Determination of the Metabolites and Metabolic Pathways for Three β-Receptor Agonists in Rats Based on LC-MS/MS
Abstract
:Simple Summary
Abstract
1. Introduction
2. Experimental
2.1. Reagents and Materials
2.2. Animals and Sample Collection
2.3. Analysis of Salbutamol, Ractopamine, and Clenbuterol
2.3.1. Sample Extraction and Pretreatment
2.3.2. High Performance Liquid Chromatography-Tandem Mass Spectrometry Analysis
2.4. Method Validation
3. Results
3.1. Methodological Evaluation
3.2. Mass Spectrometric Analysis of Three β-Agonist Standard Products
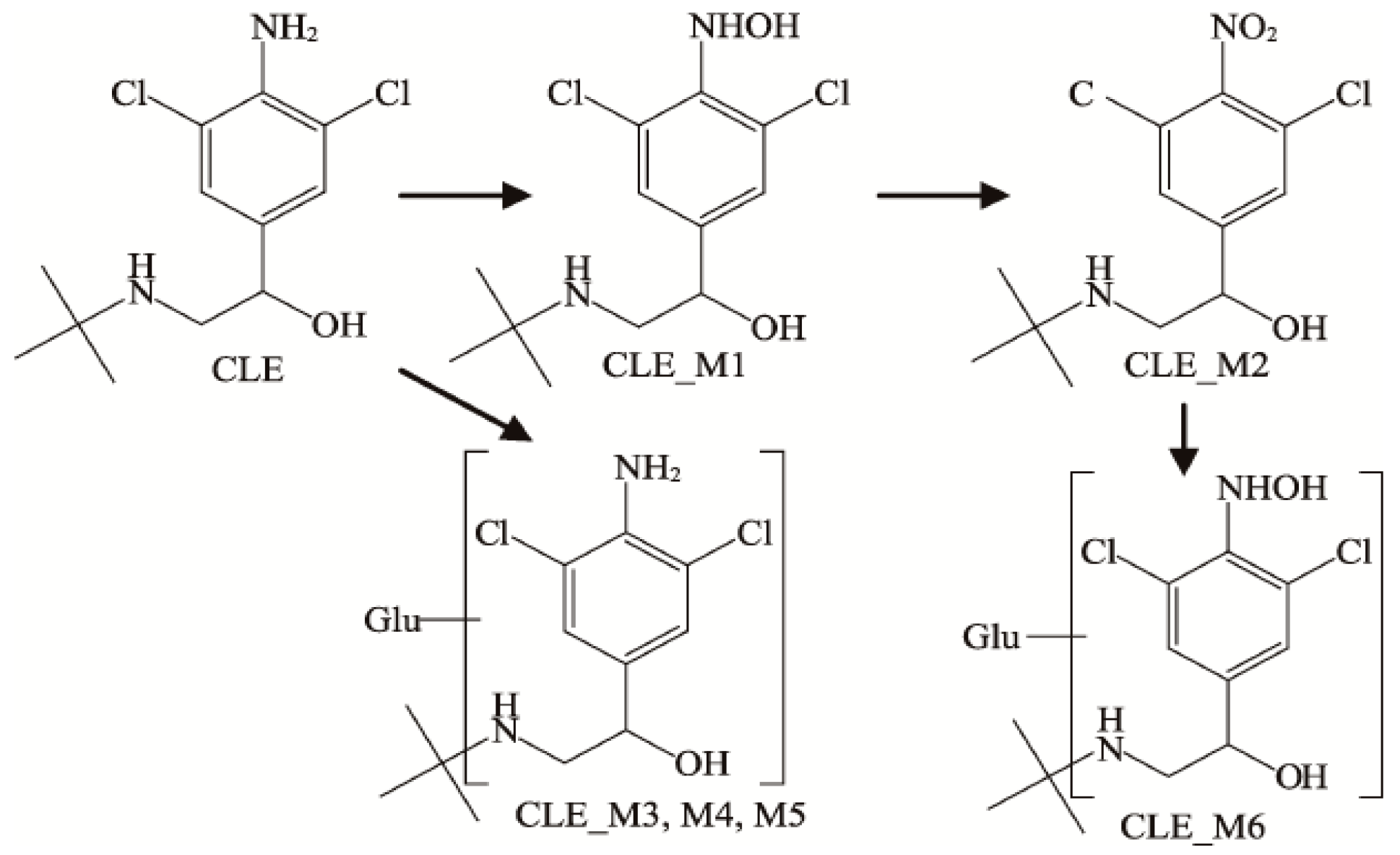
3.3. Identification of Three Metabolites for β-Receptor Agonists
3.4. Metabolism of Three Receptor Agonists In Vivo
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abramson, M.J.; Walters, J.; Walters, E.H. Adverse effects of -agonists: Are they clinically relevant? Am. J. Respir. Med. Drugs Devices Other Interv. 2003, 2, 287–297. [Google Scholar] [CrossRef]
- Bjermer, L.; Abbott-Banner, K.; Newman, K. Efficacy and safety of a first-in-class inhaled PDE3/4 inhibitor (ensifentrine) vs. salbutamol in asthma. Pulm. Pharmacol. Ther. 2019, 58, 101814. [Google Scholar] [CrossRef]
- Keränen, T.; Hömmö, T.; Moilanen, E.; Korhonen, R. β2-receptor agonists salbutamol and terbutaline attenuated cytokine production by suppressing ERK pathway through cAMP in macrophages. Cytokine 2017, 94, 1–7. [Google Scholar] [CrossRef]
- Ferreira, A.z.S.; Silva, E.P.D. Ractopamine for pigs: A review about nutritional requirements. J. Basic Appl. Ences. 2013, 9, 276. [Google Scholar] [CrossRef] [Green Version]
- Shishani, E.; Chai, S.C.; Jamokha, S.; Aznar, G.; Hoffman, M.K. Determination of ractopamine in animal tissues by liquid chromatography-fluorescence and liquid chromatography/tandem mass spectrometry. Anal. Chim. Acta. 2003, 483, 137–145. [Google Scholar] [CrossRef]
- Lehmann, S.; Thomas, A.; Schiwy-Bochat, K.H.; Geyer, H.; Thevis, M.; Glenewinkel, F.; Rothschild, M.A.; Andresen-Streichert, H.; Juebner, M. Death after misuse of anabolic substances (clenbuterol, stanozolol and metandienone). Forensic Sci. Int. 2019, 303, 109925. [Google Scholar] [CrossRef]
- Niño, A.M.; Granja, R.H.; Wanschel, A.C.; Salerno, A.G. The challenges of ractopamine use in meat production for export to European Union and Russia. Food Control 2017, 72, 289–292. [Google Scholar] [CrossRef]
- Biolatti, B.; Bollo, E.; Re, G.; Appino, S.; Tartari, E.; Benatti GElliott, C.T.; McCaughey, W.J. Pathology and residues in veal calves treated experimentally with clenbuterol. Res. Vet. Sci. 1994, 57, 365–371. [Google Scholar] [CrossRef]
- Huckins, D.S.; Lemons, M.F. Myocardial ischemia associated with clenbuterol abuse: Reportoftwo cases. J. Emerg. Med. 2013, 44, 444–449. [Google Scholar] [CrossRef]
- Yang, X.; Feng, B.; Yang, P.; Ding, Y.; Chen, Y.; Fei, J. Electrochemical determination of toxic ractopamine at an ordered mesoporous carbon modified electrode. Food Chem. 2014, 145, 619–624. [Google Scholar] [CrossRef]
- Zou, Y.; Wu, H.; Guo, X.; Peng, L.; Ding, Y.; Tang, J.; Guo, F. MK-FSVM-SVDD: A multiple Kernel-based fuzzy SVM model for predicting DNA-binding proteins via support vector data description. Curr. Bioinform. 2021, 16, 274–283. [Google Scholar]
- Zhang, L.; Buatois, L.A.; Mángano, M.G. Potential and problems in evaluating secular changes in the diversity of animal-substrate interactions at ichnospecies rank. Terra Nova 2022. [Google Scholar] [CrossRef]
- Reshef, R.; Aderka, D.; Suprun, H.; Manelis, G.; Manelis, J. Myocardial infarction associated with the Riley-Day syndrome. Am. Heart J. 1977, 94, 486–490. [Google Scholar] [CrossRef]
- Li, H.; Wang, F. Core-shell chitosan microsphere with antimicrobial and vascularized functions for promoting skin wound healing. Mater. Des. 2021, 204, 109683. [Google Scholar] [CrossRef]
- Pleadin, J.; Perši, N.; Vulić, A.; Milić, D.; Vahčić, N. Determination of residual ractopamine concentrations by enzyme immunoassay in treated pig’s tissues on days after withdrawal. Meat Sci. 2012, 90, 755–758. [Google Scholar] [CrossRef]
- Ji, X.; He, Z.; Ai, X.; Yang, H.; Xu, C. Determination of clenbuterol by capillary electrophoresis immunoassay with chemiluminescence detection. Talanta 2006, 70, 353–357. [Google Scholar] [CrossRef]
- He, L.; Su, Y.; Zeng, Z.; Liu, Y.; Huang, X. Determination of ractopamine and clenbuterol in feeds by gas chromatography–mass spectrometry. Anim. Feed. Sci. Technol. 2007, 132, 316–323. [Google Scholar] [CrossRef]
- Juan, C.; Igualada, C.; Moragues, F.; León, N.; Mañes, J. Development and validation of a liquid chromatography tandem mass spectrometry method for the analysis of β-agonists in animal feed and drinking water. J. Chromatogr. A 2010, 1217, 6061–6068. [Google Scholar] [CrossRef]
- Zhang, G.J.; Fang, B.H.; Liu, Y.H.; Wang, X.F.; Xu, L.X.; Zhang, Y.P.; He, L.M. Development of a multi-residue method for fast screening and confirmation of 20 prohibited veterinary drugs in feedstuffs by liquid chromatography tandem mass spectrometry. J. Chromatogr. B 2013, 936, 10–17. [Google Scholar] [CrossRef]
- Qie, M.; Zhao, Y.; Yang, S.; Wang, W.; Xu, Z. Rapid simultaneous determination of 160 drugs in urine and blood of livestock and poultry by ultra-high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2019, 1608, 460423. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Q.; Qi, Y.; Li, L.; Wang, S.; Wang, X. An ultrasensitive sensor based on polyoxometalate and zirconium dioxide nanocomposites hybrids material for simultaneous detection of toxic clenbuterol and ractopamine. Sens. Actuators B Chem. 2019, 288, 347–355. [Google Scholar] [CrossRef]
- Yaxin, W. Preliminary study on enzyme-linked immunosorbent assay for multi-residue detection of organic phosphorus pesticides. Chin. Acad. Agric. Sci. 2009. [Google Scholar]
- Cao, B.; He, G.; Yang, H.; Chang, H.; Li, S.; Deng, A. Development of a highly sensitive and specific enzyme-linked immunosorbent assay (ELISA) for the detection of phenylethanolamine A in tissue and feed samples and confirmed by liquid chromatography tandem mass spectrometry (LC-MS/MS). Talanta 2013, 115, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, Y.; Wang, J.; Shi, X.; Ye, J. Determination of beta-agonists in pig feed, pig urine and pig liver using capillary electrophoresis with electrochemical detection. Meat Sci. 2010, 85, 302–305. [Google Scholar] [CrossRef]
- Jiang, S.; Xu, Y.H.; Zhao, Y.; Jin, Y.; Liu, Y.; Zhong, Y.; Zeng, F.; Li, X.D. Multi residue simultaneous determination study of 120 pesticides in ginger by gas chromatography-tandem mass spectrometry. Agrochemicals 2011, 50, 672–676+696. [Google Scholar]
- Zhu, J.; Bo, L.I.; Fang, X.M.; Chen, J.H.; Yang, J.X. Analysis of 11 β-agonist residues in liver, kidney and meat by gas chromatography-mass spectrometry. J. Chin. Mass Spectrom. Soc. 2005, 26, 129–137. [Google Scholar]
- Hong, H.; Su-Jie, X.; Chang, L.; Ke, A. Determination of 22 β-agonists in pork and pork liver by high performance liquid chromatography-tandem mass spectrometry. J. Food Saf. Qual. 2014, 5, 1155–1165. [Google Scholar]
- Shao, B.; Jia, X.; Zhang, J.; Meng, J.; Wu, Y.; Duan, H.; Tu, X. Multi-residual analysis of 16 β-agonists in pig liver, kidney and muscle by ultra-performance liquid chromatography tandem mass spectrometry. Food Chem. 2009, 114, 1115–1121. [Google Scholar] [CrossRef]
- Smith, D.J. The pharmacokinetics, metabolism, and tissue residues of beta-adrenergic agonists in livestock. J. Anim. Sci. 1998, 76, 173–194. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.U.; Liu, Y.M.; Yao, T.; Shi, H.L.; Jun, L.I.; Zhao, Z.; Yu-Chang, Q.I.N. Identification of major metabolites of salbutamol in swine urine and plasma using ultra-high performance liquid chromatography-electrospray-time of flight-mass spectrometry. Chin. J. Anal. Chem. 2014, 42, 1692–1696. [Google Scholar]
- Shi, J.F. Metabolism and Tissue Residue Prediction of Salbutamol and Ractopamine in Swine and Goats. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2014. [Google Scholar]
- Wang, H.; Li, D.; Bi, Y.; Sun, L.; Xu, S. Identification of major metabolites of clenbuterol in swine urine using ultra-performance liquid chromatography-quadrupole time-of-flight mass spectrometry. J. Food Saf. Qual. 2014, 5, 3203–3209. [Google Scholar]
- Dalidowicz, J.E.; Thomson, T.D.; Babbitt, G.E. Ractopamine hydrochloride, a phenethanolamine repartitioning agent, xenobiotics and food-producing animals. Am. Chem. Soc. 1992, 234–243. [Google Scholar] [CrossRef]
- Smith, D.J.; Feil, V.J.; Paulson, G.D. Identification of turkey biliary metabolites of ractopamine hydrochloride and the metabolism and distribution of synthetic [14C] ractopamine glucuronides in the turkey. Xenobiotica 2008, 30, 427–440. [Google Scholar] [CrossRef]
- Smith, D.J.; Giddings, J.M.; Feil, V.J.; Paulson, G.D. Identification of ractopamine hydrochloride metabolites excreted in rat bile. Xenobiotica 1995, 25, 511–520. [Google Scholar] [CrossRef]
- Smith, D.J.; Shelver, W.L. Tissue residues of ractopamine and urinary excretion of ractopamine and metabolites in animals treated for 7 days with dietary ractopamine. J. Anim. Sci. 2002, 80, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Malucelli, A.; Ellendorff, F.; Meyer, H.H. Tissue distribution and residues of clenbuterol, salbutamol, and terbutaline in tissues of treated broiler chickens. J. Anim. Sci. 1994, 72, 1555–1560. [Google Scholar] [CrossRef] [PubMed]
- Jelka, P.; Ana, V.; Svjetlana, T.; Nada, V.; Ksenija, Š.; Eleonora, P. Comparison of accumulation of clenbuterol and salbutamol residues in animal internal tissues, non-pigmented eyes and hair. J. Anal. Toxicol. 2014, 38, 681–685. [Google Scholar]
- Zhang, K.; Zhang, J.M.; Qing-yu Tang, C.H.; Liang, X.W. Research advancement of residue, depletion and monitoring technology of salbutamol in animal body. Food Nutr. China 2015, 21, 9–13. [Google Scholar]
- Yu, Z. Studies on Pharmacokinetics and Redidues of Salbutamol in Sheep; Chinese Academy of Agricultural Sciences: Beijing, China, 2015. [Google Scholar]
- Zhang, G.; Wang, F.M.; Tang, Z.X.; Wang, S.F.; Guan, E.P.; Yan, X.H. Determination of clenbuterol residues in pig hairs by high performance liquid chromatography coupled with tandem mass spectrometry and investigation of the residue and elimination regularity of clenbuterol. J. Food Saf. Qual. 2014, 5, 3778–3783. [Google Scholar]
- Pleadin, J.; Vulić, A.; Perši, N.; Vahčić, N. Clenbuterol residues in pig muscle after repeat administration in a growth-promoting dose. Meat Sci. 2010, 86, 733–737. [Google Scholar] [CrossRef]
- Daniel, Z.; Elisabeth, P.D.; Laurent, D.; Marie-Pierre, B.F.; Jacques, T. Comparative metabolism of clenbuterol by rat and bovine liver microsomes and slices. Drug Metab. Dispos. 1998, 26, 28–35. [Google Scholar]
- Feddern, V.; Aroeira, C.N.; Molognoni, L.; Gressler, V.; Daguer, H.; Dalla Costa, O.A.; Castillo, C.J.C.; de Lima, C.J.M.M. Ractopamine analysis in pig kidney, liver and lungs: A validation of the method scope extension using QuEChERS as a sample preparation step. J. Chromatogr. B 2018, 1091, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Yongfei, Y.; Congying, Z.; Wei, Z.; Jian, Q.; Chunbo, L.; Yizhen, W. β- Study on the fragmentation characteristics of receptor agonists by mass spectrometry. Chin. J. Vet. Med. 2015, 49, 52–57. [Google Scholar]
- Cong, W.; Ruiqiang, L.; Zhengrui, N.; Jin, C. 7 β- Study on the fragmentation law of receptor agonists by electrospray spray mass spectrometry. J. Pharm. Anal. 2018, 38, 1419–1426. [Google Scholar]
- Montrade, M.P.; Bizec, B.L.; Monteau, F.; Andre, F. Analysis of beta-agonists in urine and tissues by capillary gas chromatography-mass spectrometry: In vivo study of salbutamol disposition in calves. Food Addit. Contam. 1995, 12, 625–636. [Google Scholar] [CrossRef]
- Pleadin, J.; Vulic, A.; Perai, N.; Terzic, S.; Andriaic, M.; Arkovic, I.; Andor, K.; Perak, E. Comparison of ractopamine residue depletion from internal tissues. Immunopharmacol. Immunotoxicol. 2013, 35, 88–92. [Google Scholar] [CrossRef]
- Qiang, Z.; Shentu, F.; Wang, B.; Wang, J.; Shen, J. Residue depletion of ractopamine and its metabolites in swine tissues, urine, and serum. J. Agric. Food Chem. 2007, 55, 4319–4326. [Google Scholar] [CrossRef]
- Dong, Y.; Xia, X.; Wang, X.; Ding, S.; Li, X.; Zhang, S.; Jiang, H.; Liu, J.; Li, J.; Feng, Z.; et al. Validation of an ultra-performance liquid chromatography-tandem mass spectrometry method for determination of ractopamine: Application to residue depletion study in swine. Food Chem. 2011, 127, 327–332. [Google Scholar] [CrossRef]
- Meyer, H.H.; Rinke, L.M. The pharmacokinetics and residues of clenbuterol in veal calves. J. Anim. Sci. 1991, 69, 4538–4544. [Google Scholar] [CrossRef]
- Liu, M. Studies on Accumulation, Depletion, Residual Prediction Model of the Clenbuterol Hydrochloride in Sheep. Master’s Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2014. [Google Scholar]




| Organization | Added Recovery Level (μg/mL) | n | Salbutamol | Clenbuterol | Ractopamine | |||
|---|---|---|---|---|---|---|---|---|
| Average Recovery (%) | Coefficient of Variation (%) | Average Recovery (%) | Coefficient of Variation (%) | Average Recovery (%) | Coefficient of Variation (%) | |||
| Muscle of rat | 0.5 | 3 | 95.18 | 8.0 | 93.82 | 8.9 | 94.84 | 8.2 |
| 1 | 3 | 94.22 | 8.9 | 92.52 | 4.8 | 90.55 | 7.1 | |
| 1.5 | 3 | 92.63 | 5.3 | 94.64 | 8.2 | 93.45 | 6.3 | |
| Liver of rat | 0.5 | 3 | 91.71 | 4.1 | 92.63 | 6.3 | 89.85 | 5.2 |
| 1 | 3 | 92.96 | 6.6 | 95.18 | 6.9 | 92.70 | 5.4 | |
| 1.5 | 3 | 89.85 | 7.9 | 94.23 | 8.9 | 88.95 | 5.2 | |
| Kidney of rat | 0.5 | 3 | 92.05 | 6.5 | 94.70 | 7.9 | 91.63 | 7.0 |
| 1 | 3 | 89.44 | 5.7 | 92.34 | 4.8 | 86.77 | 7.2 | |
| 1.5 | 3 | 91.18 | 7.8 | 90.87 | 7.7 | 90.45 | 7.4 | |
| Blood of rat | 0.5 | 3 | 89.64 | 6.7 | 90.08 | 8.0 | 89.99 | 6.7 |
| 1 | 3 | 88.21 | 6.2 | 90.15 | 7.0 | 88.47 | 6.1 | |
| 1.5 | 3 | 90.48 | 6.8 | 91.41 | 6.1 | 90.15 | 7.5 | |
| Feces of rat | 0.5 | 3 | 92.54 | 9.3 | 89.00 | 7.3 | 89.69 | 7.0 |
| 1 | 3 | 89.99 | 7.7 | 87.33 | 6.5 | 90.48 | 6.1 | |
| 1.5 | 3 | 87.00 | 6.9 | 88.66 | 7.4 | 87.78 | 7.4 | |
| Muscle of pig | 0.5 | 3 | 95.26 | 6.3 | 96.45 | 6.0 | 89.96 | 7.8 |
| 1 | 3 | 89.26 | 4.4 | 94.86 | 8.2 | 95.11 | 6.02 | |
| 1.5 | 3 | 95.63 | 5.9 | 96.73 | 6.1 | 92.97 | 3.5 | |
| Muscle of sheep | 0.5 | 3 | 90.78 | 9.0 | 91.70 | 4.0 | 92.33 | 2.6 |
| 1 | 3 | 90.12 | 3.6 | 94.08 | 7.4 | 89.56 | 3.9 | |
| 1.5 | 3 | 93.63 | 4.3 | 94.67 | 7.7 | 94.33 | 6.5 | |
| Name of Lean Meat Essence | Chemical Formula | Measured Relative Molecular Mass [M + H]+ | Retention Time (min) | Major Fragment Ions (m/z) |
|---|---|---|---|---|
| Salbutamol | C13H21NO3 | 240.100 | 4.069 | 221.9000 |
| 166.1000 | ||||
| 148.1000 | ||||
| 131.8000 | ||||
| Clenbuterol | C12H18Cl2N2O | 277.100 | 6.734 | 259.0000 |
| 203.0000 | ||||
| 168.0000 | ||||
| 131.9000 | ||||
| Ractopamine | C18H23NO3 | 302.000 | 6.264 | 284.1000 |
| 164.1000 | ||||
| 136.0000 | ||||
| 120.7000 | ||||
| 107.0000 |
| Name of β-Receptor Agonists | Product | Forms of Metabolites | Measured Relative Molecular Mass [M + H]+ |
|---|---|---|---|
| Salbutamol | A | Original form | m/z 240.1000 |
| B | Methylated conjugate | m/z 254.0000 | |
| Clenbuterol | C | Original form | m/z 277.0000 |
| D | Methylated conjugate | m/z 293.0000 | |
| Ractopamine | E | Original form | m/z 302.1000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.; Wang, L.; Zhang, R.; Pan, J.; Wu, W.; Huang, Y.; Zhang, Z.; Zhao, R. Determination of the Metabolites and Metabolic Pathways for Three β-Receptor Agonists in Rats Based on LC-MS/MS. Animals 2022, 12, 1885. https://doi.org/10.3390/ani12151885
Liang Y, Wang L, Zhang R, Pan J, Wu W, Huang Y, Zhang Z, Zhao R. Determination of the Metabolites and Metabolic Pathways for Three β-Receptor Agonists in Rats Based on LC-MS/MS. Animals. 2022; 12(15):1885. https://doi.org/10.3390/ani12151885
Chicago/Turabian StyleLiang, Ying, Lin Wang, Ruipeng Zhang, Jiadi Pan, Wenhong Wu, Yuanyuan Huang, Zifan Zhang, and Renbang Zhao. 2022. "Determination of the Metabolites and Metabolic Pathways for Three β-Receptor Agonists in Rats Based on LC-MS/MS" Animals 12, no. 15: 1885. https://doi.org/10.3390/ani12151885





