Hoxa5 Inhibits the Proliferation and Induces Adipogenic Differentiation of Subcutaneous Preadipocytes in Goats
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Cell Culture
2.2. Vectors Construction, Chemical Synthesis of siRNA, and Transfection
2.3. Induced Differentiation of Goat Subcutaneous Preadipocytes
2.4. Oil Red O Staining and Bodipy Staining
2.5. MTT Analysis
2.6. EdU Staining
2.7. RNA Isolation and Real-Time Quantitative PCR (qRT-PCR) Analysis
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
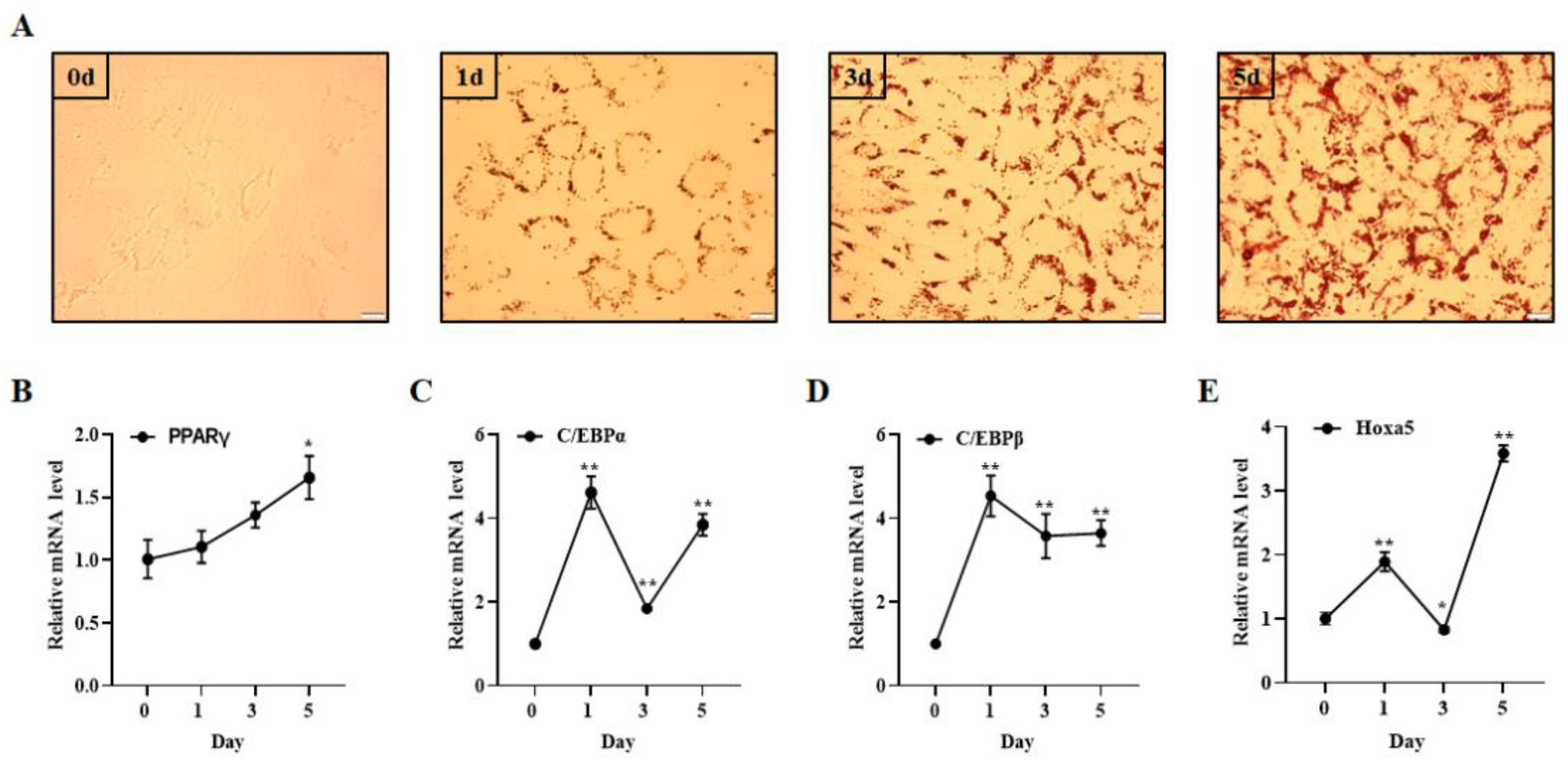
3.1. Hoxa5 Expression Changed during Goat Subcutaneous Preadipocytes Differentiation
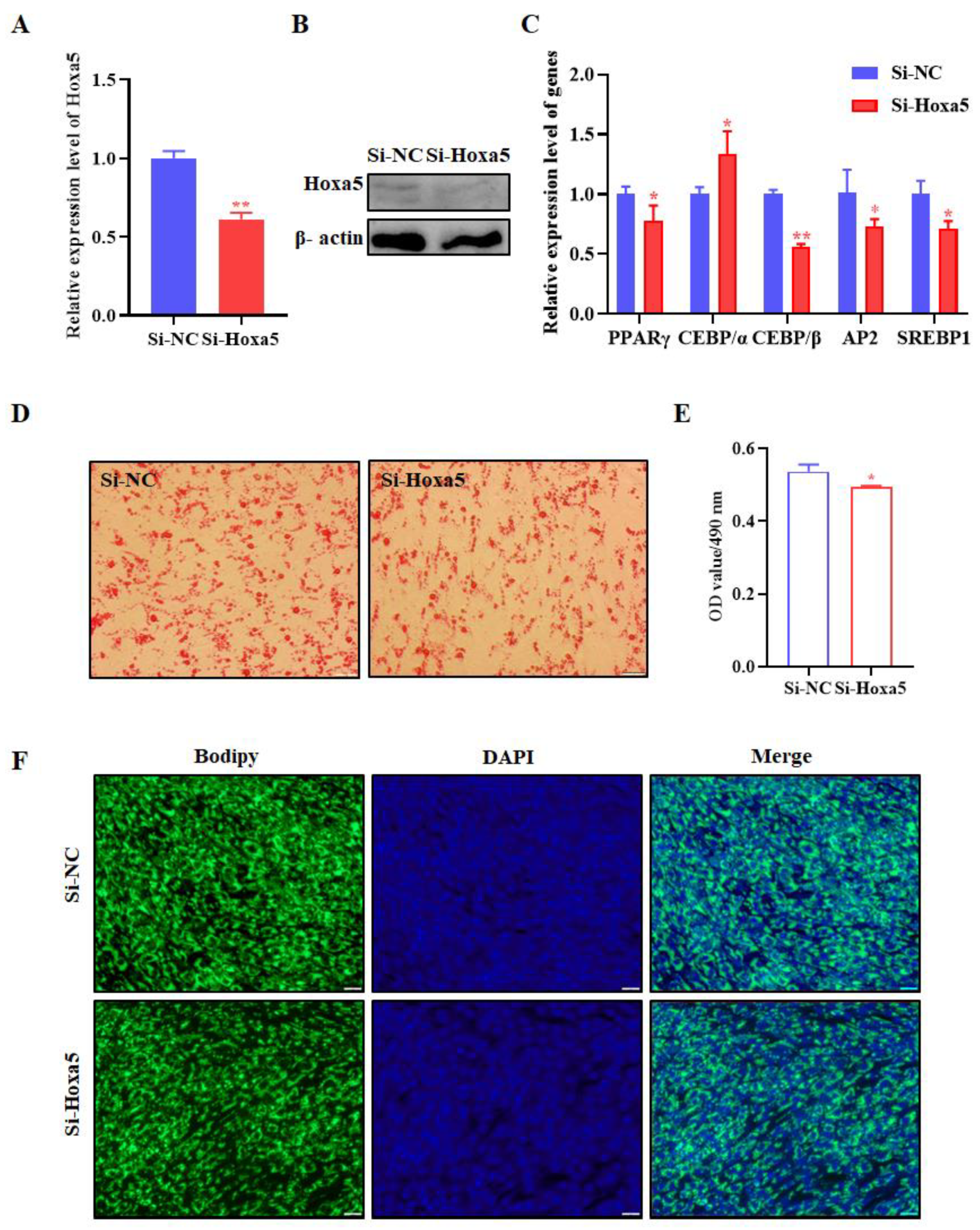
3.2. Hoxa5 Promotes Adipogenic Differentiation of Goat Subcutaneous Preadipocytes
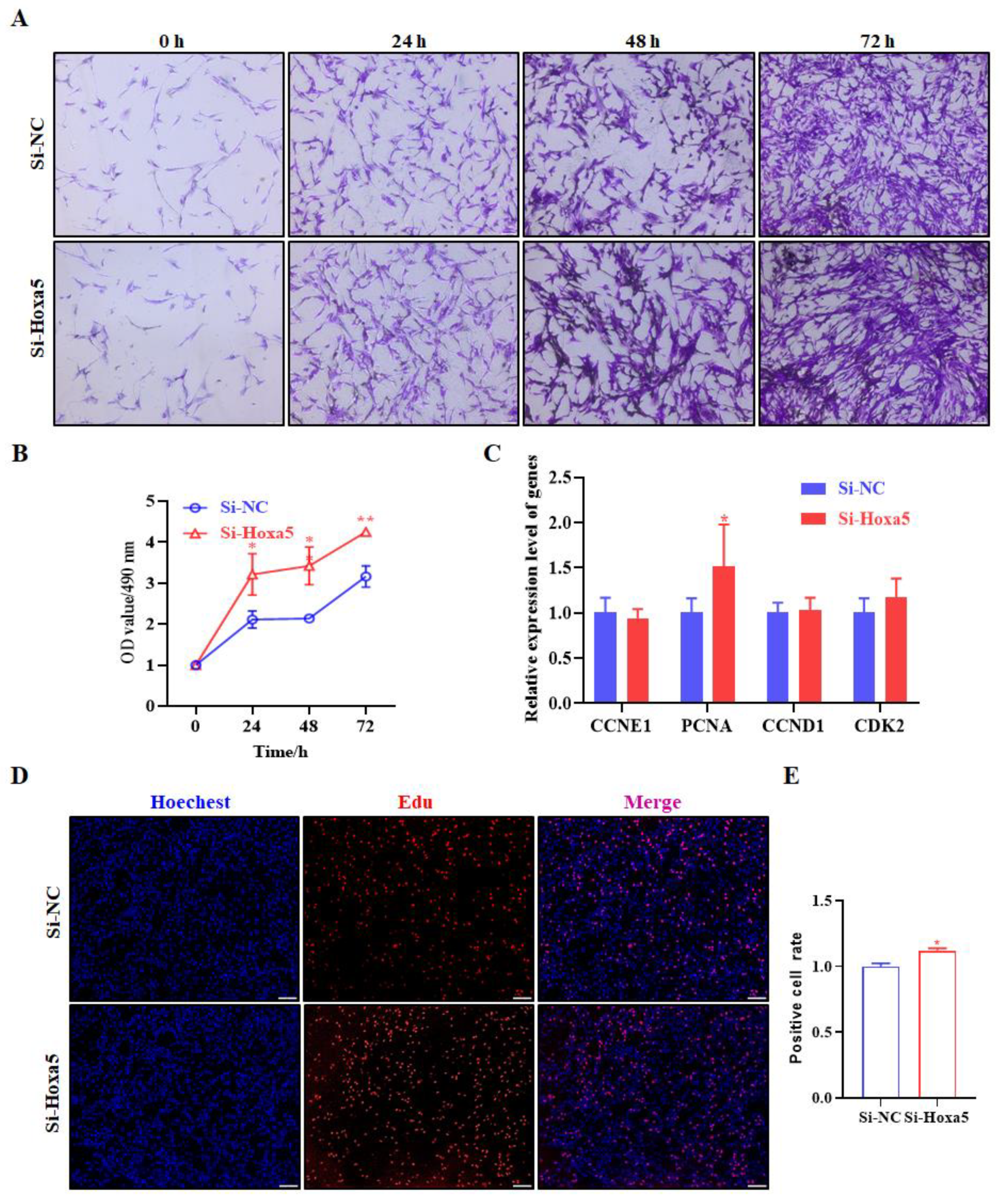
3.3. Hoxa5 Suppresses the Proliferation of Goat Subcutaneous Preadipocytes
3.4. Hoxa5 Affects Subcutaneous Preadipocytes Proliferation and Differentiation Possibly Targets the Promoter of PPARγ, SREBP1, and PCNA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ottaviani, E.; Malagoli, D.; Franceschi, C. The evolution of the adipose tissue: A neglected enigma. Gen. Comp Endocrinol. 2011, 174, 1–4. [Google Scholar] [PubMed]
- Coelho, M.; Oliveira, T.; Fernandes, R. Biochemistry of adipose tissue: An endocrine organ. Arch. Med. Sci. 2013, 9, 191–200. [Google Scholar] [PubMed] [Green Version]
- Halberg, N.; Wernstedt-Asterholm, I.; Scherer, P.E. The Adipocyte as an Endocrine Cell. Endocrinol. Metabol. Clin. North Am. 2008, 37, 753–768. [Google Scholar]
- Becher, T.; Palanisamy, S.; Kramer, D.J.; Eljalby, M.; Marx, S.J.; Wibmer, A.G.; Butler, S.D.; Jiang, C.S.; Vaughan, R.; Schoder, H.; et al. Brown adipose tissue is associated with cardiometabolic health. Nat. Med. 2021, 27, 58–65. [Google Scholar]
- Neeland, I.J.; Turer, A.T.; Ayers, C.R.; Powell-Wiley, T.M.; Vega, G.L.; Farzaneh-Far, R.; Grundy, S.M.; Khera, A.; McGuire, D.K.; de Lemos, J.A. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA 2012, 308, 1150–1159. [Google Scholar]
- Bloomgarden, Z.T. Approaches to treatment of pre-diabetes and obesity and promising new approaches to type 2 diabetes. Diabetes Care 2008, 31, 1461–1466. [Google Scholar]
- Rodriguez, A.; Ezquerro, S.; Mendez-Gimenez, L.; Becerril, S.; Fruhbeck, G. Revisiting the adipocyte: A model for integration of cytokine signaling in the regulation of energy metabolism. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E691–E714. [Google Scholar]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010, 11, 11–18. [Google Scholar]
- Roush, G.C. Obesity-Induced Hypertension: Heavy on the Accelerator. J. Am. Heart Assoc. 2019, 8, e012334. [Google Scholar]
- Landsberg, L. Obesity, metabolism, and hypertension. Yale J. Biol. Med. 1989, 62, 511–519. [Google Scholar]
- Liu, R.; Liu, X.; Bai, X.; Xiao, C.; Dong, Y. A Study of the Regulatory Mechanism of the CB1/PPARgamma2/PLIN1/HSL Pathway for Fat Metabolism in Cattle. Front. Genet. 2021, 12, 631187. [Google Scholar] [PubMed]
- Anderson, F.; Pannier, L.; Pethick, D.W.; Gardner, G.E. Intramuscular fat in lamb muscle and the impact of selection for improved carcass lean meat yield. Animal 2015, 9, 1081–1090. [Google Scholar] [PubMed] [Green Version]
- Wang, Y.; Zhang, Y.; Su, X.; Wang, H.; Yang, W.; Zan, L. Cooperative and Independent Functions of the miR-23a~27a~24-2 Cluster in Bovine Adipocyte Adipogenesis. Int. J. Mol. Sci. 2018, 19, 3957. [Google Scholar]
- Lambert, S.A.; Jolma, A.; Campitelli, L.F.; Das, P.K.; Yin, Y.; Albu, M.; Chen, X.; Taipale, J.; Hughes, T.R.; Weirauch, M.T. The Human Transcription Factors. Cell 2018, 172, 650–665. [Google Scholar] [PubMed] [Green Version]
- Spitz, F.; Furlong, E.E. Transcription factors: From enhancer binding to developmental control. Nat. Rev. Genet. 2012, 13, 613–626. [Google Scholar] [PubMed]
- Rosen, E.D.; MacDougald, O.A. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006, 7, 885–896. [Google Scholar] [PubMed]
- Ali, A.T.; Hochfeld, W.E.; Myburgh, R.; Pepper, M.S. Adipocyte and adipogenesis. Eur. J. Cell Biol. 2013, 92, 229–236. [Google Scholar]
- de Sa, P.M.; Richard, A.J.; Hang, H.; Stephens, J.M. Transcriptional Regulation of Adipogenesis. Compr. Physiol. 2017, 7, 635–674. [Google Scholar]
- Desvergne, B.; Wahli, W. Peroxisome proliferator-activated receptors: Nuclear control of metabolism. Endocr. Rev. 1999, 20, 649–688. [Google Scholar]
- He, Z.; Liu, H.; Agostini, M.; Yousefi, S.; Perren, A.; Tschan, M.P.; Mak, T.W.; Melino, G.; Simon, H.U. p73 regulates autophagy and hepatocellular lipid metabolism through a transcriptional activation of the ATG5 gene. Cell Death Differ. 2013, 20, 1415–1424. [Google Scholar]
- Jeannotte, L.; Lemieux, M.; Charron, J.; Poirier, F.; Robertson, E.J. Specification of axial identity in the mouse: Role of the Hoxa-5 (Hox1.3) gene. Genes Dev. 1993, 7, 2085–2096. [Google Scholar] [PubMed] [Green Version]
- Jeannotte, L.; Gotti, F.; Landry-Truchon, K. Hoxa5: A Key Player in Development and Disease. J. Dev. Biol. 2016, 4, 13. [Google Scholar]
- Zhu, Q.; Lv, T.; Wu, Y.; Shi, X.; Liu, H.; Song, Y. Long non-coding RNA 00312 regulated by HOXA5 inhibits tumour proliferation and promotes apoptosis in Non-small cell lung cancer. J. Cell Mol. Med. 2017, 21, 2184–2198. [Google Scholar]
- Ordonez-Moran, P.; Dafflon, C.; Imajo, M.; Nishida, E.; Huelsken, J. HOXA5 Counteracts Stem Cell Traits by Inhibiting Wnt Signaling in Colorectal Cancer. Cancer Cell 2015, 28, 815–829. [Google Scholar]
- Holzman, M.A.; Ryckman, A.; Finkelstein, T.M.; Landry-Truchon, K.; Schindler, K.A.; Bergmann, J.M.; Jeannotte, L.; Mansfield, J.H. HOXA5 Participates in Brown Adipose Tissue and Epaxial Skeletal Muscle Patterning and in Brown Adipocyte Differentiation. Front. Cell Dev. Biol. 2021, 9, 632303. [Google Scholar] [PubMed]
- Pan, M.; Sun, Q.; Li, C.; Tai, R.; Shi, X.; Sun, C. HOXA5 inhibits adipocytes proliferation through transcriptional regulation of Ccne1 and blocking JAK2/STAT3 signaling pathway in mice. Biochem. Cell Biol. 2022. [Google Scholar] [CrossRef]
- Parrillo, L.; Spinelli, R.; Costanzo, M.; Florese, P.; Cabaro, S.; Desiderio, A.; Prevenzano, I.; Raciti, G.A.; Smith, U.; Miele, C.; et al. Epigenetic Dysregulation of the Homeobox A5 (HOXA5) Gene Associates with Subcutaneous Adipocyte Hypertrophy in Human Obesity. Cells 2022, 11, 728. [Google Scholar]
- Parrillo, L.; Costa, V.; Raciti, G.A.; Longo, M.; Spinelli, R.; Esposito, R.; Nigro, C.; Vastolo, V.; Desiderio, A.; Zatterale, F.; et al. Hoxa5 undergoes dynamic DNA methylation and transcriptional repression in the adipose tissue of mice exposed to high-fat diet. Int. J. Obes. 2016, 40, 929–937. [Google Scholar]
- Cao, W.; Xu, Y.; Luo, D.; Saeed, M.; Sun, C. Hoxa5 Promotes Adipose Differentiation via Increasing DNA Methylation Level and Inhibiting PKA/HSL Signal Pathway in Mice. Cell Physiol. Biochem. 2018, 45, 1023–1033. [Google Scholar]
- Feng, F.; Ren, Q.; Wu, S.; Saeed, M.; Sun, C. Hoxa5 increases mitochondrial apoptosis by inhibiting Akt/mTORC1/S6K1 pathway in mice white adipocytes. Oncotarget 2017, 8, 95332–95345. [Google Scholar]
- Cao, W.; Zhang, T.; Feng, R.; Xia, T.; Huang, H.; Liu, C.; Sun, C. Hoxa5 alleviates obesity-induced chronic inflammation by reducing ER stress and promoting M2 macrophage polarization in mouse adipose tissue. J. Cell Mol. Med. 2019, 23, 7029–7042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Zhang, H.; Wang, Y.; Li, Y.; He, C.; Zhu, J.; Xiong, Y.; Lin, Y. RNA-seq analysis reveals the positive role of KLF5 in the differentiation of subcutaneous adipocyte in goats. Gene 2022, 808, 145969. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Li, Y.; Lin, S.; Wang, Y.; Zhu, J.; Lin, Y. KLF4 Inhibits the Differentiation of Goat Intramuscular Preadipocytes Through Targeting C/EBPbeta Directly. Front. Genet 2021, 12, 663759. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Tontonoz, P.; Hu, E.; Spiegelman, B.M. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell 1994, 79, 1147–1156. [Google Scholar] [CrossRef]
- Asghar, U.; Witkiewicz, A.K.; Turner, N.C.; Knudsen, E.S. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat. Rev. Drug Discov. 2015, 14, 130–146. [Google Scholar] [CrossRef] [Green Version]
- Kubben, F.J.; Peeters-Haesevoets, A.; Engels, L.G.; Baeten, C.G.; Schutte, B.; Arends, J.W.; Stockbrugger, R.W.; Blijham, G.H. Proliferating cell nuclear antigen (PCNA): A new marker to study human colonic cell proliferation. Gut 1994, 35, 530–535. [Google Scholar] [CrossRef] [Green Version]
- Zschemisch, N.H.; Liedtke, C.; Dierssen, U.; Nevzorova, Y.A.; Wustefeld, T.; Borlak, J.; Manns, M.P.; Trautwein, C. Expression of a cyclin E1 isoform in mice is correlated with the quiescent cell cycle status of hepatocytes in vivo. Hepatology 2006, 44, 164–173. [Google Scholar] [CrossRef]
- Qie, S.; Diehl, J.A. Cyclin D1, cancer progression, and opportunities in cancer treatment. J. Mol. Med. 2016, 94, 1313–1326. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Yu, C.; Wang, H. HOXA5 inhibits the proliferation and induces the apoptosis of cervical cancer cells via regulation of protein kinase B and p27. Oncol. Rep. 2019, 41, 1122–1130. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.M.; Cui, N.; Zheng, P.S. HOXA5 inhibits the proliferation and neoplasia of cervical cancer cells via downregulating the activity of the Wnt/beta-catenin pathway and transactivating TP53. Cell Death Dis. 2020, 11, 420. [Google Scholar] [PubMed]
- Zhang, H.; Zhao, J.H.; Suo, Z.M. Knockdown of HOXA5 inhibits the tumorigenesis in esophageal squamous cell cancer. Biomed. Pharm. 2017, 86, 149–154. [Google Scholar]
- Rosen, E.D.; Spiegelman, B.M. Molecular regulation of adipogenesis. Annu. Rev. Cell Dev. Biol. 2000, 16, 145–171. [Google Scholar] [PubMed]
- Rosen, E.D.; Hsu, C.H.; Wang, X.; Sakai, S.; Freeman, M.W.; Gonzalez, F.J.; Spiegelman, B.M. C/EBPalpha induces adipogenesis through PPARgamma: A unified pathway. Genes Dev. 2002, 16, 22–26. [Google Scholar]
- Wu, Z.; Rosen, E.D.; Brun, R.; Hauser, S.; Adelmant, G.; Troy, A.E.; McKeon, C.; Darlington, G.J.; Spiegelman, B.M. Cross-regulation of C/EBP alpha and PPAR gamma controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol. Cell 1999, 3, 151–158. [Google Scholar]
- Kim, S.W.; Xie, Y.; Nguyen, P.Q.; Bui, V.T.; Huynh, K.; Kang, J.S.; Brown, D.J.; Jester, J.V. PPARgamma regulates meibocyte differentiation and lipid synthesis of cultured human meibomian gland epithelial cells (hMGEC). Ocul. Surf. 2018, 16, 463–469. [Google Scholar]
- Liang, Y.; Zhou, R.; Fu, X.; Wang, C.; Wang, D. HOXA5 counteracts the function of pathological scar-derived fibroblasts by partially activating p53 signaling. Cell Death Dis. 2021, 12, 40. [Google Scholar]



| Gene Name | Forward Sequence (5′–3′) | Reverse Sequence (5′–3′) |
|---|---|---|
| Hoxa5 | CTCATTTTGCGGTCGCTATC | ACGCTGAGATCCATGCCATT |
| C/EBPα | CTCCGGATCTCAAGACTGCC | CCCCTCATCTTAGACGCACC |
| C/EBPβ | CCGCCTTTAAATCCATGGAA | CTCGTGCTCTCCGATGCTAC |
| PPARγ | AAGCGTCAGGGTTCCACTATG | GAACCTGATGGCGTTATGAGAC |
| AP2 | GTCCTTCAAATGGGCCAGGA | CTGGTGGTAGTGACACCGTT |
| SREBP1 | AACATCTGTTGGAGCGAGCA | TCCAGCCATATCCGAACAGC |
| CCNE1 | CTCCCTGATTCCCACACCTG | CATAAGATGCTTGTCCCTCA |
| PCNA | AGTGGAGAACTTGGAAAGGAA | GAGACAGTGGAGTGGCTTTTGT |
| CCND1 | TGAACTACCTGGACCGCT | CAGGTTCCACTTGAGTTTGT |
| CDK2 | GCCAGGAGTTACTTCTATGC | TGGAAGAAAGGGTGAGCC |
| UXT | GCAAGTGGATTTGGGCTGTAAC | ATGGAGTCCTTGGTGAGGTTGT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Lin, Y.; Zhao, N.; Wang, Y.; Li, Y. Hoxa5 Inhibits the Proliferation and Induces Adipogenic Differentiation of Subcutaneous Preadipocytes in Goats. Animals 2022, 12, 1859. https://doi.org/10.3390/ani12141859
Chen D, Lin Y, Zhao N, Wang Y, Li Y. Hoxa5 Inhibits the Proliferation and Induces Adipogenic Differentiation of Subcutaneous Preadipocytes in Goats. Animals. 2022; 12(14):1859. https://doi.org/10.3390/ani12141859
Chicago/Turabian StyleChen, Dingshuang, Yaqiu Lin, Nan Zhao, Yong Wang, and Yanyan Li. 2022. "Hoxa5 Inhibits the Proliferation and Induces Adipogenic Differentiation of Subcutaneous Preadipocytes in Goats" Animals 12, no. 14: 1859. https://doi.org/10.3390/ani12141859
APA StyleChen, D., Lin, Y., Zhao, N., Wang, Y., & Li, Y. (2022). Hoxa5 Inhibits the Proliferation and Induces Adipogenic Differentiation of Subcutaneous Preadipocytes in Goats. Animals, 12(14), 1859. https://doi.org/10.3390/ani12141859





