New Data on Pterygodermatites (Pterygodermatites) plagiostoma Wedl, 1861 (Nematoda, Rictulariidae) Parasite of the Algerian Hedgehog Atelerix algirus Linnaeus, 1758 (Eulipotyphla: Erinaceidae) from the Canary Islands †
Abstract
Simple Summary
Abstract
1. Introduction
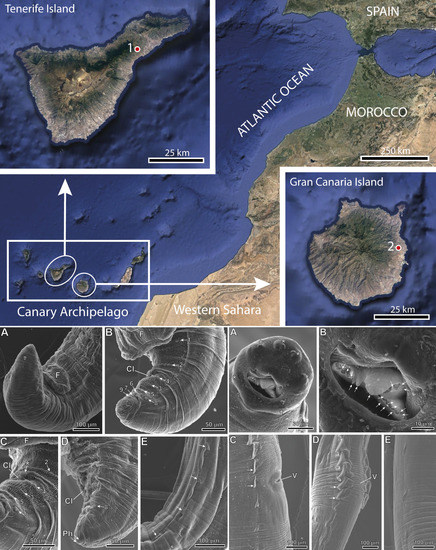
2. Materials and Methods
2.1. Specimens
2.2. Scanning Electron Microscopy Study
2.3. Molecular Analyses
3. Results
3.1. Taxonomic Summary
3.2. Phylogenetic Tree
3.3. Description


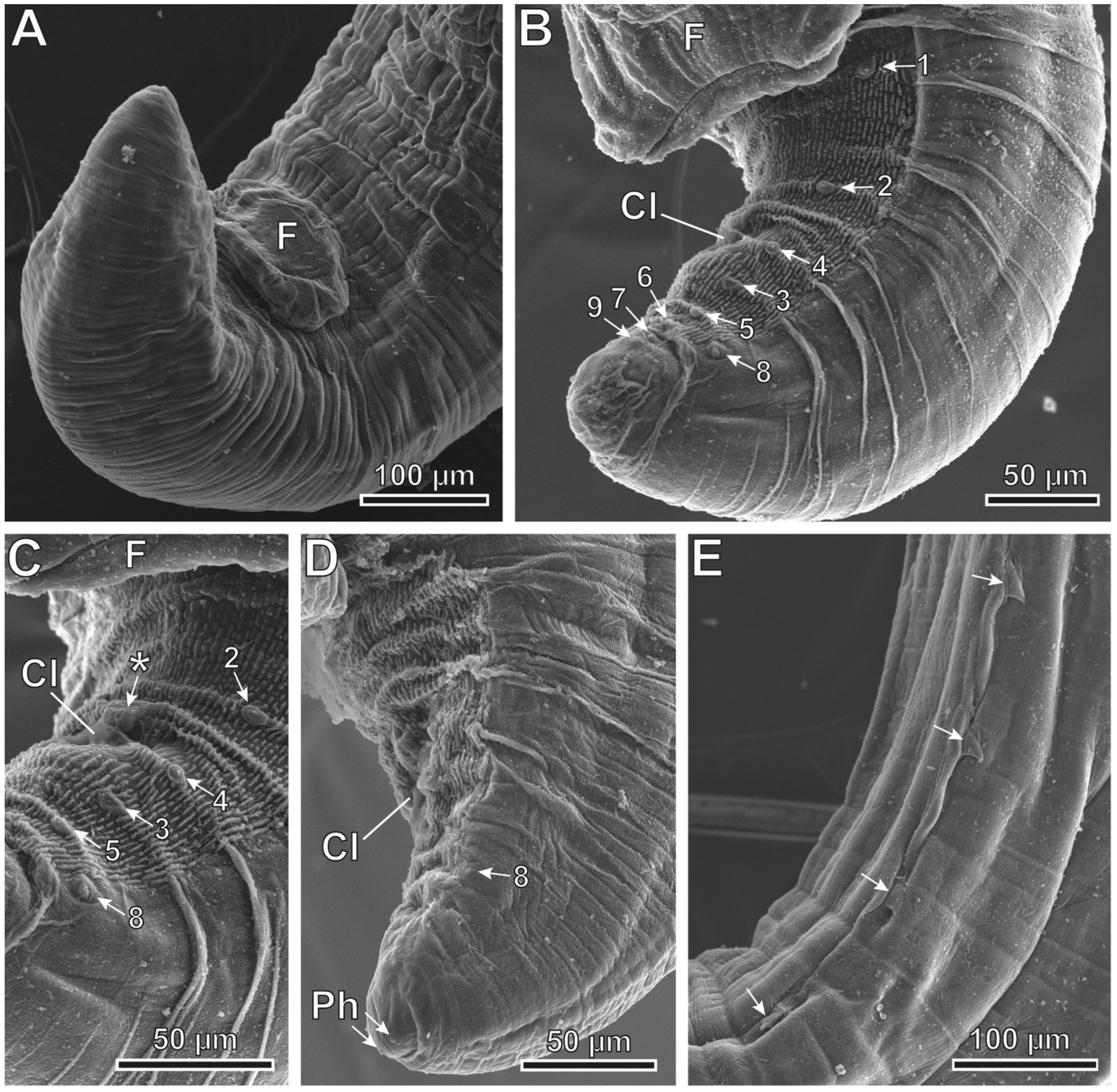


4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amin, O.M.; Heckmann, R.A. Nematodes and cestodes from the desert hedgehog, Paraechinus aethiopicus (Ehrenberg) in Central Saudi Arabia, revealed by SEM and microscopy, with notes on histopathology. Sci. Parasitol. 2016, 17, 26–35. [Google Scholar]
- Feliu, C.; Blasco, S.; Torres, J.; Miquel, J.; Casanova, J.C. On the helminthfauna of Erinaceus europaeus Linnaeus, 1758 (Insectivora, Erinaceidae) in the Iberian Peninsula. Rev. Ibér. Parasitol. 2001, 61, 31–37. [Google Scholar]
- Jägerskiöld, L.A. Nematodem aus Ägypten und dem Sudan (eingesammelt von der Schwedischen Zoologischen Expedition). I. Rictularia und Dichelyne. In Results of the Swedish Zoological Expedition to Egypt and the White Nile 1901 under the Direction of LA Jägerskiöld; Part I. K.W.; Appelberg: Upsala, Sweden, 1904; pp. 1–66. [Google Scholar]
- Jrijer, J.; Bordes, F.; Morand, S.; Neifar, L. A survey of nematode parasites of small mammals in Tunisia, North Africa: Diversity of species and zoonotic implications. Comp. Parasitol. 2015, 82, 204–210. [Google Scholar] [CrossRef]
- Khaldi, M.; Torres, J.; Samsó, B.; Miquel, J.; Biche, M.; Benyettou, M.; Barech, G.; Benelkadi, H.A.; Ribas, A. Endoparasites (helminths and coccidians) in the hedgehogs Atelerix algirus and Paraechinus aethiopicus from Algeria. Afr. Zool. 2012, 47, 48–54. [Google Scholar] [CrossRef]
- Mas-Coma, S.; Esteban, J.G.; Fuentes, M.V.; Bargues, M.D.; Valero, M.A.; Galán-Puchades, M.T. Helminth parasites of small mammals (insectivores and rodents) on the Pityusic Island of Eivissa (Balearic Archipelago). Res. Rev. Parasitol. 2000, 60, 41–49. [Google Scholar]
- Miquel, J.; Blasco, S.; Marchand, B.; Torres, J.; Feliu, C. Étude en microscopie électronique à balayage de la femelle de Pterygodermatites (Pterygodermatites) plagiostoma (Nematoda: Rictulariidae) chez un nouvel hôte. Vie Milieu 1997, 47, 213–220. [Google Scholar]
- Wedl, K. Zur Helminthenfauna Äegyptens. III Nematoda. Sitz. Akad. Wiss. Math-Naturwiss. Kl. 1861, 44, 463–482. [Google Scholar]
- Leiper, R.T. Demonstration or Nematode Parasites obtained from animals in the Zoological gardens during the year ending November 1910. Proc. Zool. Soc. Lond. 1911, XLIV, 620–621. [Google Scholar]
- Parona, C. Sopra alcuni elminti di Vertebrati Birmani raccolti da Leonardo Fea. Ann. Mus. Civ. Stor. Nat. Genova Ser. 2 1889, 7, 765–780. [Google Scholar]
- Sonsino, P. Notices helminthologiques. II. Rictularia plagiostoma et espèces semblables. Arch. Ital. De Biol. 1888, 10, 192–196. [Google Scholar]
- von Willemoes-Suhm, R. Helminlhologische Notizen. Z. Wiss. Zool. 1869, XIX, 469–475. [Google Scholar]
- Quentin, J.-C. Essai de classification des Nématodes Rictulaires. Mém. Mus. Natn. Hist. Nat. Sér. A, Zool. 1969, 54, 55–115. [Google Scholar]
- von Willemoes-Suhm, R. Helminlhologische Notizen III. Z. Wiss. Zool. 1873, XXIII, 331–345. [Google Scholar]
- Blanchard, R. Notices helminthologiques (Première série). Bull. Soc. Zool. Fr. 1886, XI, 294–304. [Google Scholar]
- Caspeta-Mandujano, J.M.; Jiménez, F.A.; Peralta-Rodríguez, J.L.; Guerrero, J.A. Pterygodermatites (Pterygodermatites) mexicana n. sp. (Nematoda: Rictulariidae), a parasite of Balantiopteryx plicata (Chiroptera) in Mexico. Parasite 2013, 20, 47. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, P.L. A New Nematode (Rictularia Aethechini sp. nov.) a Physaloptera and an Acanthocephala from the Hedgehog (Aethechinus Frontalis); XVIth Report Director Veterinary Services and Animal Industry: Pretoria, South Africa, 1930; pp. 217–232. [Google Scholar]
- Simões, M.B.; Moreira, N.I.B.; Leite, Y.L. First record of Pterygodermatites (Pterygodermatites) (Nematoda: Rictulariidae) in South America, with the description of a new species from the Atlantic Forest, southeast Brazil. Zootaxa 2019, 4629, 96–108. [Google Scholar] [CrossRef]
- Simões, M.B.; Pinto, H.A.; Moreira, N.I.B. An annotated checklist of the genus Pterygodermatites Wedl, 1861 (Nematoda: Rictulariidae), with notes on hosts and geographical distribution. Syst. Parasitol. 2022, 99, 253–283. [Google Scholar] [CrossRef]
- López, C.; Clemente, S.; Almeida, C.; Brito, A.; Hernández, M. A genetic approach to the origin of Millepora sp. in the eastern Atlantic. Coral Reefs 2015, 34, 631–638. [Google Scholar] [CrossRef]
- Prosser, S.W.J.; Velarde-Aguilar, M.G.; León-Règagnon, V.; Hebert, P.D.N. Advancing nematode barcoding: A primer cocktail for the cytochrome c oxidase subunit I gene from vertebrate parasitic nematodes. Mol. Ecol. Resour. 2013, 13, 1108–1115. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [PubMed]
- Babaev, Y.A.; Kolodenko, A.I. Studies on helminth fauna of insectivorous mammals from the Turkmen-SSR USSR. Izv. Akad. Nauk Turkm. SSR Ser. Biol. Nauk 1975, 4, 71–75. [Google Scholar]
- Tinnin, D.S.; Ganzorig, S.; Gardner, S.L. Helminths of small mammals (Erinaceomorpha, Soricomorpha, Chiroptera, Rodentia, and Lagomorpha) of Mongolia. Fac. Publ. Harold W. Manter Lab. Parasitol. Special Publ. Mus. Texas Tech. Univ. 2011, 59, 1–50. [Google Scholar]
- Chabaud, A.G.; Petter, A.J. Remarques sur l’évolution des papilles cloacales chez les Nématodes phasmidiens parasites de Vertébrés. Parassitologia 1961, 3, 51–70. [Google Scholar]
- Cardia, D.F.; Tebaldi, J.H.; Fornazari, F.; Menozzi, B.D.; Langoni, H.; Nascimento, A.A.; Bresciani, K.D.S.; Hoppe, E.G.L. Pterygodermatites (Paucipectines) andyraicola n. sp. (Spirurida: Rictulariidae), an intestinal nematode of Neotropical Molossidae bats from Brazil. Comp. Parasitol. 2015, 82, 296–300. [Google Scholar] [CrossRef]
- Ezquiaga, M.C.; Rios, T.A.; Abba, A.M.; Navone, G.T. A new Rictulariid (Nematoda: Spirurida) in xenarthrans from Argentina and new morphological data of Pterygodermatites (Paucipectines) chaetophracti. J. Parasitol. 2017, 103, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Lynggaard, C.; García-Prieto, L.; Guzmán-Cornejo, C.; Osorio-Sarabia, D. Pterygodermatites (Paucipectines) baiomydis n. sp. (Nematoda: Rictulariidae), a parasite of Baiomys taylori (Cricetidae). Parasite 2014, 21, 58. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Quentin, J.-C. Un nouveau nématode Rictulaire Pterygodermatites hispanica n. sp., parasite de rongeurs en Espagne. Bull. Mus. Natn. Hist. Nat. 1973, 183, 1395–1401. [Google Scholar]
- Dollfus, R.P.; Desportes, C. Sur le genre Rictularia Froelich 1802 (Nématodes Spiruroidea). Ann. Parasitol. Hum. Comp. 1945, 20, 6–34. [Google Scholar] [CrossRef][Green Version]

| Species | Males | Females | Host Group | Geographical Distribution | References | ||||
|---|---|---|---|---|---|---|---|---|---|
| CP | Spicule length right/left (in µm) | Fans | CP diff. * | VP # | Prevulvar CP/ Total CP | ||||
| P. (P.) aethechini | 50 | Unequal 118–132/190–225 | 1 | Post | Post | 42/75 | Eulipotyphla | South Africa | [13,17] |
| P. (P.) atlanticaensis | – | – | – | Post | Ant | 44–47/56–72 | Chiroptera | Brazil | [18] |
| P. (P.) mexicana | 40 | Unequal 30–50/83–111 | 3–4 | Post | Post | 40/66 | Chiroptera | Mexico | [16] |
| P. (P.) plagiostoma | 49–53 1 | Unequal 98–123/185–236 1 | 1–2 1 | Vulva-Post 1 | Post 1 | 43–46/71–77 1 43–46/72–75 2 43–44/74–77 3 | Eulipotyphla Carnivora? | Saudi Arabia Mainland Spain Egypt Tunisia Algeria Eivissa Island (Spain) Canary Islands (Spain) London Zoo | [1] [2,7] [3,8,13] [4] [5] [6] [Present study] [9,13] |
| P. (P.) shaldybini | – | Unequal 70/140 | – | – | – | 42/84 | Chiroptera Eulipotyphla | Kazakhstan Turkmenistan Mongolia | [13] [25] [26] |
| P. (P.) spinosa | – | – | – | – | – | 43–44/77 | Chiroptera | Germany | [12,13] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miquel, J.; Ribas, A.; Pino-Vera, R.; Izquierdo-Rodríguez, E.; Martín-Carrillo, N.; Feliu, C.; Foronda, P. New Data on Pterygodermatites (Pterygodermatites) plagiostoma Wedl, 1861 (Nematoda, Rictulariidae) Parasite of the Algerian Hedgehog Atelerix algirus Linnaeus, 1758 (Eulipotyphla: Erinaceidae) from the Canary Islands. Animals 2022, 12, 1991. https://doi.org/10.3390/ani12151991
Miquel J, Ribas A, Pino-Vera R, Izquierdo-Rodríguez E, Martín-Carrillo N, Feliu C, Foronda P. New Data on Pterygodermatites (Pterygodermatites) plagiostoma Wedl, 1861 (Nematoda, Rictulariidae) Parasite of the Algerian Hedgehog Atelerix algirus Linnaeus, 1758 (Eulipotyphla: Erinaceidae) from the Canary Islands. Animals. 2022; 12(15):1991. https://doi.org/10.3390/ani12151991
Chicago/Turabian StyleMiquel, Jordi, Alexis Ribas, Román Pino-Vera, Elena Izquierdo-Rodríguez, Natalia Martín-Carrillo, Carlos Feliu, and Pilar Foronda. 2022. "New Data on Pterygodermatites (Pterygodermatites) plagiostoma Wedl, 1861 (Nematoda, Rictulariidae) Parasite of the Algerian Hedgehog Atelerix algirus Linnaeus, 1758 (Eulipotyphla: Erinaceidae) from the Canary Islands" Animals 12, no. 15: 1991. https://doi.org/10.3390/ani12151991
APA StyleMiquel, J., Ribas, A., Pino-Vera, R., Izquierdo-Rodríguez, E., Martín-Carrillo, N., Feliu, C., & Foronda, P. (2022). New Data on Pterygodermatites (Pterygodermatites) plagiostoma Wedl, 1861 (Nematoda, Rictulariidae) Parasite of the Algerian Hedgehog Atelerix algirus Linnaeus, 1758 (Eulipotyphla: Erinaceidae) from the Canary Islands. Animals, 12(15), 1991. https://doi.org/10.3390/ani12151991