. Gene 2019, 707, 151–171. [Google Scholar] [CrossRef]
- Miller, E.J.; Gay, S. The collagens: An overview and update. Methods Enzymol. 1987, 144, 3–41. [Google Scholar] [CrossRef] [PubMed]
- Badid, C.; Vincent, M.; McGregor, B.; Melin, M.; Hadj-Aissa, A.; Veysseyre, C.; Hartmann, D.J.; Desmouliere, A.; Laville, M. My-cophenolate mofetil reduces myofibroblast infiltration and collagen III deposition in rat remnant kidney. Kidney Int. 2000, 58, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.J.; Veidal, S.S.; Karsdal, M.A.; Ørsnes-Leeming, D.J.; Vainer, B.; Gardner, S.D.; Hamatake, R.; Goodman, Z.D.; Schuppan, D.; Patel, K. Plasma Pro-C3 (N-terminal type III collagen propeptide) predicts fibrosis progression in patients with chronic hepatitis C. Liver Int. 2015, 35, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Leeming, D.J.; Dolman, G.; Nielsen, M.J.; Karsdal, M.A.; Patel, K.; Irving, W.; Guha, I.N. True collagen type III formation (Pro-C3) is predictive of outcome in HCV patients with advanced liver fibrosis with in the trent study. J. Hepatol. 2016, 64, S719–S720. [Google Scholar] [CrossRef]
- Gressner, O.; Weiskirchen, R.; Gressner, A. Biomarkers of liver fibrosis: Clinical translation of molecular pathogenesis or based on liver-dependent malfunction tests. Clin. Chim. Acta 2007, 381, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, E.M.; Ginther, O.J. Relationship of age to uterine function and reproductive efficiency in mares. Theriogenology 1992, 37, 1101–1115. [Google Scholar] [CrossRef]
- Rebordão, M.R.; Amaral, A.; Lukasik, K.; Szóstek-Mioduchowska, A.; Pinto-Bravo, P.; Galvão, A.; Skarzynski, D.J.; Ferreira-Dias, G. Impairment of the antifibrotic prostaglandin E2 pathway may influence neutrophil extracellular traps-induced fibrosis in the mare endometrium. Domest. Anim. Endocrinol. 2019, 67, 1–10. [Google Scholar] [CrossRef]
- Pinto-Bravo, P.; Rebordão, M.R.; Amaral, A.; Fernandes, C.; Cuello, C.; Parrilla, I.; Martínez, E.; Roberto da Costa, R.P.; Skarzynski, D.J.; Ferreira-Dias, G. Is mare endometrosis linked to oviduct fibrosis? Pferdeheilkunde Equine Med. 2018, 34, 43–46. [Google Scholar] [CrossRef]
- Price, J.S.; Jackson, B.; Eastell, R.; Goodship, A.E.; Blumsohn, A.; Wright, I.; Stoneham, S.; Lanyon, L.E.; Russell, R.G. Age related changes in biochemical markers of bone metabolism in horses. Equine Vet. J. 1995, 27, 201–207. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alpoim-Moreira, J.; Fernandes, C.; Rebordão, M.R.; Costa, A.L.; Bliebernicht, M.; Nunes, T.; Szóstek-Mioduchowska, A.; Skarzynski, D.J.; Ferreira-Dias, G. Collagen Type III as a Possible Blood Biomarker of Fibrosis in Equine Endometrium. Animals 2022, 12, 1854. https://doi.org/10.3390/ani12141854
Alpoim-Moreira J, Fernandes C, Rebordão MR, Costa AL, Bliebernicht M, Nunes T, Szóstek-Mioduchowska A, Skarzynski DJ, Ferreira-Dias G. Collagen Type III as a Possible Blood Biomarker of Fibrosis in Equine Endometrium. Animals. 2022; 12(14):1854. https://doi.org/10.3390/ani12141854
Chicago/Turabian StyleAlpoim-Moreira, Joana, Carina Fernandes, Maria Rosa Rebordão, Ana Luísa Costa, Miguel Bliebernicht, Telmo Nunes, Anna Szóstek-Mioduchowska, Dariusz J. Skarzynski, and Graça Ferreira-Dias. 2022. "Collagen Type III as a Possible Blood Biomarker of Fibrosis in Equine Endometrium" Animals 12, no. 14: 1854. https://doi.org/10.3390/ani12141854
APA StyleAlpoim-Moreira, J., Fernandes, C., Rebordão, M. R., Costa, A. L., Bliebernicht, M., Nunes, T., Szóstek-Mioduchowska, A., Skarzynski, D. J., & Ferreira-Dias, G. (2022). Collagen Type III as a Possible Blood Biomarker of Fibrosis in Equine Endometrium. Animals, 12(14), 1854. https://doi.org/10.3390/ani12141854








