Effects of Intestinal Microorganisms on Metabolism and Toxicity Mitigation of Zearalenone in Broilers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Analytes and Reagents
2.2. In Vitro Test
2.2.1. Anaerobic Incubation of ZEN with the Microflora
2.2.2. Analysis of ZEN and its Metabolites
2.3. Animal Experiment
2.3.1. Animal
2.3.2. Sample Collection
2.3.3. DNA Extraction and PCR Amplification
2.3.4. Illumina MiSeq Sequencing
2.3.5. Processing of Sequencing Data
2.3.6. ZEN and its Metabolites of Excreta Analysis
2.3.7. Antioxidant Analysis
2.3.8. Histopathology
2.4. Statistical Analysis
3. Results
3.1. Effect of In Vitro Incubation with Chicken Gut Microbiota on ZEN
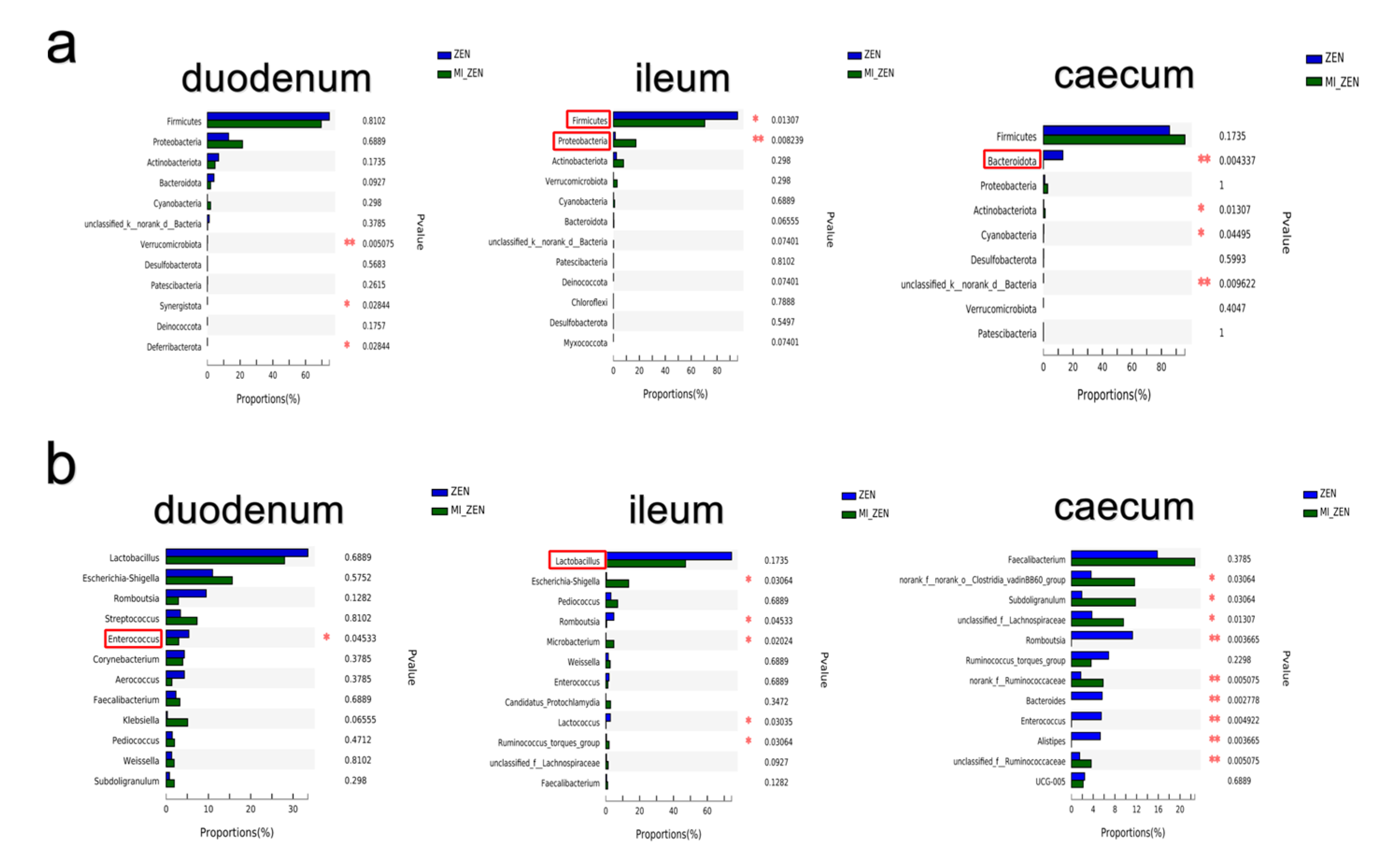
3.2. ZEN and Microbial Inhibition Alter the Gut Microbiota In Vivo
3.2.1. Beta Diversity Analysis
3.2.2. ZEN Alter the Bacterial Abundance
3.2.3. Microbial Inhibition Alter the Gut Microbiota
3.3. Metabolism of ZEN in Broilers
3.4. Oxidative Stress and Liver Injury
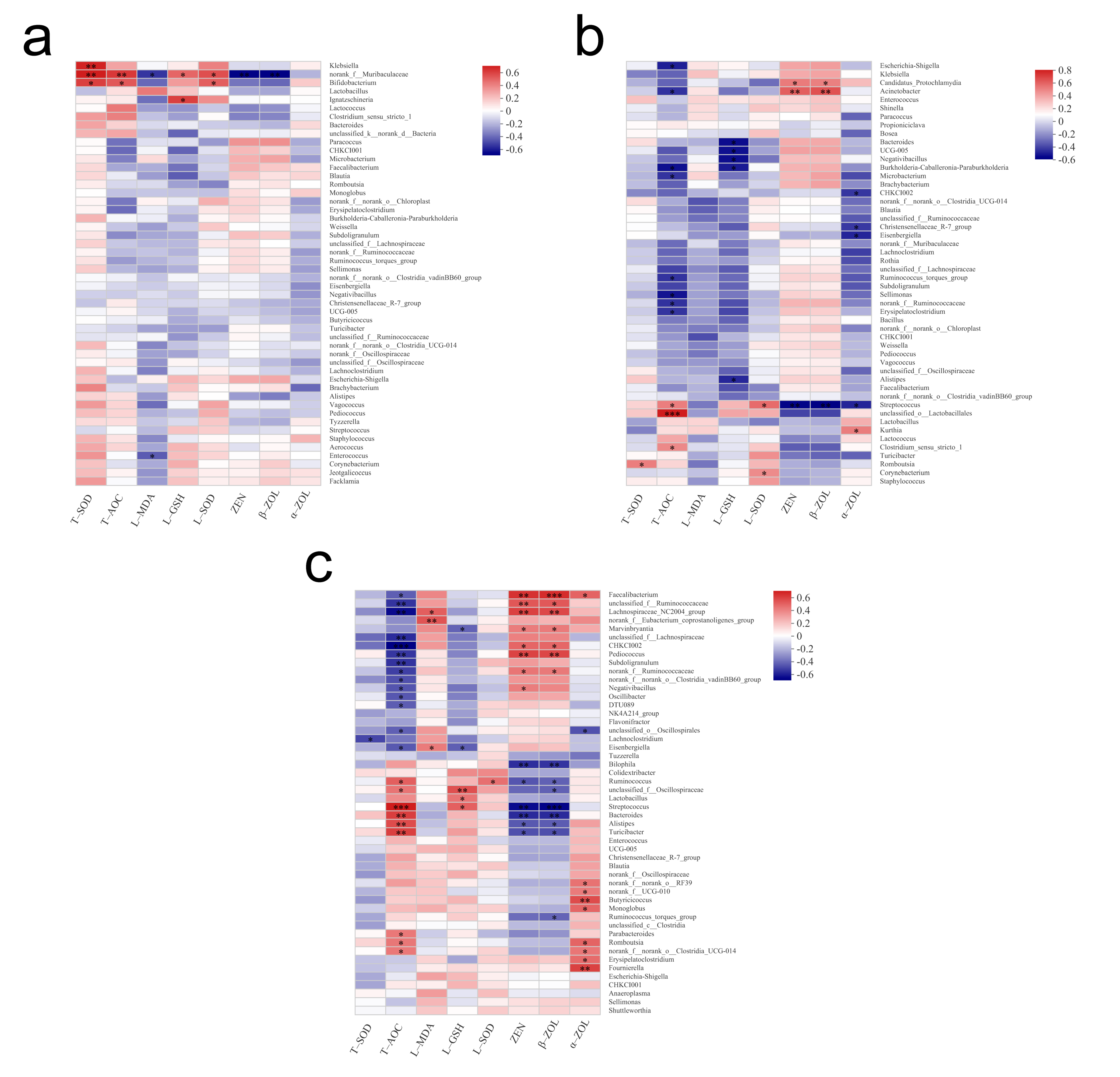
3.5. Spearman Correlation Heatmap
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abid-Essefi, S.; Baudrimont, I.; Hassen, W.; Ouanes, Z.; Mobio, T.A.; Anane, R.; Creppy, E.E.; Bacha, H. DNA fragmentation, apoptosis and cell cycle arrest induced by zearalenone in cultured DOK, Vero and Caco-2 cells: Prevention by Vitamin E. Toxicology 2003, 192, 237–248. [Google Scholar] [CrossRef]
- Ma, R.; Zhang, L.; Liu, M.; Su, Y.-T.; Xie, W.-M.; Zhang, N.-Y.; Dai, J.-F.; Wang, Y.; Rajput, S.A.; Qi, D.-S. Individual and combined occurrence of mycotoxins in feed ingredients and complete feeds in China. Toxins 2018, 10, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, S.Z.; Yang, Z.B.; Yang, W.R.; Wang, S.J.; Wang, Y.; Broomhead, J.; Johnston, S.L.; Chi, F. Effect on hepatonephric organs, serum metabolites and oxidative stress in post-weaning piglets fed purified zearalenone-contaminated diets with or without Calibrin-Z. J. Anim. Physiol. Anim. Nutr. 2012, 96, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Gajecki, M. Zearalenone--undesirable substances in feed. Pol. J. Vet. Sci. 2002, 5, 117–122. [Google Scholar] [PubMed]
- Ouanes, Z.; Ayed-Boussema, I.; Baati, T.; Creppy, E.E.; Bacha, H. Zearalenone induces chromosome aberrations in mouse bone marrow: Preventive effect of 17β-estradiol, progesterone and Vitamin E. Mutat. Res./Fundam. Mol. Mech. Mutagenesis 2005, 565, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Gajęcka, M.; Waśkiewicz, A.; Zielonka, Ł.; Goliński, P.; Rykaczewska, A.; Lisieska-Żołnierczyk, S.; Gajęcki, M.T. Mycotoxin levels in the digestive tissues of immature gilts exposed to zearalenone and deoxynivalenol. Toxicon 2018, 153, 1–11. [Google Scholar] [CrossRef]
- Jiang, S.Z.; Yang, Z.B.; Yang, W.R.; Gao, J.; Liu, F.X.; Broomhead, J.; Chi, F. Effects of purified zearalenone on growth performance, organ size, serum metabolites, and oxidative stress in postweaning gilts. J. Anim. Sci. 2011, 89, 3008. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Applegate, T. Zearalenone (ZEN) in Livestock and Poultry: Dose, Toxicokinetics, Toxicity and Estrogenicity. Toxins 2020, 12, 377. [Google Scholar] [CrossRef]
- Zhang, G.-L.; Feng, Y.-L.; Song, J.-L.; Zhou, X.-S. Zearalenone: A mycotoxin with different toxic effect in domestic and laboratory animals’ granulosa cells. Front. Genet. 2018, 9, 667. [Google Scholar] [CrossRef] [Green Version]
- Malekinejad, H.; Maas-Bakker, R.; Fink-Gremmels, J. Species differences in the hepatic biotransformation of zearalenone. Vet. J. 2006, 172, 96–102. [Google Scholar] [CrossRef]
- Chi, M.; Mirocha, C.; Weaver, G.; Kurtz, H. Effect of zearalenone on female white leghorn chickens. Appl. Environ. Microbiol. 1980, 39, 1026–1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, N.K.; Mirocha, C.J.; Weaver, G.; Aakhus-Allen, S.; Bates, F. Effects of dietary zearalenone on finishing broiler chickens and young turkey poults. Poult. Sci. 1981, 60, 124. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cheng, Y.; Wen, C.; Wang, W.; Kang, Y.; Wang, A.; Zhou, Y. The protective effects of modified palygorskite on the broilers fed a purified zearalenone-contaminated diet. Poult. Sci. 2019, 98, 3802–3810. [Google Scholar] [CrossRef] [PubMed]
- Gresakova, L.; Borutova, R.; Faix, S.; Placha, I.; Cobanova, K.; Kosikova, B.; Leng, L. Effect of lignin on oxidative stress in chickens fed a diet contaminated with zearalenone. Acta Vet. Hung. 2012, 60, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, H.; Sun, F.; De Ruyck, K.; Zhang, J.; Jin, Y.; Li, Y.; Wang, Z.; Zhang, S.; De Saeger, S. Metabolic Profile of Zearalenone in Liver Microsomes from Different Species and Its in Vivo Metabolism in Rats and Chickens Using Ultra High-Pressure Liquid Chromatography-Quadrupole/Time-of-Flight Mass Spectrometry. J. Agric. Food Chem. 2017, 65, 11292–11303. [Google Scholar] [CrossRef]
- Völkel, I.; Schröer-Merker, E.; Czerny, C.-P. The carry-over of mycotoxins in products of animal origin with special regard to its implications for the European food safety legislation. Food Nutr. Sci. 2011, 2, 852–867. [Google Scholar] [CrossRef] [Green Version]
- Devreese, M.; Antonissen, G.; Broekaert, N.; De Baere, S.; Vanhaecke, L.; De Backer, P.; Croubels, S. Comparative Toxicokinetics, Absolute Oral Bioavailability, and Biotransformation of Zearalenone in Different Poultry Species. J. Agric. Food Chem. 2015, 63, 5092. [Google Scholar] [CrossRef]
- Gratz, S.W.; Dinesh, R.; Yoshinari, T.; Holtrop, G.; Richardson, A.J.; Duncan, G.; MacDonald, S.; Lloyd, A.; Tarbin, J. Masked trichothecene and zearalenone mycotoxins withstand digestion and absorption in the upper GI tract but are efficiently hydrolyzed by human gut microbiota in vitro. Mol. Nutr. Food Res. 2017, 61, 1600680. [Google Scholar] [CrossRef]
- Zinedine, A.; Soriano, J.M.; Moltó, J.C.; Mañes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef]
- Malekinejad, H.; Maasbakker, R.F.; Finkgremmels, J. Bioactivation of zearalenone by porcine hepatic biotransformation. Vet. Res. 2005, 36, 799–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Zhang, Y.; Wang, Y.; Sheng, C. Toxic effects of zearalenone and alpha-zearalenol on the regulation of steroidogenesis and testosterone production in mouse Leydig cells. Toxicol. Vitr. 2007, 21, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Ropejko, K.; Twarużek, M. Zearalenone and Its Metabolites—General Overview, Occurrence, and Toxicity. Toxins 2021, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Rogowska, A.; Pomastowski, P.; Sagandykova, G.N.; Buszewski, B. Zearalenone and its metabolites: Effect on human health, metabolism and neutralisation methods. Toxicon 2019, 162, 46–56. [Google Scholar] [CrossRef]
- Mendez-Catala, D.M.; Spenkelink, A.; Rietjens, I.M.C.M.; Beekmann, K. An in vitromodel to quantify interspecies differences in kinetics for intestinal microbial bioactivation and detoxification of zearalenone. Toxicol. Rep. 2020, 7, 938–946. [Google Scholar] [CrossRef]
- Zhang, T.; Ding, H.; Chen, L.; Lin, Y.; Gong, Y.; Pan, Z.; Zhang, G.; Xie, K.; Dai, G.; Wang, J. Antibiotic-Induced Dysbiosis of Microbiota Promotes Chicken Lipogenesis by Altering Metabolomics in the Cecum. Metabolites 2021, 11, 487. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Wang, L.; Wang, J.; Tan, Y.; Yu, D.; Chang, X.; Fan, Y.; Zhao, D.; Wang, C.; De Boevre, M. A QuEChERS-based liquid chromatography-tandem mass spectrometry method for the simultaneous determination of nine zearalenone-like mycotoxins in pigs. Toxins 2018, 10, 129. [Google Scholar] [CrossRef] [Green Version]
- Złoch, M.; Rogowska, A.; Pomastowski, P.; Railean-Plugaru, V.; Walczak-Skierska, J.; Rudnicka, J.; Buszewski, B. Use of Lactobacillus paracasei strain for zearalenone binding and metabolization. Toxicon 2020, 181, 9–18. [Google Scholar] [CrossRef]
- Videmann, B.; Mazallon, M.; Tep, J.; Lecoeur, S. Metabolism and transfer of the mycotoxin zearalenone in human intestinal Caco-2 cells. Food Chem. Toxicol. 2008, 46, 3279–3286. [Google Scholar] [CrossRef]
- Koraichi, F.; Videmann, B.; Mazallon, M.; Benahmed, M.; Prouillac, C.; Lecoeur, S. Zearalenone exposure modulates the expression of ABC transporters and nuclear receptors in pregnant rats and fetal liver. Toxicol. Lett. 2012, 211, 246–256. [Google Scholar] [CrossRef]
- Wang, W.; Zhai, S.; Xia, Y.; Wang, H.; Ruan, D.; Zhou, T.; Zhu, Y.; Zhang, H.; Zhang, M.; Ye, H.; et al. Ochratoxin A induces liver inflammation: Involvement of intestinal microbiota. Microbiome 2019, 7, 151. [Google Scholar] [CrossRef]
- Bao, W.; He, Y.; Liu, W. Diversity Analysis of Bacterial and Function Prediction in Hurunge From Mongolia. Front. Nutr. 2022, 9, 835123. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Stackebrandt, E.; GOEBEL, B.M. Taxonomic note: A place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int. J. Syst. Evol. Microbiol. 1994, 44, 846–849. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [Green Version]
- Mao, T.; Jiang, X. Changes in microbial community and enzyme activity in soil under continuous pepper cropping in response to Trichoderma hamatum MHT1134 application. Sci. Rep. 2021, 11, 21585. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Z.; Wang, X.; Li, Q.; Gao, X.; Yi, X.; Ma, Y. High-throughput sequencing method to study the dynamic changes of microbial communities in second-generation fermented lettuce. IOP Conf. Ser. Earth Environ. Sci. 2021, 680, 012064. [Google Scholar] [CrossRef]
- De Baere, S.; Osselaere, A.; Devreese, M.; Vanhaecke, L.; De Backer, P.; Croubels, S. Development of a liquid–chromatography tandem mass spectrometry and ultra-high-performance liquid chromatography high-resolution mass spectrometry method for the quantitative determination of zearalenone and its major metabolites in chicken and pig plasma. Anal. Chim. Acta 2012, 756, 37–48. [Google Scholar] [CrossRef]
- Han, Z.; Jiang, K.; Fan, Z.; Di Mavungu, J.D.; Dong, M.; Guo, W.; Fan, K.; Campbell, K.; Zhao, Z.; Wu, Y. Multi-walled carbon nanotubes-based magnetic solid-phase extraction for the determination of zearalenone and its derivatives in maize by ultra-high performance liquid chromatography-tandem mass spectrometry. Food Control 2017, 79, 177–184. [Google Scholar] [CrossRef]
- Akbaş, C.E. Classification of Histopathological Cancer Stem Cell Images in h&e Stained Liver Tissues. Master’s Thesis, Bilkent University, Ankara, Turkey, 2016. [Google Scholar]
- Liew, W.-P.-P.; Mohd-Redzwan, S. Mycotoxin: Its impact on gut health and microbiota. Front. Cell. Infect. Microbiol. 2018, 8, 60. [Google Scholar] [CrossRef] [Green Version]
- Devi, P.; Khan, A.; Chattopadhyay, P.; Garg, A.; Pandey, R. 2.31—Gut Microbiota and the Liver: Interaction Shaping Interactome. In Comprehensive Gut Microbiota; Glibetic, M., Ed.; Elsevier: Oxford, UK, 2022; pp. 400–411. [Google Scholar]
- Osselaere, A.; Devreese, M.; Goossens, J.; Vandenbroucke, V.; De Baere, S.; De Backer, P.; Croubels, S. Toxicokinetic study and absolute oral bioavailability of deoxynivalenol, T-2 toxin and zearalenone in broiler chickens. Food Chem. Toxicol. 2013, 51, 350–355. [Google Scholar] [CrossRef]
- Tardieu, D.; Travel, A.; Metayer, J.-P.; Le Bourhis, C.; Guerre, P. Zearalenone and Metabolites in Livers of Turkey Poults and Broiler Chickens Fed with Diets Containing Fusariotoxins. Toxins 2020, 12, 525. [Google Scholar] [CrossRef] [PubMed]
- Dänicke, S.; Brezina, U. Kinetics and metabolism of the Fusarium toxin deoxynivalenol in farm animals: Consequences for diagnosis of exposure and intoxication and carry over. Food Chem. Toxicol. 2013, 60, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Guerre, P. Mycotoxin and Gut Microbiota Interactions. Toxins 2020, 12, 769. [Google Scholar] [CrossRef]
- Appell, M.; Wang, L.C.; Bosma, W.B. Analysis of the photophysical properties of zearalenone using density functional theory. J. Lumin. 2017, 188, 551–557. [Google Scholar] [CrossRef]
- Reddy, K.E.; Jeong, J.Y.; Song, J.; Lee, Y.; Lee, H.-J.; Kim, D.-W.; Jung, H.J.; Kim, K.H.; Kim, M.; Oh, Y.K.; et al. Colon Microbiome of Pigs Fed Diet Contaminated with Commercial Purified Deoxynivalenol and Zearalenone. Toxins 2018, 10, 347. [Google Scholar] [CrossRef] [Green Version]
- Long, M.; Li, P.; Zhang, W.; Li, X.; Zhang, Y.; Wang, Z.; Liu, G. Removal of zearalenone by strains of Lactobacillus sp. isolated from rumen in vitro. J. Anim. Vet. Adv. 2012, 11, 2417–2422. [Google Scholar]
- Niderkorn, V.; Morgavi, D.P.; Pujos, E.; Tissandier, A.; Boudra, H. Screening of fermentative bacteria for their ability to bind and biotransform deoxynivalenol, zearalenone and fumonisins in an in vitro simulated corn silage model. Food Addit. Contam. 2007, 24, 406–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kollarczik, B.; Gareis, M.; Hanelt, M. In vitro transformation of the Fusarium mycotoxins deoxynivalenol and zearalenone by the normal gut microflora of pigs. Nat. Toxins 1994, 2, 105–110. [Google Scholar] [CrossRef]
- Biehl, M.L.; Prelusky, D.B.; Koritz, G.D.; Hartin, K.E.; Buck, W.B.; Trenholm, H.L. Biliary excretion and enterohepatic cycling of zearalenone in immature pigs. Toxicol. Appl. Pharmacol. 1993, 121, 152–159. [Google Scholar] [CrossRef]
- Frey, J.C.; Pell, A.N.; Berthiaume, R.; Lapierre, H.; Lee, S.; Ha, J.K.; Mendell, J.E.; Angert, E.R. Comparative studies of microbial populations in the rumen, duodenum, ileum and faeces of lactating dairy cows. J. Appl. Microbiol. 2010, 108, 1982–1993. [Google Scholar] [CrossRef] [PubMed]
- Grenier, B.; Applegate, T.J. Modulation of Intestinal Functions Following Mycotoxin Ingestion: Meta-Analysis of Published Experiments in Animals. Toxins 2013, 5, 396–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gajęcka, M.; Zielonka, Ł.; Gajęcki, M. Activity of Zearalenone in the Porcine Intestinal Tract. Molecules 2017, 22, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zielonka, Ł.; Waśkiewicz, A.; Beszterda, M.; Kostecki, M.; Dąbrowski, M.; Obremski, K.; Goliński, P.; Gajęcki, M. Zearalenone in the Intestinal Tissues of Immature Gilts Exposed per os to Mycotoxins. Toxins 2015, 7, 3210–3223. [Google Scholar] [CrossRef] [Green Version]
- Król, A.; Pomastowski, P.; Rafińska, K.; Railean-Plugaru, V.; Walczak, J.; Buszewski, B. Microbiology neutralization of zearalenone using Lactococcus lactis and Bifidobacterium sp. Anal. Bioanal. Chem. 2018, 410, 943–952. [Google Scholar] [CrossRef] [PubMed]
- El-Nezami, H.; Polychronaki, N.; Salminen, S.; Mykkänen, H. Binding Rather Than Metabolism May Explain the Interaction of Two Food-Grade Lactobacillus Strains with Zearalenone and Its Derivative α-Zearalenol. Appl. Environ. Microbiol. 2002, 68, 3545–3549. [Google Scholar] [CrossRef] [Green Version]
- Sangsila, A.; Faucet-Marquis, V.; Pfohl-Leszkowicz, A.; Itsaranuwat, P. Detoxification of zearalenone by Lactobacillus pentosus strains. Food Control 2016, 62, 187–192. [Google Scholar] [CrossRef]
- Cui, C.; Shen, C.; Jia, G.; Wang, K. Effect of dietary Bacillus subtilis on proportion of Bacteroidetes and Firmicutes in swine intestine and lipid metabolism. Genet. Mol. Res. 2013, 12, 1766–1776. [Google Scholar] [CrossRef]
- Buranatragool, K.; Poapolathep, S.; Isariyodom, S.; Imsilp, K.; Klangkaew, N.; Poapolathep, A. Dispositions and tissue residue of zearalenone and its metabolites α-zearalenol and β-zearalenol in broilers. Toxicol. Rep. 2015, 2, 351–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robert, H.; Payros, D.; Pinton, P.; Théodorou, V.; Mercier-Bonin, M.; Oswald, I.P. Impact of mycotoxins on the intestine: Are mucus and microbiota new targets? J. Toxicol. Environ. Health Part B 2017, 20, 249–275. [Google Scholar] [CrossRef] [PubMed]
- Martens, E.C.; Neumann, M.; Desai, M.S. Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier. Nat. Rev. Microbiol. 2018, 16, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Bibo, Z.; Lei, Z.; Weili, Y.; Yefei, C.; Chao, W.; Yanmin, Z. Effect of zearalenone on serum parameters, hepatic oxidative damage and residue of zearalenone in broilers. Anim. Husb. Vet. Med. 2016, 48, 10–14. [Google Scholar]
- Lee, K.S.; Palatinszky, M.; Pereira, F.C.; Nguyen, J.; Fernandez, V.I.; Mueller, A.J.; Menolascina, F.; Daims, H.; Berry, D.; Wagner, M. An automated Raman-based platform for the sorting of live cells by functional properties. Nat. Microbiol. 2019, 4, 1035–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danicke, S.; Ueberschar, K.; Halle, I.; Matthes, S.; Valenta, H.; Flachowsky, G. Effect of addition of a detoxifying agent to laying hen diets containing uncontaminated or Fusarium toxin-contaminated maize on performance of hens and on carryover of zearalenone. Poult. Sci. 2002, 81, 1671–1680. [Google Scholar] [CrossRef]
- Schneweis, I.; Meyer, K.; Ritzmann, M.; Hoffmann, P.; Dempfle, L.; Bauer, J. Influence of organically or conventionally produced wheat on health, performance and mycotoxin residues in tissues and bile of growing pigs. Arch. Anim. Nutr. 2005, 59, 155–163. [Google Scholar] [CrossRef]




| Analyte | Retention Time (min) | Molecular Weight | Parent Ion (m/z) | Product ion (m/z) | Collision Energy (eV) | Crushing Voltage (V) |
|---|---|---|---|---|---|---|
| ZEN | 6.9 | 318.36 | 317 | 174.6 130.6 | 20 25 | 135 |
| α-ZOL | 6.1 | 320.38 | 319.1 | 275.1 159.8 | 15 25 | 135 |
| β-ZOL | 5.5 | 320.38 | 319.1 | 275.1 159.8 | 15 25 | 105 |
| Intestinal Contents | ZEN Degradation Rate (%) | Conversion Rate (%) | |
|---|---|---|---|
| α-ZOL | β-ZOL | ||
| Duodenum | 55.7 ± 4.6 | 1.6 ± 0.6 | 1.8 ± 1.0 |
| Jejunum | 0 | / | / |
| Ileum | 11.9 ± 5.6 | / | / |
| Cecum | 15.1 ± 5.9 | 1.1 ± 0.3 | / |
| Colon | 16.6 ± 9.8 | 1.7 ± 0.6 | 3.4 ± 2.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, S.; Ren, C.; Yang, P.; Qi, D. Effects of Intestinal Microorganisms on Metabolism and Toxicity Mitigation of Zearalenone in Broilers. Animals 2022, 12, 1962. https://doi.org/10.3390/ani12151962
Jia S, Ren C, Yang P, Qi D. Effects of Intestinal Microorganisms on Metabolism and Toxicity Mitigation of Zearalenone in Broilers. Animals. 2022; 12(15):1962. https://doi.org/10.3390/ani12151962
Chicago/Turabian StyleJia, Sifan, Chenxi Ren, Ping Yang, and Desheng Qi. 2022. "Effects of Intestinal Microorganisms on Metabolism and Toxicity Mitigation of Zearalenone in Broilers" Animals 12, no. 15: 1962. https://doi.org/10.3390/ani12151962
APA StyleJia, S., Ren, C., Yang, P., & Qi, D. (2022). Effects of Intestinal Microorganisms on Metabolism and Toxicity Mitigation of Zearalenone in Broilers. Animals, 12(15), 1962. https://doi.org/10.3390/ani12151962






