Bibliometric Analysis of Literature in Snake Venom-Related Research Worldwide (1933–2022)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
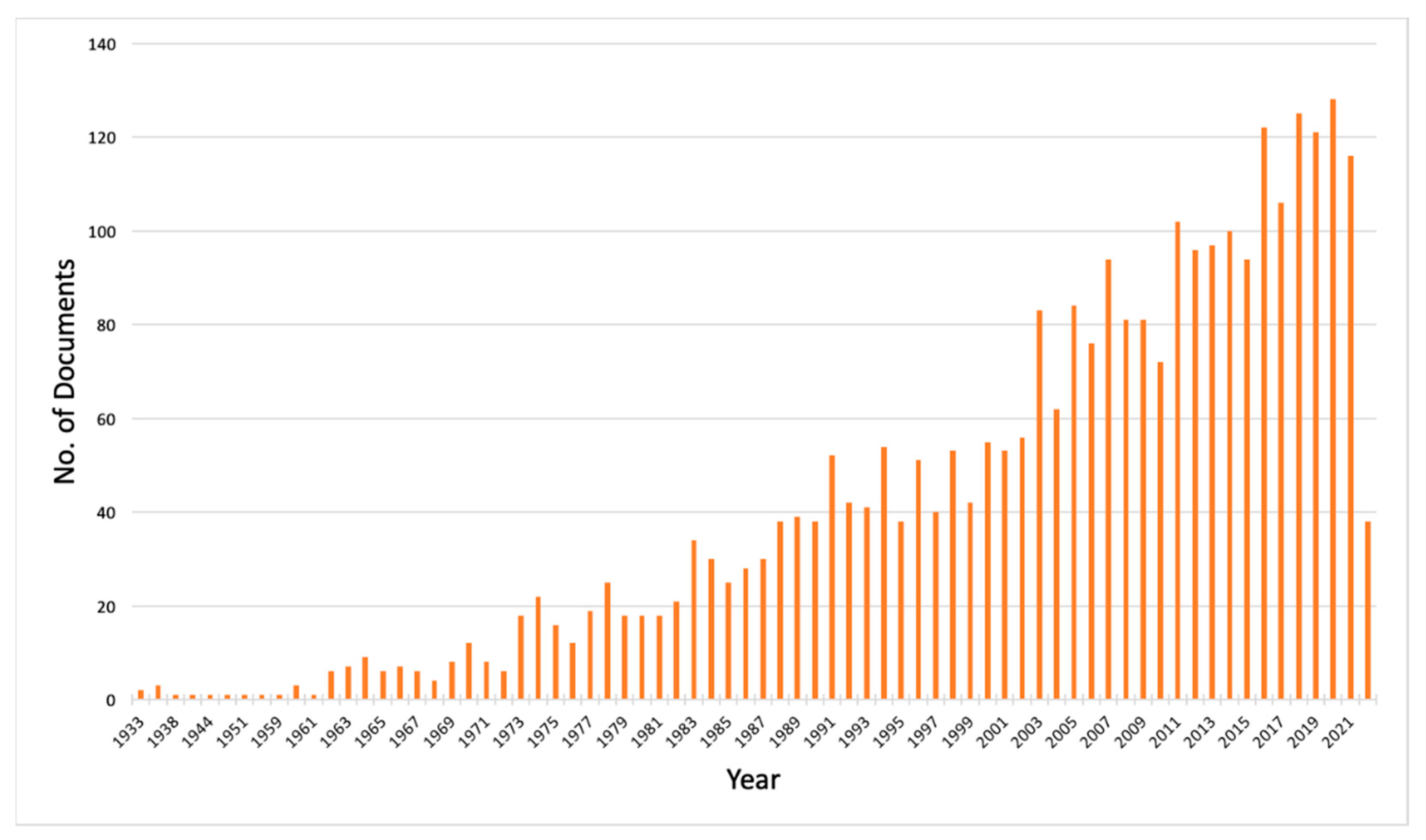
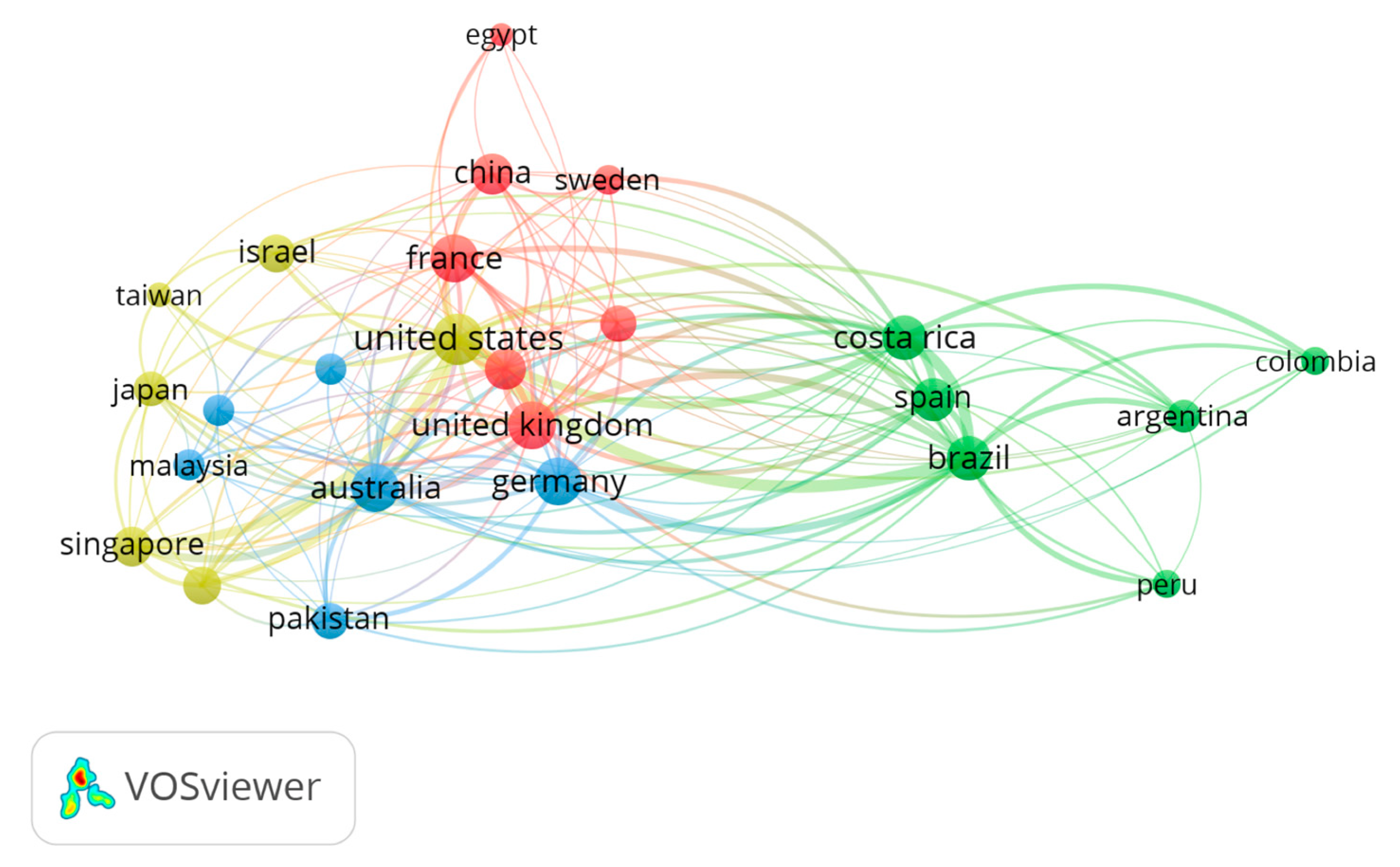
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lüddecke, T.; Herzig, V.; Reumont, B.M.; Vilcinskas, A. The Biology and Evolution of Spider Venoms. Biol. Rev. 2022, 97, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Schendel, V.; Rash, L.D.; Jenner, R.A.; Undheim, E.A. The Diversity of Venom: The Importance of Behavior and Venom System Morphology in Understanding Its Ecology and Evolution. Toxins 2019, 11, 666. [Google Scholar] [CrossRef] [PubMed]
- Suranse, V.; Srikanthan, A.; Sunagar, K. Animal Venoms: Origin, Diversity and Evolution. In eLS; John Wiley & Sons, Ltd., Ed.; Wiley: Hoboken, NJ, USA, 2018; pp. 1–20. ISBN 978-0-470-01617-6. [Google Scholar]
- Walker, A.A. The Evolutionary Dynamics of Venom Toxins Made by Insects and Other Animals. Biochem. Soc. Trans. 2020, 48, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Scheib, H.; van der Weerd, L.; Young, B.; McNaughtan, J.; Ramjan, S.F.R.; Vidal, N.; Poelmann, R.E.; Norman, J.A. Evolution of an Arsenal: Structural and Functional Diversification of the Venom System in the Advanced Snakes (Caenophidia). Mol. Cell. Proteom. 2008, 7, 215–246. [Google Scholar] [CrossRef]
- Bordon, K.d.C.F.; Cologna, C.T.; Fornari-Baldo, E.C.; Pinheiro-Júnior, E.L.; Cerni, F.A.; Amorim, F.G.; Anjolette, F.A.P.; Cordeiro, F.A.; Wiezel, G.A.; Cardoso, I.A.; et al. From Animal Poisons and Venoms to Medicines: Achievements, Challenges and Perspectives in Drug Discovery. Front. Pharm. 2020, 11, 1132. [Google Scholar] [CrossRef]
- Coulter-Parkhill, A.; McClean, S.; Gault, V.A.; Irwin, N. Therapeutic Potential of Peptides Derived from Animal Venoms: Current Views and Emerging Drugs for Diabetes. Clin. Med. Insights Endocrinol Diabetes 2021, 14, 117955142110060. [Google Scholar] [CrossRef]
- Sung, S.-H.; Kim, J.-W.; Han, J.-E.; Shin, B.-C.; Park, J.-K.; Lee, G. Animal Venom for Medical Usage in Pharmacopuncture in Korean Medicine: Current Status and Clinical Implication. Toxins 2021, 13, 105. [Google Scholar] [CrossRef]
- Utkin, Y.N. Animal Venom Studies: Current Benefits and Future Developments. WJBC 2015, 6, 28. [Google Scholar] [CrossRef]
- Chan, Y.S.; Cheung, R.C.F.; Xia, L.; Wong, J.H.; Ng, T.B.; Chan, W.Y. Snake Venom Toxins: Toxicity and Medicinal Applications. Appl. Microbiol. Biotechnol. 2016, 100, 6165–6181. [Google Scholar] [CrossRef]
- Ferraz, C.R.; Arrahman, A.; Xie, C.; Casewell, N.R.; Lewis, R.J.; Kool, J.; Cardoso, F.C. Multifunctional Toxins in Snake Venoms and Therapeutic Implications: From Pain to Hemorrhage and Necrosis. Front. Ecol. Evol. 2019, 7, 218. [Google Scholar] [CrossRef]
- Mohamed Abd El-Aziz, T.; Soares, A.G.; Stockand, J.D. Snake Venoms in Drug Discovery: Valuable Therapeutic Tools for Life Saving. Toxins 2019, 11, 564. [Google Scholar] [CrossRef]
- Ahmed, S.; Koudou, G.B.; Bagot, M.; Drabo, F.; Bougma, W.R.; Pulford, C.; Bockarie, M.; Harrison, R.A. Health and Economic Burden Estimates of Snakebite Management upon Health Facilities in Three Regions of Southern Burkina Faso. PLoS Negl. Trop. Dis. 2021, 15, e0009464. [Google Scholar] [CrossRef]
- Martins, S.B.; Bolon, I.; Alcoba, G.; Ochoa, C.; Torgerson, P.; Sharma, S.K.; Ray, N.; Chappuis, F.; Ruiz de Castañeda, R. Assessment of the Effect of Snakebite on Health and Socioeconomic Factors Using a One Health Perspective in the Terai Region of Nepal: A Cross-Sectional Study. Lancet Glob. Health 2022, 10, e409–e415. [Google Scholar] [CrossRef]
- Kasturiratne, A.; Lalloo, D.G.; Janaka de Silva, H. Chronic Health Effects and Cost of Snakebite. Toxicon X 2021, 9–10, 100074. [Google Scholar] [CrossRef]
- Patikorn, C.; Leelavanich, D.; Ismail, A.K.; Othman, I.; Taychakhoonavudh, S.; Chaiyakunapruk, N. Global Systematic Review of Cost of Illness and Economic Evaluation Studies Associated with Snakebite. J. Glob. Health 2020, 10, 020415. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite Envenoming. Nat. Rev. Dis. Primers 2017, 3, 17063. [Google Scholar] [CrossRef]
- Harrison, R.A.; Casewell, N.R.; Ainsworth, S.A.; Lalloo, D.G. The Time Is Now: A Call for Action to Translate Recent Momentum on Tackling Tropical Snakebite into Sustained Benefit for Victims. Trans. R. Soc. Trop. Med. Hyg. 2019, 113, 835–838. [Google Scholar] [CrossRef]
- Kasturiratne, A.; Wickremasinghe, A.R.; de Silva, N.; Gunawardena, N.K.; Pathmeswaran, A.; Premaratna, R.; Savioli, L.; Lalloo, D.G.; de Silva, H.J. The Global Burden of Snakebite: A Literature Analysis and Modelling Based on Regional Estimates of Envenoming and Deaths. PLoS Med. 2008, 5, e218. [Google Scholar] [CrossRef]
- The Lancet Snake-Bite Envenoming: A Priority Neglected Tropical Disease. Lancet 2017, 390, 2. [CrossRef]
- Slagboom, J.; Kool, J.; Harrison, R.A.; Casewell, N.R. Haemotoxic Snake Venoms: Their Functional Activity, Impact on Snakebite Victims and Pharmaceutical Promise. Br. J. Haematol. 2017, 177, 947–959. [Google Scholar] [CrossRef]
- Tasoulis, T.; Isbister, G.K. A Review and Database of Snake Venom Proteomes. Toxins 2017, 9, 290. [Google Scholar] [CrossRef]
- Vonk, F.J.; Jackson, K.; Doley, R.; Madaras, F.; Mirtschin, P.J.; Vidal, N. Snake Venom: From Fieldwork to the Clinic: Recent Insights into Snake Biology, Together with New Technology Allowing High-Throughput Screening of Venom, Bring New Hope for Drug Discovery. Bioessays 2011, 33, 269–279. [Google Scholar] [CrossRef]
- Casewell, N.R.; Wüster, W.; Vonk, F.J.; Harrison, R.A.; Fry, B.G. Complex Cocktails: The Evolutionary Novelty of Venoms. Trends Ecol. Evol. 2013, 28, 219–229. [Google Scholar] [CrossRef]
- Fry, B.G.; Wüster, W.; Kini, R.M.; Brusic, V.; Khan, A.; Venkataraman, D.; Rooney, A.P. Molecular Evolution and Phylogeny of Elapid Snake Venom Three-Finger Toxins. J. Mol. Evol. 2003, 57, 110–129. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Rucavado, A.; Escalante, T.; Díaz, C. Hemorrhage Induced by Snake Venom Metalloproteinases: Biochemical and Biophysical Mechanisms Involved in Microvessel Damage. Toxicon 2005, 45, 997–1011. [Google Scholar] [CrossRef]
- Gutiérrez, J.; Rucavado, A. Snake Venom Metalloproteinases: Their Role in the Pathogenesis of Local Tissue Damage. Biochimie 2000, 82, 841–850. [Google Scholar] [CrossRef]
- Harris, J.; Scott-Davey, T. Secreted Phospholipases A2 of Snake Venoms: Effects on the Peripheral Neuromuscular System with Comments on the Role of Phospholipases A2 in Disorders of the CNS and Their Uses in Industry. Toxins 2013, 5, 2533–2571. [Google Scholar] [CrossRef]
- Lynch, V.J. Inventing an Arsenal: Adaptive Evolution and Neofunctionalization of Snake Venom Phospholipase A2 Genes. BMC Evol. Biol. 2007, 7, 2. [Google Scholar] [CrossRef]
- Rivel, M.; Solano, D.; Herrera, M.; Vargas, M.; Villalta, M.; Segura, Á.; Arias, A.S.; León, G.; Gutiérrez, J.M. Pathogenesis of Dermonecrosis Induced by Venom of the Spitting Cobra, Naja Nigricollis: An Experimental Study in Mice. Toxicon 2016, 119, 171–179. [Google Scholar] [CrossRef]
- Tsetlin, V.I. Three-Finger Snake Neurotoxins and Ly6 Proteins Targeting Nicotinic Acetylcholine Receptors: Pharmacological Tools and Endogenous Modulators. Trends Pharmacol. Sci. 2015, 36, 109–123. [Google Scholar] [CrossRef]
- Seo, Y.H.; Park, M.R.; Yoo, S.H. Development of Complex Regional Pain Syndrome after a Snake Bite: A Case Report. Korean J. Pain. 2014, 27, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Kleggetveit, I.P.; Skulberg, P.K.; Jørum, E. Complex Regional Pain Syndrome Following Viper-Bite. Scand. J. Pain 2016, 10, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Jayawardana, S.; Gnanathasan, A.; Arambepola, C.; Chang, T. Chronic Musculoskeletal Disabilities Following Snake Envenoming in Sri Lanka: A Population-Based Study. PLoS Negl. Trop. Dis. 2016, 10, e0005103. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Peinado, C.; Defaus, S.; Andreu, D. Hitchhiking with Nature: Snake Venom Peptides to Fight Cancer and Superbugs. Toxins 2020, 12, 255. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.H. Snake Venomics: Fundamentals, Recent Updates, and a Look to the Next Decade. Toxins 2022, 14, 247. [Google Scholar] [CrossRef]
- Li, S.-A.; Lee, W.-H.; Zhang, Y. Efficacy of OH-CATH30 and Its Analogs against Drug-Resistant Bacteria In Vitro and in Mouse Models. Antimicrob. Agents Chemother. 2012, 56, 3309–3317. [Google Scholar] [CrossRef]
- Cai, S.; Qiao, X.; Feng, L.; Shi, N.; Wang, H.; Yang, H.; Guo, Z.; Wang, M.; Chen, Y.; Wang, Y.; et al. Python Cathelicidin CATHPb1 Protects against Multidrug-Resistant Staphylococcal Infections by Antimicrobial-Immunomodulatory Duality. J. Med. Chem. 2018, 61, 2075–2086. [Google Scholar] [CrossRef]
- Zhao, F.; Lan, X.-Q.; Du, Y.; Chen, P.-Y.; Zhao, J.; Zhao, F.; Lee, W.-H.; Zhang, Y. King Cobra Peptide OH-CATH30 as a Potential Candidate Drug through Clinic Drug-Resistant Isolates. Zool. Res. 2018, 39, 87–96. [Google Scholar] [CrossRef]
- Tajbakhsh, M.; Karimi, A.; Tohidpour, A.; Abbasi, N.; Fallah, F.; Akhavan, M.M. The Antimicrobial Potential of a New Derivative of Cathelicidin from Bungarus Fasciatus against Methicillin-Resistant Staphylococcus Aureus. J. Microbiol. 2018, 56, 128–137. [Google Scholar] [CrossRef]
- Perumal Samy, R.; Pachiappan, A.; Gopalakrishnakone, P.; Thwin, M.M.; Hian, Y.E.; Chow, V.T.; Bow, H.; Weng, J.T. In Vitro Antimicrobial Activity of Natural Toxins and Animal Venoms Tested against Burkholderia Pseudomallei. BMC Infect Dis. 2006, 6, 100. [Google Scholar] [CrossRef]
- Samy, R.P.; Gopalakrishnakone, P.; Chow, V.T.K.; Ho, B. Viper Metalloproteinase (Agkistrodon halys Pallas) with Antimicrobial Activity against Multi-Drug Resistant Human Pathogens. J. Cell. Physiol. 2008, 216, 54–68. [Google Scholar] [CrossRef]
- Samy, R.P.; Stiles, B.G.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Franco, O.L.; Rowan, E.G.; Kumar, A.P.; Lim, L.H.K.; Sethi, G. Viperatoxin-II: A Novel Viper Venom Protein as an Effective Bactericidal Agent. FEBS Open Bio 2015, 5, 928–941. [Google Scholar] [CrossRef]
- Xie, J.P.; Yue, J.; Xiong, Y.L.; Wang, W.Y.; Yu, S.Q.; Wang, H.H. In Vitro Activities of Small Peptides from Snake Venom against Clinical Isolates of Drug-Resistant Mycobacterium Tuberculosis. Int. J. Antimicrob. Agents 2003, 22, 172–174. [Google Scholar] [CrossRef]
- Almeida, J.R.; Mendes, B.; Lancellotti, M.; Marangoni, S.; Vale, N.; Passos, Ó.; Ramos, M.J.; Fernandes, P.A.; Gomes, P.; Da Silva, S.L. A Novel Synthetic Peptide Inspired on Lys49 Phospholipase A 2 from Crotalus Oreganus Abyssus Snake Venom Active against Multidrug-Resistant Clinical Isolates. Eur. J. Med. Chem. 2018, 149, 248–256. [Google Scholar] [CrossRef]
- Trikha, M.; De Clerck, Y.A.; Markland, F.S. Contortrostatin, a Snake Venom Disintegrin, Inhibits Β1 Integrin-Mediated Human Metastatic Melanoma Cell Adhesion and Blocks Experimental Metastasis. Cancer Res. 1994, 54, 4993–4998. [Google Scholar]
- Zhou, Q.; Sherwin, R.P.; Parrish, C.; Richters, V.; Groshen, S.G.; Tsao-Wei, D.; Markland, F.S. Contortrostatin, a Dimeric Disintegrin from Contortrix Contortrix, Inhibits Breast Cancer Progression. Breast Cancer Res. Treat. 2000, 61, 249–259. [Google Scholar] [CrossRef]
- Schmitmeier, S.; Markland, F.S.; Ritter, M.R.; Sawcer, D.E.; Chen, T.C. Functional Effect of Contortrostatin, a Snake Venom Disintegrin, on Human Glioma Cell Invasion In Vitro. Cell Commun. Adhes. 2003, 10, 1–16. [Google Scholar] [CrossRef]
- Huang, M.; Wu, S.; Hu, Q.; Wu, H.; Wei, S.; Xie, H.; Sun, K.; Li, X.; Fang, L. Agkihpin, a Novel SVAE May Inhibit the Migration and Invasion of Liver Cancer Cells Associated with the Inversion of EMT Induced by Wnt/β-Catenin Signaling Inhibition. Biochem. Biophys. Res. Commun. 2016, 479, 283–289. [Google Scholar] [CrossRef]
- Yen, C.-Y.; Liang, S.-S.; Han, L.-Y.; Chou, H.-L.; Chou, C.-K.; Lin, S.-R.; Chiu, C.-C. Cardiotoxin III Inhibits Proliferation and Migration of Oral Cancer Cells through MAPK and MMP Signaling. Sci. World J. 2013, 2013, 650946. [Google Scholar] [CrossRef]
- Latinović, Z.; Leonardi, A.; Petan, T.; Žlajpah, M.; Križaj, I. Disintegrins from the Venom of Vipera Ammodytes Ammodytes Efficiently Inhibit Migration of Breast Cancer Cells. ACSi 2017, 64, 555–559. [Google Scholar] [CrossRef]
- Naumann, G.B.; Silva, L.F.; Silva, L.; Faria, G.; Richardson, M.; Evangelista, K.; Kohlhoff, M.; Gontijo, C.M.F.; Navdaev, A.; de Rezende, F.F.; et al. Cytotoxicity and Inhibition of Platelet Aggregation Caused by an L-Amino Acid Oxidase from Bothrops Leucurus Venom. Biochim. Biophys Acta Gen. Subj. 2011, 1810, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; He, Q.; Yu, X.; Li, B.; Song, Z. The Anti-Ovarian Carcinoma Activity of l-Amino Acid Oxidase from Crotalus Adamanteus Venom in Vivo and in Vitro. Med. Oncol. 2022, 39, 112. [Google Scholar] [CrossRef] [PubMed]
- Daghestani, M.; Hakami, H.H.; Hassan, Z.K.; Badr, G.; Amin, M.H.; Amin, M.H.; Shafi Bhat, R. The Anti-Cancer Effect of Echis Coloratus and Walterinnesia Aegyptia Venoms on Colon Cancer Cells. Toxin Rev. 2021, 40, 257–266. [Google Scholar] [CrossRef]
- Alves, R.M.; Antonucci, G.A.; Paiva, H.H.; Cintra, A.C.O.; Franco, J.J.; Mendonça-Franqueiro, E.P.; Dorta, D.J.; Giglio, J.R.; Rosa, J.C.; Fuly, A.L.; et al. Evidence of Caspase-Mediated Apoptosis Induced by l-Amino Acid Oxidase Isolated from Bothrops Atrox Snake Venom. Comp. Biochem. Physiol. Part. A Mol. Integr. Physiol. 2008, 151, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Son, D.J.; Park, M.H.; Chae, S.J.; Moon, S.O.; Lee, J.W.; Song, H.S.; Moon, D.C.; Kang, S.S.; Kwon, Y.E.; Hong, J.T. Inhibitory Effect of Snake Venom Toxin from Vipera Lebetina Turanica on Hormone-Refractory Human Prostate Cancer Cell Growth: Induction of Apoptosis through Inactivation of Nuclear Factor ΚB. Mol. Cancer Ther. 2007, 6, 675–683. [Google Scholar] [CrossRef]
- Mukherjee, A.K.; Saviola, A.J.; Burns, P.D.; Mackessy, S.P. Apoptosis Induction in Human Breast Cancer (MCF-7) Cells by a Novel Venom l-Amino Acid Oxidase (Rusvinoxidase) Is Independent of Its Enzymatic Activity and Is Accompanied by Caspase-7 Activation and Reactive Oxygen Species Production. Apoptosis 2015, 20, 1358–1372. [Google Scholar] [CrossRef]
- Park, M.H.; Jo, M.; Won, D.; Song, H.S.; Han, S.B.; Song, M.J.; Hong, J.T. Snake Venom Toxin from Vipera Lebetina Turanicainduces Apoptosis of Colon Cancer Cells via Upregulation of ROS- and JNK-Mediated Death Receptor Expression. BMC Cancer 2012, 12, 228. [Google Scholar] [CrossRef]
- Song, J.K.; Jo, M.R.; Park, M.H.; Song, H.S.; An, B.J.; Song, M.J.; Han, S.B.; Hong, J.T. Cell Growth Inhibition and Induction of Apoptosis by Snake Venom Toxin in Ovarian Cancer Cell via Inactivation of Nuclear Factor ΚB and Signal Transducer and Activator of Transcription 3. Arch. Pharm. Res. 2012, 35, 867–876. [Google Scholar] [CrossRef]
- Park, M.H.; Son, D.J.; Kwak, D.H.; Song, H.S.; Oh, K.-W.; Yoo, H.-S.; Lee, Y.M.; Song, M.J.; Hong, J.T. Snake Venom Toxin Inhibits Cell Growth through Induction of Apoptosis in Neuroblastoma Cells. Arch. Pharm. Res. 2009, 32, 1545–1554. [Google Scholar] [CrossRef]
- Zhang, L.; Cui, L. A Cytotoxin Isolated from Agkistrodon Acutus Snake Venom Induces Apoptosis via Fas Pathway in A549 Cells. Toxicol. In Vitro 2007, 21, 1095–1103. [Google Scholar] [CrossRef]
- Zakraoui, O.; Marcinkiewicz, C.; Aloui, Z.; Othman, H.; Grépin, R.; Haoues, M.; Essafi, M.; Srairi-Abid, N.; Gasmi, A.; Karoui, H.; et al. Lebein, a Snake Venom Disintegrin, Suppresses Human Colon Cancer Cells Proliferation and Tumor-Induced Angiogenesis through Cell Cycle Arrest, Apoptosis Induction and Inhibition of VEGF Expression: Mechanisms and Targets for Lebein in Colorectal Cancer. Mol. Carcinog. 2017, 56, 18–35. [Google Scholar] [CrossRef]
- Costa, T.R.; Menaldo, D.L.; Zoccal, K.F.; Burin, S.M.; Aissa, A.F.; Castro, F.A.d.; Faccioli, L.H.; Greggi Antunes, L.M.; Sampaio, S.V. CR-LAAO, an L-Amino Acid Oxidase from Calloselasma Rhodostoma Venom, as a Potential Tool for Developing Novel Immunotherapeutic Strategies against Cancer. Sci. Rep. 2017, 7, 42673. [Google Scholar] [CrossRef]
- Prinholato da Silva, C.; Costa, T.R.; Paiva, R.M.A.; Cintra, A.C.O.; Menaldo, D.L.; Antunes, L.M.G.; Sampaio, S.V. Antitumor Potential of the Myotoxin BthTX-I from Bothrops Jararacussu Snake Venom: Evaluation of Cell Cycle Alterations and Death Mechanisms Induced in Tumor Cell Lines. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015, 21, 44. [Google Scholar] [CrossRef]
- Ebrahim, K.; Vatanpour, H.; Zare, A.; Shirazi, F.H.; Nakhjavani, M. Anticancer Activity a of Caspian Cobra (Naja naja oxiana) Snake Venom in Human Cancer Cell Lines Via Induction of Apoptosis. IJPR 2016, 15, 101–112. [Google Scholar]
- Al-Asmari, A.; Anvarbatcha, R.; Al-Shahrani, M.; Islam, M. Snake Venom Causes Apoptosis by Increasing the Reactive Oxygen Species in Colorectal and Breast Cancer Cell Lines. OTT 2016, 9, 6485–6498. [Google Scholar] [CrossRef]
- Lee, H.L.; Park, M.H.; Hong, J.E.; Kim, D.H.; Kim, J.Y.; Seo, H.O.; Han, S.-B.; Yoon, J.H.; Lee, W.H.; Song, H.S.; et al. Inhibitory Effect of Snake Venom Toxin on NF-ΚB Activity Prevents Human Cervical Cancer Cell Growth via Increase of Death Receptor 3 and 5 Expression. Arch. Toxicol. 2016, 90, 463–477. [Google Scholar] [CrossRef]
- Bernardes-Oliveira, E.; Gomes, D.L.; Martelli Palomino, G.; Juvenal Silva Farias, K.; da Silva, W.D.; Rocha, H.A.O.; Gonçalves, A.K.; Fernandes-Pedrosa, M.d.F.; Crispim, J.C.d.O. Bothrops Jararaca and Bothrops Erythromelas Snake Venoms Promote Cell Cycle Arrest and Induce Apoptosis via the Mitochondrial Depolarization of Cervical Cancer Cells. Evid. Based Complementary Altern. Med. 2016, 2016, 1574971. [Google Scholar] [CrossRef]
- Tran, T.V.; Siniavin, A.E.; Hoang, A.N.; Le, M.; Pham, C.D.; Phung, T.V.; Nguyen, K.C.; Ziganshin, R.H.; Tsetlin, V.I.; Weng, C.F.; et al. Phospholipase A2 from Krait Bungarus Fasciatus Venom Induces Human Cancer Cell Death In Vitro. PeerJ 2019, 7, 22. [Google Scholar] [CrossRef]
- Jiménez–Charris, E.; Lopes, D.S.; Gimenes, S.N.C.; Teixeira, S.C.; Montealegre–Sánchez, L.; Solano–Redondo, L.; Fierro–Pérez, L.; Rodrigues Ávila, V. de M. Antitumor Potential of Pllans–II, an Acidic Asp49–PLA2 from Porthidium Lansbergii Lansbergii Snake Venom on Human Cervical Carcinoma HeLa Cells. Int. J. Biol. Macromol. 2019, 122, 1053–1061. [Google Scholar] [CrossRef]
- Hammouda, M.; Montenegro, M.; Sánchez-del-Campo, L.; Zakraoui, O.; Aloui, Z.; Riahi-Chebbi, I.; Karoui, H.; Rodríguez-López, J.; Essafi-Benkhadir, K. Lebein, a Snake Venom Disintegrin, Induces Apoptosis in Human Melanoma Cells. Toxins 2016, 8, 206. [Google Scholar] [CrossRef]
- Bezerra, P.H.A.; Ferreira, I.M.; Franceschi, B.T.; Bianchini, F.; Ambrósio, L.; Cintra, A.C.O.; Sampaio, S.V.; Castro, F.A.d.; Torqueti, M.R. BthTX-I from Bothrops Jararacussu Induces Apoptosis in Human Breast Cancer Cell Lines and Decreases Cancer Stem Cell Subpopulation. J. Venom. Anim. Toxins Incl. Trop. Dis. 2019, 25, e20190010. [Google Scholar] [CrossRef] [PubMed]
- Ri, S. Cerastes Cerastes and Vipera Lebetina Snake Venoms Apoptotic—Stimulating Activity to Human Breast Cancer Cells and Related Gene Modulation. JCST 2012, 4, 317–323. [Google Scholar] [CrossRef]
- Nikodijević, D.D.; Jovankić, J.V.; Cvetković, D.M.; Anđelković, M.Z.; Nikezić, A.G.; Milutinović, M.G. L-Amino Acid Oxidase from Snake Venom: Biotransformation and Induction of Apoptosis in Human Colon Cancer Cells. Eur. J. Pharm. 2021, 910, 174466. [Google Scholar] [CrossRef] [PubMed]
- Bazaa, A.; Luis, J.; Srairi-Abid, N.; Kallech-Ziri, O.; Kessentini-Zouari, R.; Defilles, C.; Lissitzky, J.-C.; El Ayeb, M.; Marrakchi, N. MVL-PLA2, a Phospholipase A2 from Macrovipera Lebetina Transmediterranea Venom, Inhibits Tumor Cells Adhesion and Migration. Matrix Biol. 2009, 28, 188–193. [Google Scholar] [CrossRef]
- Sánchez, E.E.; Rodríguez-Acosta, A.; Palomar, R.; Lucena, S.E.; Bashir, S.; Soto, J.G.; Pérez, J.C. Colombistatin: A Disintegrin Isolated from the Venom of the South American Snake (Bothrops colombiensis) That Effectively Inhibits Platelet Aggregation and SK-Mel-28 Cell Adhesion. Arch. Toxicol. 2009, 83, 271–279. [Google Scholar] [CrossRef]
- Saviola, A.J.; Burns, P.D.; Mukherjee, A.K.; Mackessy, S.P. The Disintegrin Tzabcanin Inhibits Adhesion and Migration in Melanoma and Lung Cancer Cells. Int. J. Biol. Macromol. 2016, 88, 457–464. [Google Scholar] [CrossRef]
- Li Lee, M.; Chung, I.; Yee Fung, S.; Kanthimathi, M.S.; Hong Tan, N. Antiproliferative Activity of King Cobra (Ophiophagus Hannah) Venom l -Amino Acid Oxidase. Basic Clin. Pharm. Toxicol. 2014, 114, 336–343. [Google Scholar] [CrossRef]
- Teixeira, T.L.; Oliveira Silva, V.A.; da Cunha, D.B.; Polettini, F.L.; Thomaz, C.D.; Pianca, A.A.; Zambom, F.L.; da Silva Leitão Mazzi, D.P.; Reis, R.M.; Mazzi, M.V. Isolation, Characterization and Screening of the in Vitro Cytotoxic Activity of a Novel L-Amino Acid Oxidase (LAAOcdt) from Crotalus Durissus Terrificus Venom on Human Cancer Cell Lines. Toxicon 2016, 119, 203–217. [Google Scholar] [CrossRef]
- Kim, D.S.; Jang, Y.-J.; Jeon, O.-H.; Kim, D.-S. Saxatilin Inhibits TNF-α-Induced Proliferation by Suppressing AP-1-Dependent IL-8 Expression in the Ovarian Cancer Cell Line MDAH 2774. Mol. Immunol. 2007, 44, 1409–1416. [Google Scholar] [CrossRef]
- Karthikeyan, R.; Karthigayan, S.; Sri Balasubashini, M.; Somasundaram, S.T.; Balasubramanian, T. Inhibition of Hep2 and HeLa Cell Proliferation in Vitro and EAC Tumor Growth in Vivo by Lapemis Curtus (Shaw 1802) Venom. Toxicon 2008, 51, 157–161. [Google Scholar] [CrossRef]
- Chernyshenko, V.; Petruk, N.; Korolova, D.; Kasatkina, L.; Gornytska, O.; Platonova, T.; Chernyshenko, T.; Rebriev, A.; Dzhus, O.; Garmanchuk, L.; et al. Antiplatelet and Anti-Proliferative Action of Disintegrin from Echis Multisquamatis Snake Venom. Croat. Med. J. 2017, 58, 118–127. [Google Scholar] [CrossRef]
- Karthikeya, R.; Karthigaya, S.; Balasubash, M.S.; Vijayalaks, S.; Somasundar, S.T.; Balasubram, T. Inhibition of Cancer Cell Proliferation in Vitro and Tumor Growth in Vivo by Hydrophis Spiralis Sea Snake Venom. Int. J. Cancer Res. 2007, 3, 186–190. [Google Scholar] [CrossRef]
- Malekara, E.; Pazhouhi, M.; Rashidi, I.; Jalili, C. Anti-Proliferative and Cytotoxic Effect of Iranian Snake (Vipera raddei kurdistanica) Venom on Human Breast Cancer Cells via Reactive Oxygen Species-Mediated Apoptosis. Res. Pharma Sci. 2020, 15, 76. [Google Scholar] [CrossRef]
- Jang, Y.-J.; Kim, D.-S.; Jeon, O.-H.; Kim, D.-S. Saxatilin Suppresses Tumor-Induced Angiogenesis by Regulating VEGF Expression in NCI-H460 Human Lung Cancer Cells. BMB Rep. 2007, 40, 439–443. [Google Scholar] [CrossRef]
- Momic, T.; Cohen, G.; Reich, R.; Arlinghaus, F.T.; Eble, J.A.; Marcinkiewicz, C.; Lazarovici, P. Vixapatin (VP12), a C-Type Lectin-Protein from Vipera Xantina Palestinae Venom: Characterization as a Novel Anti-Angiogenic Compound. Toxins 2012, 4, 862–877. [Google Scholar] [CrossRef]
- Guimarães, D.d.O.; Lopes, D.S.; Azevedo, F.V.P.V.; Gimenes, S.N.C.; Silva, M.A.; Achê, D.C.; Gomes, M.S.R.; Vecchi, L.; Goulart, L.R.; Yoneyama, K.A.G.; et al. In Vitro Antitumor and Antiangiogenic Effects of Bothropoidin, a Metalloproteinase from Bothrops Pauloensis Snake Venom. Int. J. Biol. Macromol. 2017, 97, 770–777. [Google Scholar] [CrossRef]
- Zhao, Y.S.; Yang, H.L.; Liu, C.Z. Inhibitory Effects of Immunotargeting of Chinese Cobra Cytotoxin and Iodine-131 against Nasopharyngeal Carcinoma Cells in Vitro. J. South. Med. Univ. 2008, 28, 1235–1236. [Google Scholar]
- Al-Sadoon, M.K.; Abdel-Maksoud, M.A.; Rabah, D.M.; Badr, G. Induction of Apoptosis and Growth Arrest in Human Breast Carcinoma Cells by a Snake (Walterinnesia Aegyptia) Venom Combined with Silica Nanoparticles: Crosstalk Between Bcl2 and Caspase 3. Cell. Physiol Biochem. 2012, 30, 653–665. [Google Scholar] [CrossRef]
- Al-Sadoon, M.K.; Rabah, D.M.; Badr, G. Enhanced Anticancer Efficacy of Snake Venom Combined with Silica Nanoparticles in a Murine Model of Human Multiple Myeloma: Molecular Targets for Cell Cycle Arrest and Apoptosis Induction. Cell. Immunol. 2013, 284, 129–138. [Google Scholar] [CrossRef]
- Chippaux, J.-P. Snakebite Envenomation Turns Again into a Neglected Tropical Disease! J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 38. [Google Scholar] [CrossRef]
- Hanney, S.R.; González-Block, M.A. Organising Health Research Systems as a Key to Improving Health: The World Health Report 2013 and How to Make Further Progress. Health Res. Policy Syst. 2013, 11, 47. [Google Scholar] [CrossRef]
- Bai, J.; Li, W.; Huang, Y.-M.; Guo, Y. Bibliometric Study of Research and Development for Neglected Diseases in the BRICS. Infect. Dis. Poverty 2016, 5, 89. [Google Scholar] [CrossRef]
- Fontecha, G.; Sánchez, A.; Ortiz, B. Publication Trends in Neglected Tropical Diseases of Latin America and the Caribbean: A Bibliometric Analysis. Pathogens 2021, 10, 356. [Google Scholar] [CrossRef]
- Sweileh, W.M. Contribution of Researchers in Arab Countries to Scientific Publications on Neglected Tropical Diseases (1971–2020). Trop. Dis. Travel Med. Vaccines 2022, 8, 14. [Google Scholar] [CrossRef]
- Burnham, J.F. Scopus Database: A Review. Biomed. Digit. Libr. 2006, 3, 1. [Google Scholar] [CrossRef]
- Falagas, M.E.; Pitsouni, E.I.; Malietzis, G.A.; Pappas, G. Comparison of PubMed, Scopus, Web of Science, and Google Scholar: Strengths and Weaknesses. FASEB J. 2008, 22, 338–342. [Google Scholar] [CrossRef]
- Kulkarni, A.V. Comparisons of Citations in Web of Science, Scopus, and Google Scholar for Articles Published in General Medical Journals. JAMA 2009, 302, 1092. [Google Scholar] [CrossRef]
- Pranckutė, R. Web of Science (WoS) and Scopus: The Titans of Bibliographic Information in Today’s Academic World. Publications 2021, 9, 12. [Google Scholar] [CrossRef]
- van Eck, N.J.; Waltman, L. Software Survey: VOSviewer, a Computer Program for Bibliometric Mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef]
- Bode, W.; Gomis-Rüth, F.-X.; Stöckler, W. Astacins, Serralysins, Snake Venom and Matrix Metalloproteinases Exhibit Identical Zinc-Binding Environments (HEXXHXXGXXH and Met-Turn) and Topologies and Should Be Grouped into a Common Family, the ‘Metzincins’. FEBS Lett. 1993, 331, 134–140. [Google Scholar] [CrossRef]
- Markland, F.S. Snake Venoms and the Hemostatic System. Toxicon 1998, 36, 1749–1800. [Google Scholar] [CrossRef]
- Bjarnason, J.B.; Foxt, J.W. Hemorrhagic Metalloproteinase from Snake Venoms. Pharmacol. Ther. 1994, 62, 325–372. [Google Scholar] [CrossRef]
- Theakston, R.D.G.; Reid, H.A. Development of Simple Standard Assay Procedures for the Characterization of Snake Venoms. Bull. World Health Organ. 1983, 61, 949–956. [Google Scholar] [PubMed]
- Daltry, J.C.; Wüster, W.; Thorpe, R.S. Diet and Snake Venom Evolution. Nature 1996, 379, 537–540. [Google Scholar] [CrossRef]
- Fry, B.G.; Vidal, N.; Norman, J.A.; Vonk, F.J.; Scheib, H.; Ramjan, S.F.R.; Kuruppu, S.; Fung, K.; Blair Hedges, S.; Richardson, M.K.; et al. Evolution of an Arsenal: Structural and Functional Diversification of the Venom System in the Advanced Snakes (Caenophidia). Nature 2006, 439, 584–588. [Google Scholar] [CrossRef]
- Gutiérrez, J.; Lomonte, B. Phospholipase A2 Myotoxins from Bothrops Snake Venoms. Toxicon 1995, 33, 1405–1424. [Google Scholar] [CrossRef]
- Fox, J.W.; Serrano, S.M.T. Structural Considerations of the Snake Venom Metalloproteinases, Key Members of the M12 Reprolysin Family of Metalloproteinases. Toxicon 2005, 45, 969–985. [Google Scholar] [CrossRef]
- Matsui, T.; Fujimura, Y.; Titani, K. Snake Venom Proteases Affecting Hemostasis and Thrombosis. Biochim. Biophys. Acta 2000, 1477, 146–156. [Google Scholar] [CrossRef]
- Mebs, D. History of the International Society on Toxinology—A Personal View. Toxicon 2013, 69, 21–28. [Google Scholar] [CrossRef]
- Russell, F.E. History of the International Society on Toxinology and Toxicon. Toxicon 1987, 25, 3–21. [Google Scholar] [CrossRef]
- De Vries, A.; Kirschmann, C.; Klibansky, C.; Condrea, E.; Gitter, S. Hemolytic Action of Indirect Lytic Snake Venom In Vivo. Toxicon 1962, 1, 19–23. [Google Scholar] [CrossRef]
- Nagamizu, M.; Komori, Y.; Uchiya, K.; Nikai, T.; Tu, A. Isolation and Chemical Characterization of a Toxin Isolated from the Venom of the Sea Snake, Hydrophis Torquatus Aagardi. Toxins 2009, 1, 162–172. [Google Scholar] [CrossRef]
- Nogueira, C.C.; Argôlo, A.J.S.; Arzamendia, V.; Azevedo, J.A.; Barbo, F.E.; Bérnils, R.S.; Bolochio, B.E.; Borges-Martins, M.; Brasil-Godinho, M.; Braz, H.; et al. Atlas of Brazilian Snakes: Verified Point-Locality Maps to Mitigate the Wallacean Shortfall in a Megadiverse Snake Fauna. S. Am. J. Herpetol. 2019, 14, 1. [Google Scholar] [CrossRef]
- Guedes, T.B.; Nogueira, C.; Marques, O.A.V. Diversity, Natural History, and Geographic Distribution of Snakes in the Caatinga, Northeastern Brazil. Zootaxa 2014, 3863, 1. [Google Scholar] [CrossRef]
- Feitosa, E.S.; Sampaio, V.; Sachett, J.; Castro, D.B.d.; Noronha, M.d.D.N.; Lozano, J.L.L.; Muniz, E.; Ferreira, L.C.d.L.; Lacerda, M.V.G.d.; Monteiro, W.M. Snakebites as a Largely Neglected Problem in the Brazilian Amazon: Highlights of the Epidemiological Trends in the State of Amazonas. Rev. Soc. Bras. Med. Trop. 2015, 48, 34–41. [Google Scholar] [CrossRef]
- Melo Araújo, S.C.; Ceron, K.; Guedes, T.B. Use of Geospatial Analyses to Address Snakebite Hotspots in Mid-Northern Brazil—A Direction to Health Planning in Shortfall Biodiversity Knowledge Areas. Toxicon 2022, 213, 43–51. [Google Scholar] [CrossRef]
- Mise, Y.; Lira-da-Silva, R.; Carvalho, F. Time to Treatment and Severity of Snake Envenoming in Brazil. Rev. Panam. Salud Publica 2018, 42, e52. [Google Scholar] [CrossRef]
- Rocha, G.d.S.; Farias, A.S.; Alcântara, J.A.; Machado, V.A.; Murta, F.; Val, F.; Cristino, J.S.; Santos, A.C.; Ferreira, M.B.; Marques, L.; et al. Validation of a Culturally Relevant Snakebite Envenomation Clinical Practice Guideline in Brazil. Toxins 2022, 14, 376. [Google Scholar] [CrossRef]
- Roriz, K.R.P.S.; Zaqueo, K.D.; Setubal, S.S.; Katsuragawa, T.H.; Silva, R.R.d.; Fernandes, C.F.C.; Cardoso, L.A.P.; Rodrigues, M.M.d.S.; Soares, A.M.; Stábeli, R.G.; et al. Epidemiological Study of Snakebite Cases in Brazilian Western Amazonia. Rev. Soc. Bras. Med. Trop. 2018, 51, 338–346. [Google Scholar] [CrossRef]
- Schneider, M.C.; Min, K.; Hamrick, P.N.; Montebello, L.R.; Ranieri, T.M.; Mardini, L.; Camara, V.M.; Raggio Luiz, R.; Liese, B.; Vuckovic, M.; et al. Overview of Snakebite in Brazil: Possible Drivers and a Tool for Risk Mapping. PLoS Negl. Trop. Dis. 2021, 15, e0009044. [Google Scholar] [CrossRef]
- Chakma, J.; Menon, J.; Dhaliwal, R. Indian Council of Medical Research White Paper on Venomous Snakebite in India. Indian J. Med. Res. 2020, 152, 568. [Google Scholar] [CrossRef]
- Fernández, P.; Gutiérrez, J.M. Mortality Due to Snakebite Envenomation in Costa Rica (1993–2006). Toxicon 2008, 52, 530–533. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Maduwage, K.; Iliyasu, G.; Habib, A. Snakebite Envenoming in Different National Contexts: Costa Rica, Sri Lanka, and Nigeria. Toxicon X 2021, 9–10, 100066. [Google Scholar] [CrossRef]
- Rojas, G.; Bogarín, G.; Gutiérrez, J. Snakebite Mortality in Costa Rica. Toxicon 1997, 35, 1639–1643. [Google Scholar] [CrossRef]
- Salve, P.S.; Vatavati, S.; Hallad, J. Clustering the Envenoming of Snakebite in India: The District Level Analysis Using Health Management Information System Data. Clin. Epidemiol. Glob. Health 2020, 8, 733–738. [Google Scholar] [CrossRef]
- Sasa, M.; Segura Cano, S.E. New Insights into Snakebite Epidemiology in Costa Rica: A Retrospective Evaluation of Medical Records. Toxicon X 2020, 7, 100055. [Google Scholar] [CrossRef]
- Suraweera, W.; Warrell, D.; Whitaker, R.; Menon, G.; Rodrigues, R.; Fu, S.H.; Begum, R.; Sati, P.; Piyasena, K.; Bhatia, M.; et al. Trends in Snakebite Deaths in India from 2000 to 2019 in a Nationally Representative Mortality Study. eLife 2020, 9, e54076. [Google Scholar] [CrossRef]
- Aksnes, D.W.; Langfeldt, L.; Wouters, P. Citations, Citation Indicators, and Research Quality: An Overview of Basic Concepts and Theories. SAGE Open 2019, 9, 215824401982957. [Google Scholar] [CrossRef]
- Mammola, S.; Fontaneto, D.; Martínez, A.; Chichorro, F. Impact of the Reference List Features on the Number of Citations. Scientometrics 2021, 126, 785–799. [Google Scholar] [CrossRef]
- Tahamtan, I.; Safipour Afshar, A.; Ahamdzadeh, K. Factors Affecting Number of Citations: A Comprehensive Review of the Literature. Scientometrics 2016, 107, 1195–1225. [Google Scholar] [CrossRef]
- Lentz, T.L.; Wilson, P.T.; Hawrot, E.; Speicher, D.W. Amino Acid Sequence Similarity Between Rabies Virus Glycoprotein and Snake Venom Curaremimetic Neurotoxins. Science 1984, 226, 847–848. [Google Scholar] [CrossRef] [PubMed]
- Metz, M.; Piliponsky, A.M.; Chen, C.-C.; Lammel, V.; Åbrink, M.; Pejler, G.; Tsai, M.; Galli, S.J. Mast Cells Can Enhance Resistance to Snake and Honeybee Venoms. Science 2006, 313, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Tsernoglou, D.; Petsko, G.A.; McQueen, J.E.; Hermans, J. Molecular Graphics: Application to the Structure Determination of a Snake Venom Neurotoxin. Science 1977, 197, 1378–1381. [Google Scholar] [CrossRef] [PubMed]
- Laustsen, A.H.; Gutiérrez, J.M.; Rasmussen, A.R.; Engmark, M.; Gravlund, P.; Sanders, K.L.; Lohse, B.; Lomonte, B. Danger in the Reef: Proteome, Toxicity, and Neutralization of the Venom of the Olive Sea Snake, Aipysurus Laevis. Toxicon 2015, 107, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Doley, R.; Tram, N.N.B.; Reza, M.A.; Kini, R.M. Unusual Accelerated Rate of Deletions and Insertions in Toxin Genes in the Venom Glands of the Pygmy Copperhead (Austrelaps labialis) from Kangaroo Island. BMC Evol Biol. 2008, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Oh, A.M.F.; Tan, C.H.; Ariaranee, G.C.; Quraishi, N.; Tan, N.H. Venomics of Bungarus caeruleus (Indian krait): Comparable Venom Profiles, Variable Immunoreactivities among Specimens from Sri Lanka, India and Pakistan. J. Proteom. 2017, 164, 1–18. [Google Scholar] [CrossRef]
- Rusmili, M.R.A.; Yee, T.T.; Mustafa, M.R.; Hodgson, W.C.; Othman, I. Proteomic Characterization and Comparison of Malaysian Bungarus Candidus and Bungarus Fasciatus Venoms. J. Proteom. 2014, 110, 129–144. [Google Scholar] [CrossRef]
- Ziganshin, R.H.; Kovalchuk, S.I.; Arapidi, G.P.; Starkov, V.G.; Hoang, A.N.; Thi Nguyen, T.T.; Nguyen, K.C.; Shoibonov, B.B.; Tsetlin, V.I.; Utkin, Y.N. Quantitative Proteomic Analysis of Vietnamese Krait Venoms: Neurotoxins Are the Major Components in Bungarus Multicinctus and Phospholipases A2 in Bungarus Fasciatus. Toxicon 2015, 107, 197–209. [Google Scholar] [CrossRef]
- Shan, L.-L.; Gao, J.-F.; Zhang, Y.-X.; Shen, S.-S.; He, Y.; Wang, J.; Ma, X.-M.; Ji, X. Proteomic Characterization and Comparison of Venoms from Two Elapid Snakes (Bungarus multicinctus and Naja atra) from China. J. Proteom. 2016, 138, 83–94. [Google Scholar] [CrossRef]
- Lauridsen, L.P.; Laustsen, A.H.; Lomonte, B.; Gutiérrez, J.M. Toxicovenomics and Antivenom Profiling of the Eastern Green Mamba Snake (Dendroaspis angusticeps). J. Proteom. 2016, 136, 248–261. [Google Scholar] [CrossRef]
- Laustsen, A.H.; Lomonte, B.; Lohse, B.; Fernández, J.; Gutiérrez, J.M. Unveiling the Nature of Black Mamba (Dendroaspis polylepis) Venom through Venomics and Antivenom Immunoprofiling: Identification of Key Toxin Targets for Antivenom Development. J. Proteom. 2015, 119, 126–142. [Google Scholar] [CrossRef]
- Chatrath, S.T.; Chapeaurouge, A.; Lin, Q.; Lim, T.K.; Dunstan, N.; Mirtschin, P.; Kumar, P.P.; Kini, R.M. Identification of Novel Proteins from the Venom of a Cryptic Snake Drysdalia Coronoides by a Combined Transcriptomics and Proteomics Approach. J. Proteome Res. 2011, 10, 739–750. [Google Scholar] [CrossRef]
- Calvete, J.J.; Ghezellou, P.; Paiva, O.; Matainaho, T.; Ghassempour, A.; Goudarzi, H.; Kraus, F.; Sanz, L.; Williams, D.J. Snake Venomics of Two Poorly Known Hydrophiinae: Comparative Proteomics of the Venoms of Terrestrial Toxicocalamus Longissimus and Marine Hydrophis Cyanocinctus. J. Proteom. 2012, 75, 4091–4101. [Google Scholar] [CrossRef]
- Tan, C.H.; Tan, K.Y.; Lim, S.E.; Tan, N.H. Venomics of the Beaked Sea Snake, Hydrophis Schistosus: A Minimalist Toxin Arsenal and Its Cross-Neutralization by Heterologous Antivenoms. J. Proteom. 2015, 126, 121–130. [Google Scholar] [CrossRef]
- Fernández, J.; Vargas-Vargas, N.; Pla, D.; Sasa, M.; Rey-Suárez, P.; Sanz, L.; Gutiérrez, J.M.; Calvete, J.J.; Lomonte, B. Snake Venomics of Micrurus Alleni and Micrurus Mosquitensis from the Caribbean Region of Costa Rica Reveals Two Divergent Compositional Patterns in New World Elapids. Toxicon 2015, 107, 217–233. [Google Scholar] [CrossRef]
- Corrêa-Netto, C.; Junqueira-de-Azevedo, I.d.L.M.; Silva, D.A.; Ho, P.L.; Leitão-de-Araújo, M.; Alves, M.L.M.; Sanz, L.; Foguel, D.; Zingali, R.B.; Calvete, J.J. Snake Venomics and Venom Gland Transcriptomic Analysis of Brazilian Coral Snakes, Micrurus Altirostris and M. Corallinus. J. Proteom. 2011, 74, 1795–1809. [Google Scholar] [CrossRef]
- Margres, M.J.; Aronow, K.; Loyacano, J.; Rokyta, D.R. The Venom-Gland Transcriptome of the Eastern Coral Snake (Micrurus fulvius) Reveals High Venom Complexity in the Intragenomic Evolution of Venoms. BMC Genom. 2013, 14, 531. [Google Scholar] [CrossRef]
- Rey-Suárez, P.; Núñez, V.; Gutiérrez, J.M.; Lomonte, B. Proteomic and Biological Characterization of the Venom of the Redtail Coral Snake, Micrurus Mipartitus (Elapidae), from Colombia and Costa Rica. J. Proteom. 2011, 75, 655–667. [Google Scholar] [CrossRef]
- Fernández, J.; Alape-Girón, A.; Angulo, Y.; Sanz, L.; Gutiérrez, J.M.; Calvete, J.J.; Lomonte, B. Venomic and Antivenomic Analyses of the Central American Coral Snake, Micrurus Nigrocinctus (Elapidae). J. Proteome Res. 2011, 10, 1816–1827. [Google Scholar] [CrossRef]
- Sanz, L.; Pla, D.; Pérez, A.; Rodríguez, Y.; Zavaleta, A.; Salas, M.; Lomonte, B.; Calvete, J. Venomic Analysis of the Poorly Studied Desert Coral Snake, Micrurus Tschudii Tschudii, Supports the 3FTx/PLA2 Dichotomy across Micrurus Venoms. Toxins 2016, 8, 178. [Google Scholar] [CrossRef]
- Huang, H.-W.; Liu, B.-S.; Chien, K.-Y.; Chiang, L.-C.; Huang, S.-Y.; Sung, W.-C.; Wu, W.-G. Cobra Venom Proteome and Glycome Determined from Individual Snakes of Naja Atra Reveal Medically Important Dynamic Range and Systematic Geographic Variation. J. Proteom. 2015, 128, 92–104. [Google Scholar] [CrossRef]
- Malih, I.; Ahmad rusmili, M.R.; Tee, T.Y.; Saile, R.; Ghalim, N.; Othman, I. Proteomic Analysis of Moroccan Cobra Naja Haje Legionis Venom Using Tandem Mass Spectrometry. J. Proteom. 2014, 96, 240–252. [Google Scholar] [CrossRef]
- Tan, K.Y.; Tan, C.H.; Fung, S.Y.; Tan, N.H. Venomics, Lethality and Neutralization of Naja Kaouthia (Monocled cobra) Venoms from Three Different Geographical Regions of Southeast Asia. J. Proteom. 2015, 120, 105–125. [Google Scholar] [CrossRef]
- Xu, N.; Zhao, H.-Y.; Yin, Y.; Shen, S.-S.; Shan, L.-L.; Chen, C.-X.; Zhang, Y.-X.; Gao, J.-F.; Ji, X. Combined Venomics, Antivenomics and Venom Gland Transcriptome Analysis of the Monocoled Cobra (Naja kaouthia) from China. J. Proteom. 2017, 159, 19–31. [Google Scholar] [CrossRef]
- Petras, D.; Sanz, L.; Segura, Á.; Herrera, M.; Villalta, M.; Solano, D.; Vargas, M.; León, G.; Warrell, D.A.; Theakston, R.D.G.; et al. Snake Venomics of African Spitting Cobras: Toxin Composition and Assessment of Congeneric Cross-Reactivity of the Pan-African EchiTAb-Plus-ICP Antivenom by Antivenomics and Neutralization Approaches. J. Proteome Res. 2011, 10, 1266–1280. [Google Scholar] [CrossRef]
- Lauridsen, L.P.; Laustsen, A.H.; Lomonte, B.; Gutiérrez, J.M. Exploring the Venom of the Forest Cobra Snake: Toxicovenomics and Antivenom Profiling of Naja Melanoleuca. J. Proteom. 2017, 150, 98–108. [Google Scholar] [CrossRef]
- Dutta, S.; Chanda, A.; Kalita, B.; Islam, T.; Patra, A.; Mukherjee, A.K. Proteomic Analysis to Unravel the Complex Venom Proteome of Eastern India Naja Naja: Correlation of Venom Composition with Its Biochemical and Pharmacological Properties. J. Proteom. 2017, 156, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Sintiprungrat, K.; Watcharatanyatip, K.; Senevirathne, W.D.S.T.; Chaisuriya, P.; Chokchaichamnankit, D.; Srisomsap, C.; Ratanabanangkoon, K. A Comparative Study of Venomics of Naja Naja from India and Sri Lanka, Clinical Manifestations and Antivenomics of an Indian Polyspecific Antivenom. J. Proteom. 2016, 132, 131–143. [Google Scholar] [CrossRef]
- Herrera, M.; Fernández, J.; Vargas, M.; Villalta, M.; Segura, Á.; León, G.; Angulo, Y.; Paiva, O.; Matainaho, T.; Jensen, S.D.; et al. Comparative Proteomic Analysis of the Venom of the Taipan Snake, Oxyuranus Scutellatus, from Papua New Guinea and Australia: Role of Neurotoxic and Procoagulant Effects in Venom Toxicity. J. Proteom. 2012, 75, 2128–2140. [Google Scholar] [CrossRef] [PubMed]
- Lomonte, B.; Pla, D.; Sasa, M.; Tsai, W.-C.; Solórzano, A.; Ureña-Díaz, J.M.; Fernández-Montes, M.L.; Mora-Obando, D.; Sanz, L.; Gutiérrez, J.M.; et al. Two Color Morphs of the Pelagic Yellow-Bellied Sea Snake, Pelamis Platura, from Different Locations of Costa Rica: Snake Venomics, Toxicity, and Neutralization by Antivenom. J. Proteom. 2014, 103, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Pla, D.; Bande, B.W.; Welton, R.E.; Paiva, O.K.; Sanz, L.; Segura, Á.; Wright, C.E.; Calvete, J.J.; Gutiérrez, J.M.; Williams, D.J. Proteomics and Antivenomics of Papuan Black Snake (Pseudechis papuanus) Venom with Analysis of Its Toxicological Profile and the Preclinical Efficacy of Australian Antivenoms. J. Proteom. 2017, 150, 201–215. [Google Scholar] [CrossRef]
- Bocian, A.; Urbanik, M.; Hus, K.; Łyskowski, A.; Petrilla, V.; Andrejčáková, Z.; Petrillová, M.; Legáth, J. Proteomic Analyses of Agkistrodon Contortrix Contortrix Venom Using 2D Electrophoresis and MS Techniques. Toxins 2016, 8, 372. [Google Scholar] [CrossRef]
- Lomonte, B.; Tsai, W.-C.; Ureña-Diaz, J.M.; Sanz, L.; Mora-Obando, D.; Sánchez, E.E.; Fry, B.G.; Gutiérrez, J.M.; Gibbs, H.L.; Sovic, M.G.; et al. Venomics of New World Pit Vipers: Genus-Wide Comparisons of Venom Proteomes across Agkistrodon. J. Proteom. 2014, 96, 103–116. [Google Scholar] [CrossRef]
- Angulo, Y.; Escolano, J.; Lomonte, B.; Gutiérrez, J.M.; Sanz, L.; Calvete, J.J. Snake Venomics of Central American Pitvipers: Clues for Rationalizing the Distinct Envenomation Profiles of Atropoides Nummifer and Atropoides Picadoi. J. Proteome Res. 2008, 7, 708–719. [Google Scholar] [CrossRef]
- Calvete, J.J.; Escolano, J.; Sanz, L. Snake Venomics of Bitis Species Reveals Large Intragenus Venom Toxin Composition Variation: Application to Taxonomy of Congeneric Taxa. J. Proteome Res. 2007, 6, 2732–2745. [Google Scholar] [CrossRef]
- Fernández, J.; Lomonte, B.; Sanz, L.; Angulo, Y.; Gutiérrez, J.M.; Calvete, J.J. Snake Venomics of Bothriechis Nigroviridis Reveals Extreme Variability among Palm Pitviper Venoms: Different Evolutionary Solutions for the Same Trophic Purpose. J. Proteome Res. 2010, 9, 4234–4241. [Google Scholar] [CrossRef]
- Pla, D.; Sanz, L.; Sasa, M.; Acevedo, M.E.; Dwyer, Q.; Durban, J.; Pérez, A.; Rodriguez, Y.; Lomonte, B.; Calvete, J.J. Proteomic Analysis of Venom Variability and Ontogeny across the Arboreal Palm-Pitvipers (Genus bothriechis). J. Proteom. 2017, 152, 1–12. [Google Scholar] [CrossRef]
- Salazar-Valenzuela, D.; Mora-Obando, D.; Fernández, M.L.; Loaiza-Lange, A.; Gibbs, H.L.; Lomonte, B. Proteomic and Toxicological Profiling of the Venom of Bothrocophias Campbelli, a Pitviper Species from Ecuador and Colombia. Toxicon 2014, 90, 15–25. [Google Scholar] [CrossRef]
- Rodrigues, R.S.; Boldrini-França, J.; Fonseca, F.P.P.; Henrique-Silva, F.; Sanz, L.; Calvete, J.J.; Rodrigues, V.M. Combined Snake Venomics and Venom Gland Transcriptomic Analysis of Bothropoides Pauloensis. J. Proteom. 2012, 75, 2707–2720. [Google Scholar] [CrossRef]
- Calvete, J.J.; Sanz, L.; Pérez, A.; Borges, A.; Vargas, A.M.; Lomonte, B.; Angulo, Y.; Gutiérrez, J.M.; Chalkidis, H.M.; Mourão, R.H.V.; et al. Snake Population Venomics and Antivenomics of Bothrops Atrox: Paedomorphism along Its Transamazonian Dispersal and Implications of Geographic Venom Variability on Snakebite Management. J. Proteom. 2011, 74, 510–527. [Google Scholar] [CrossRef]
- Kohlhoff, M.; Borges, M.H.; Yarleque, A.; Cabezas, C.; Richardson, M.; Sanchez, E.F. Exploring the Proteomes of the Venoms of the Peruvian Pit Vipers Bothrops Atrox, B. Barnetti and B. Pictus. J. Proteom. 2012, 75, 2181–2195. [Google Scholar] [CrossRef]
- Núñez, V.; Cid, P.; Sanz, L.; De La Torre, P.; Angulo, Y.; Lomonte, B.; Gutiérrez, J.M.; Calvete, J.J. Snake Venomics and Antivenomics of Bothrops Atrox Venoms from Colombia and the Amazon Regions of Brazil, Perú and Ecuador Suggest the Occurrence of Geographic Variation of Venom Phenotype by a Trend towards Paedomorphism. J. Proteom. 2009, 73, 57–78. [Google Scholar] [CrossRef]
- Sousa, L.F.; Nicolau, C.A.; Peixoto, P.S.; Bernardoni, J.L.; Oliveira, S.S.; Portes-Junior, J.A.; Mourão, R.H.V.; Lima-dos-Santos, I.; Sano-Martins, I.S.; Chalkidis, H.M.; et al. Comparison of Phylogeny, Venom Composition and Neutralization by Antivenom in Diverse Species of Bothrops Complex. PLoS Negl. Trop. Dis. 2013, 7, e2442. [Google Scholar] [CrossRef]
- Sousa, L.F.; Portes-Junior, J.A.; Nicolau, C.A.; Bernardoni, J.L.; Nishiyama, M.Y., Jr.; Amazonas, D.R.; Freitas-de-Sousa, L.A.; Mourão, R.H.; Chalkidis, H.M.; Valente, R.H.; et al. Functional Proteomic Analyses of Bothrops Atrox Venom Reveals Phenotypes Associated with Habitat Variation in the Amazon. J. Proteom. 2017, 159, 32–46. [Google Scholar] [CrossRef]
- Mora-Obando, D.; Guerrero-Vargas, J.A.; Prieto-Sánchez, R.; Beltrán, J.; Rucavado, A.; Sasa, M.; Gutiérrez, J.M.; Ayerbe, S.; Lomonte, B. Proteomic and Functional Profiling of the Venom of Bothrops Ayerbei from Cauca, Colombia, Reveals Striking Interspecific Variation with Bothrops Asper Venom. J. Proteom. 2014, 96, 159–172. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Sanz, L.; Escolano, J.; Fernández, J.; Lomonte, B.; Angulo, Y.; Rucavado, A.; Warrell, D.A.; Calvete, J.J. Snake Venomics of the Lesser Antillean Pit Vipers Bothrops Caribbaeus and Bothrops Lanceolatus: Correlation with Toxicological Activities and Immunoreactivity of a Heterologous Antivenom. J. Proteome Res. 2008, 7, 4396–4408. [Google Scholar] [CrossRef]
- Calvete, J.J.; Borges, A.; Segura, Á.; Flores-Díaz, M.; Alape-Girón, A.; Gutiérrez, J.M.; Diez, N.; De Sousa, L.; Kiriakos, D.; Sánchez, E.; et al. Snake Venomics and Antivenomics of Bothrops Colombiensis, a Medically Important Pitviper of the Bothrops Atrox-Asper Complex Endemic to Venezuela: Contributing to Its Taxonomy and Snakebite Management. J. Proteom. 2009, 72, 227–240. [Google Scholar] [CrossRef]
- Tashima, A.K.; Sanz, L.; Camargo, A.C.M.; Serrano, S.M.T.; Calvete, J.J. Snake Venomics of the Brazilian Pitvipers Bothrops Cotiara and Bothrops Fonsecai. Identification of Taxonomy Markers. J. Proteom. 2008, 71, 473–485. [Google Scholar] [CrossRef]
- Gay, C.; Sanz, L.; Calvete, J.; Pla, D. Snake Venomics and Antivenomics of Bothrops Diporus, a Medically Important Pitviper in Northeastern Argentina. Toxins 2015, 8, 9. [Google Scholar] [CrossRef]
- Jorge, R.J.B.; Monteiro, H.S.A.; Gonçalves-Machado, L.; Guarnieri, M.C.; Ximenes, R.M.; Borges-Nojosa, D.M.; Luna, K.P.d.O.; Zingali, R.B.; Corrêa-Netto, C.; Gutiérrez, J.M.; et al. Venomics and Antivenomics of Bothrops Erythromelas from Five Geographic Populations within the Caatinga Ecoregion of Northeastern Brazil. J. Proteom. 2015, 114, 93–114. [Google Scholar] [CrossRef]
- Valente, R.H.; Guimarães, P.R.; Junqueira, M.; Neves-Ferreira, A.G.C.; Soares, M.R.; Chapeaurouge, A.; Trugilho, M.R.O.; León, I.R.; Rocha, S.L.G.; Oliveira-Carvalho, A.L.; et al. Bothrops Insularis Venomics: A Proteomic Analysis Supported by Transcriptomic-Generated Sequence Data. J. Proteom. 2009, 72, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves-Machado, L.; Pla, D.; Sanz, L.; Jorge, R.J.B.; Leitão-De-Araújo, M.; Alves, M.L.M.; Alvares, D.J.; De Miranda, J.; Nowatzki, J.; de Morais-Zani, K.; et al. Combined Venomics, Venom Gland Transcriptomics, Bioactivities, and Antivenomics of Two Bothrops Jararaca Populations from Geographic Isolated Regions within the Brazilian Atlantic Rainforest. J. Proteom. 2016, 135, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, C.P.; Menaldo, D.L.; Camacho, E.; Rosa, J.C.; Escalante, T.; Rucavado, A.; Lomonte, B.; Gutiérrez, J.M.; Sampaio, S.V. Proteomic Analysis of Bothrops Pirajai Snake Venom and Characterization of BpirMP, a New P-I Metalloproteinase. J. Proteom. 2013, 80, 250–267. [Google Scholar] [CrossRef] [PubMed]
- Fernández Culma, M.; Andrés Pereañez, J.; Núñez Rangel, V.; Lomonte, B. Snake Venomics of Bothrops Punctatus, a Semiarboreal Pitviper Species from Antioquia, Colombia. PeerJ 2014, 2, e246. [Google Scholar] [CrossRef]
- Tang, E.L.H.; Tan, C.H.; Fung, S.Y.; Tan, N.H. Venomics of Calloselasma Rhodostoma, the Malayan Pit Viper: A Complex Toxin Arsenal Unraveled. J. Proteom. 2016, 148, 44–56. [Google Scholar] [CrossRef]
- Fahmi, L.; Makran, B.; Pla, D.; Sanz, L.; Oukkache, N.; Lkhider, M.; Harrison, R.A.; Ghalim, N.; Calvete, J.J. Venomics and Antivenomics Profiles of North African Cerastes Cerastes and C. Vipera Populations Reveals a Potentially Important Therapeutic Weakness. J. Proteom. 2012, 75, 2442–2453. [Google Scholar] [CrossRef]
- Lomonte, B.; Rey-Suárez, P.; Tsai, W.-C.; Angulo, Y.; Sasa, M.; Gutiérrez, J.M.; Calvete, J.J. Snake Venomics of the Pit Vipers Porthidium Nasutum, Porthidium Ophryomegas, and Cerrophidion Godmani from Costa Rica: Toxicological and Taxonomical Insights. J. Proteom. 2012, 75, 1675–1689. [Google Scholar] [CrossRef]
- Rokyta, D.R.; Wray, K.P.; Lemmon, A.R.; Lemmon, E.M.; Caudle, S.B. A High-Throughput Venom-Gland Transcriptome for the Eastern Diamondback Rattlesnake (Crotalus adamanteus) and Evidence for Pervasive Positive Selection across Toxin Classes. Toxicon 2011, 57, 657–671. [Google Scholar] [CrossRef]
- Segura, Á.; Herrera, M.; Reta Mares, F.; Jaime, C.; Sánchez, A.; Vargas, M.; Villalta, M.; Gómez, A.; Gutiérrez, J.M.; León, G. Proteomic, Toxicological and Immunogenic Characterization of Mexican West-Coast Rattlesnake (Crotalus basiliscus) Venom and Its Immunological Relatedness with the Venom of Central American Rattlesnake (Crotalus simus). J. Proteom. 2017, 158, 62–72. [Google Scholar] [CrossRef]
- Boldrini-França, J.; Corrêa-Netto, C.; Silva, M.M.S.; Rodrigues, R.S.; De La Torre, P.; Pérez, A.; Soares, A.M.; Zingali, R.B.; Nogueira, R.A.; Rodrigues, V.M.; et al. Snake Venomics and Antivenomics of Crotalus Durissus Subspecies from Brazil: Assessment of Geographic Variation and Its Implication on Snakebite Management. J. Proteom. 2010, 73, 1758–1776. [Google Scholar] [CrossRef]
- Georgieva, D.; Öhler, M.; Seifert, J.; von Bergen, M.; Arni, R.K.; Genov, N.; Betzel, C. Snake Venomic of Crotalus Durissus Terrificus —Correlation with Pharmacological Activities. J. Proteome Res. 2010, 9, 2302–2316. [Google Scholar] [CrossRef]
- Rokyta, D.R.; Wray, K.P.; Margres, M.J. The Genesis of an Exceptionally Lethal Venom in the Timber Rattlesnake (Crotalus horridus) Revealed through Comparative Venom-Gland Transcriptomics. BMC Genom. 2013, 14, 394. [Google Scholar] [CrossRef]
- Castro, E.N.; Lomonte, B.; del Carmen Gutiérrez, M.; Alagón, A.; Gutiérrez, J.M. Intraspecies Variation in the Venom of the Rattlesnake Crotalus Simus from Mexico: Different Expression of Crotoxin Results in Highly Variable Toxicity in the Venoms of Three Subspecies. J. Proteom. 2013, 87, 103–121. [Google Scholar] [CrossRef]
- Saviola, A.J.; Pla, D.; Sanz, L.; Castoe, T.A.; Calvete, J.J.; Mackessy, S.P. Comparative Venomics of the Prairie Rattlesnake (Crotalus viridis viridis) from Colorado: Identification of a Novel Pattern of Ontogenetic Changes in Venom Composition and Assessment of the Immunoreactivity of the Commercial Antivenom CroFab®. J. Proteom. 2015, 121, 28–43. [Google Scholar] [CrossRef]
- Kalita, B.; Patra, A.; Mukherjee, A.K. Unraveling the Proteome Composition and Immuno-Profiling of Western India Russell’s Viper Venom for In-Depth Understanding of Its Pharmacological Properties, Clinical Manifestations, and Effective Antivenom Treatment. J. Proteome Res. 2017, 16, 583–598. [Google Scholar] [CrossRef]
- Mukherjee, A.K.; Kalita, B.; Mackessy, S.P. A Proteomic Analysis of Pakistan Daboia Russelii Russelii Venom and Assessment of Potency of Indian Polyvalent and Monovalent Antivenom. J. Proteom. 2016, 144, 73–86. [Google Scholar] [CrossRef]
- Tan, N.H.; Fung, S.Y.; Tan, K.Y.; Yap, M.K.K.; Gnanathasan, C.A.; Tan, C.H. Functional Venomics of the Sri Lankan Russell’s Viper (Daboia russelii) and Its Toxinological Correlations. J. Proteom. 2015, 128, 403–423. [Google Scholar] [CrossRef]
- Gao, J.-F.; Wang, J.; He, Y.; Qu, Y.-F.; Lin, L.-H.; Ma, X.-M.; Ji, X. Proteomic and Biochemical Analyses of Short-Tailed Pit Viper (Gloydius brevicaudus) Venom: Age-Related Variation and Composition–Activity Correlation. J. Proteom. 2014, 105, 307–322. [Google Scholar] [CrossRef]
- Yang, Z.-M.; Yang, Y.-E.; Chen, Y.; Cao, J.; Zhang, C.; Liu, L.-L.; Wang, Z.-Z.; Wang, X.-M.; Wang, Y.-M.; Tsai, I.-H. Transcriptome and Proteome of the Highly Neurotoxic Venom of Gloydius Intermedius. Toxicon 2015, 107, 175–186. [Google Scholar] [CrossRef]
- Madrigal, M.; Sanz, L.; Flores-Díaz, M.; Sasa, M.; Núñez, V.; Alape-Girón, A.; Calvete, J.J. Snake Venomics across Genus Lachesis. Ontogenetic Changes in the Venom Composition of Lachesis Stenophrys and Comparative Proteomics of the Venoms of Adult Lachesis Melanocephala and Lachesis Acrochorda. J. Proteom. 2012, 77, 280–297. [Google Scholar] [CrossRef]
- Makran, B.; Fahmi, L.; Pla, D.; Sanz, L.; Oukkache, N.; Lkhider, M.; Ghalim, N.; Calvete, J.J. Snake Venomics of Macrovipera Mauritanica from Morocco, and Assessment of the Para-Specific Immunoreactivity of an Experimental Monospecific and a Commercial Antivenoms. J. Proteom. 2012, 75, 2431–2441. [Google Scholar] [CrossRef]
- Aird, S.D.; Watanabe, Y.; Villar-Briones, A.; Roy, M.C.; Terada, K.; Mikheyev, A.S. Quantitative High-Throughput Profiling of Snake Venom Gland Transcriptomes and Proteomes (Ovophis okinavensis and Protobothrops flavoviridis). BMC Genom. 2013, 14, 790. [Google Scholar] [CrossRef]
- Jiménez-Charris, E.; Montealegre-Sanchez, L.; Solano-Redondo, L.; Mora-Obando, D.; Camacho, E.; Castro-Herrera, F.; Fierro-Pérez, L.; Lomonte, B. Proteomic and Functional Analyses of the Venom of Porthidium lansbergii lansbergii (Lansberg’s Hognose Viper) from the Atlantic Department of Colombia. J. Proteom. 2015, 114, 287–299. [Google Scholar] [CrossRef]
- Villalta, M.; Pla, D.; Yang, S.L.; Sanz, L.; Segura, A.; Vargas, M.; Chen, P.Y.; Herrera, M.; Estrada, R.; Cheng, Y.F.; et al. Snake Venomics and Antivenomics of Protobothrops Mucrosquamatus and Viridovipera Stejnegeri from Taiwan: Keys to Understand the Variable Immune Response in Horses. J. Proteom. 2012, 75, 5628–5645. [Google Scholar] [CrossRef]
- Zainal Abidin, S.; Rajadurai, P.; Chowdhury, M.; Ahmad Rusmili, M.; Othman, I.; Naidu, R. Proteomic Characterization and Comparison of Malaysian Tropidolaemus Wagleri and Cryptelytrops Purpureomaculatus Venom Using Shotgun-Proteomics. Toxins 2016, 8, 299. [Google Scholar] [CrossRef]
- Kovalchuk, S.; Ziganshin, R.; Starkov, V.; Tsetlin, V.; Utkin, Y. Quantitative Proteomic Analysis of Venoms from Russian Vipers of Pelias Group: Phospholipases A2 Are the Main Venom Components. Toxins 2016, 8, 105. [Google Scholar] [CrossRef]
- Göçmen, B.; Heiss, P.; Petras, D.; Nalbantsoy, A.; Süssmuth, R.D. Mass Spectrometry Guided Venom Profiling and Bioactivity Screening of the Anatolian Meadow Viper, Vipera Anatolica. Toxicon 2015, 107, 163–174. [Google Scholar] [CrossRef]
- Calvete, J.J.; Fasoli, E.; Sanz, L.; Boschetti, E.; Righetti, P.G. Exploring the Venom Proteome of the Western Diamondback Rattlesnake, Crotalus Atrox, via Snake Venomics and Combinatorial Peptide Ligand Library Approaches. J. Proteome Res. 2009, 8, 3055–3067. [Google Scholar] [CrossRef]
- Calvete, J.J.; Pérez, A.; Lomonte, B.; Sánchez, E.E.; Sanz, L. Snake Venomics of Crotalus Tigris: The Minimalist Toxin Arsenal of the Deadliest Neartic Rattlesnake Venom. Evolutionary Clues for Generating a Pan-Specific Antivenom against Crotalid Type II Venoms. J. Proteome Res. 2012, 11, 1382–1390. [Google Scholar] [CrossRef]
- Pla, D.; Sanz, L.; Molina-Sánchez, P.; Zorita, V.; Madrigal, M.; Flores-Díaz, M.; Alape-Girón, A.; Núñez, V.; Andrés, V.; Gutiérrez, J.M.; et al. Snake Venomics of Lachesis Muta Rhombeata and Genus-Wide Antivenomics Assessment of the Paraspecific Immunoreactivity of Two Antivenoms Evidence the High Compositional and Immunological Conservation across Lachesis. J. Proteom. 2013, 89, 112–123. [Google Scholar] [CrossRef]
- Sanz, L.; Ayvazyan, N.; Calvete, J.J. Snake Venomics of the Armenian Mountain Vipers Macrovipera Lebetina Obtusa and Vipera Raddei. J. Proteom. 2008, 71, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Sanz, L.; Gibbs, H.L.; Mackessy, S.P.; Calvete, J.J. Venom Proteomes of Closely Related Sistrurus Rattlesnakes with Divergent Diets. J. Proteome Res. 2006, 5, 2098–2112. [Google Scholar] [CrossRef] [PubMed]
- Tasoulis, T.; Pukala, T.L.; Isbister, G.K. Investigating Toxin Diversity and Abundance in Snake Venom Proteomes. Front. Pharm. 2022, 12, 768015. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, M.; McCleary, R.J.R.; Kesherwani, M.; Kini, R.M.; Velmurugan, D. Comparison of Proteomic Profiles of the Venoms of Two of the ‘Big Four’ Snakes of India, the Indian Cobra (Naja naja) and the Common Krait (Bungarus caeruleus), and Analyses of Their Toxins. Toxicon 2017, 135, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Ghezellou, P.; Albuquerque, W.; Garikapati, V.; Casewell, N.R.; Kazemi, S.M.; Ghassempour, A.; Spengler, B. Integrating Top-Down and Bottom-Up Mass Spectrometric Strategies for Proteomic Profiling of Iranian Saw-Scaled Viper, Echis Carinatus Sochureki, Venom. J. Proteome Res. 2021, 20, 895–908. [Google Scholar] [CrossRef] [PubMed]
- Kunalan, S.; Othman, I.; Syed Hassan, S.; Hodgson, W. Proteomic Characterization of Two Medically Important Malaysian Snake Venoms, Calloselasma Rhodostoma (Malayan pit viper) and Ophiophagus Hannah (King Cobra). Toxins 2018, 10, 434. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Vasudevan, K. Comparative Proteomics of Geographically Distinct Saw-Scaled Viper (Echis carinatus) Venoms from India. Toxicon X 2020, 7, 100048. [Google Scholar] [CrossRef]
- Sanz, L.; de Freitas-Lima, L.N.; Quesada-Bernat, S.; Graça-de-Souza, V.K.; Soares, A.M.; Calderón, L.d.A.; Calvete, J.J.; Caldeira, C.A.S. Comparative Venomics of Brazilian Coral Snakes: Micrurus Frontalis, Micrurus Spixii Spixii, and Micrurus Surinamensis. Toxicon 2019, 166, 39–45. [Google Scholar] [CrossRef]
- Whiteley, G.; Casewell, N.R.; Pla, D.; Quesada-Bernat, S.; Logan, R.A.E.; Bolton, F.M.S.; Wagstaff, S.C.; Gutiérrez, J.M.; Calvete, J.J.; Harrison, R.A. Defining the Pathogenic Threat of Envenoming by South African Shield-Nosed and Coral Snakes (Genus aspidelaps), and Revealing the Likely Efficacy of Available Antivenom. J. Proteom. 2019, 198, 186–198. [Google Scholar] [CrossRef]
- Petras, D.; Hempel, B.-F.; Göçmen, B.; Karis, M.; Whiteley, G.; Wagstaff, S.C.; Heiss, P.; Casewell, N.R.; Nalbantsoy, A.; Süssmuth, R.D. Intact Protein Mass Spectrometry Reveals Intraspecies Variations in Venom Composition of a Local Population of Vipera Kaznakovi in Northeastern Turkey. J. Proteom. 2019, 199, 31–50. [Google Scholar] [CrossRef]
- Junqueira-de-Azevedo, I.L.M.; Ho, P.L. A Survey of Gene Expression and Diversity in the Venom Glands of the Pitviper Snake Bothrops Insularis through the Generation of Expressed Sequence Tags (ESTs). Gene 2002, 299, 279–291. [Google Scholar] [CrossRef]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A Revolutionary Tool for Transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef]
- Fry, B.G.; Scheib, H.; Junqueira de Azevedo, I.d.L.M.; Silva, D.A.; Casewell, N.R. Novel Transcripts in the Maxillary Venom Glands of Advanced Snakes. Toxicon 2012, 59, 696–708. [Google Scholar] [CrossRef]
- Ching, A.T.C.; Rocha, M.M.T.; Paes Leme, A.F.; Pimenta, D.C.; de Fátima, D.; Furtado, M.; Serrano, S.M.T.; Ho, P.L.; Junqueira-de-Azevedo, I.L.M. Some Aspects of the Venom Proteome of the Colubridae Snake Philodryas Olfersii Revealed from a Duvernoy’s (Venom) Gland Transcriptome. FEBS Lett. 2006, 580, 4417–4422. [Google Scholar] [CrossRef]
- Ching, A.T.C.; Paes Leme, A.F.; Zelanis, A.; Rocha, M.M.T.; Furtado, M.d.F.D.; Silva, D.A.; Trugilho, M.R.O.; da Rocha, S.L.G.; Perales, J.; Ho, P.L.; et al. Venomics Profiling of Thamnodynastes Strigatus Unveils Matrix Metalloproteinases and Other Novel Proteins Recruited to the Toxin Arsenal of Rear-Fanged Snakes. J. Proteome Res. 2012, 11, 1152–1162. [Google Scholar] [CrossRef]
- Siang, A.S.; Doley, R.; Vonk, F.J.; Kini, R.M. Transcriptomic Analysis of the Venom Gland of the Red-Headed Krait (Bungarus flaviceps) Using Expressed Sequence Tags. BMC Mol. Biol. 2010, 11, 24. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y.; Lee, W.; Xu, X.; Zhang, Y.; Zhao, R.; Zhang, Y.; Wang, W. Venom Gland Transcriptomes of Two Elapid Snakes (Bungarus multicinctus and Naja atra) and Evolution of Toxin Genes. BMC Genom. 2011, 12, 1. [Google Scholar] [CrossRef]
- St Pierre, L.; Birrell, G.W.; Earl, S.T.; Wallis, T.P.; Gorman, J.J.; de Jersey, J.; Masci, P.P.; Lavin, M.F. Diversity of Toxic Components from the Venom of the Evolutionarily Distinct Black Whip Snake, Demansia Vestigiata. J. Proteome Res. 2007, 6, 3093–3107. [Google Scholar] [CrossRef]
- Zhao, H.-Y.; Wen, L.; Miao, Y.-F.; Du, Y.; Sun, Y.; Yin, Y.; Lin, C.-X.; Lin, L.-H.; Ji, X.; Gao, J.-F. Venom-Gland Transcriptomic, Venomic, and Antivenomic Profiles of the Spine-Bellied Sea Snake (Hydrophis curtus) from the South China Sea. BMC Genom. 2021, 22, 520. [Google Scholar] [CrossRef]
- Paiva, O.; Pla, D.; Wright, C.E.; Beutler, M.; Sanz, L.; Gutiérrez, J.M.; Williams, D.J.; Calvete, J.J. Combined Venom Gland CDNA Sequencing and Venomics of the New Guinea Small-Eyed Snake, Micropechis Ikaheka. J. Proteom. 2014, 110, 209–229. [Google Scholar] [CrossRef]
- Leão, L.I.; Ho, P.L.; Junqueira-de-Azevedo, I.d.L. Transcriptomic Basis for an Antiserum against Micrurus Corallinus (Coral snake) Venom. BMC Genom. 2009, 10, 112. [Google Scholar] [CrossRef]
- Rey-Suárez, P.; Acosta, C.; Torres, U.; Saldarriaga-Córdoba, M.; Lomonte, B.; Núñez, V. MipLAAO, a New L-Amino Acid Oxidase from the Redtail Coral Snake Micrurus Mipartitus. PeerJ 2018, 6, e4924. [Google Scholar] [CrossRef]
- OmPraba, G.; Chapeaurouge, A.; Doley, R.; Devi, K.R.; Padmanaban, P.; Venkatraman, C.; Velmurugan, D.; Lin, Q.; Kini, R.M. Identification of a Novel Family of Snake Venom Proteins Veficolins from Cerberus Rynchops Using a Venom Gland Transcriptomics and Proteomics Approach. J. Proteome Res. 2010, 9, 1882–1893. [Google Scholar] [CrossRef]
- Jia, Y.; Cantu, B.A.; Sánchez, E.E.; Pérez, J.C. Complementary DNA Sequencing and Identification of MRNAs from the Venomous Gland of Agkistrodon Piscivorus Leucostoma. Toxicon 2008, 51, 1457–1466. [Google Scholar] [CrossRef]
- Durban, J.; Juárez, P.; Angulo, Y.; Lomonte, B.; Flores-Diaz, M.; Alape-Girón, A.; Sasa, M.; Sanz, L.; Gutiérrez, J.M.; Dopazo, J.; et al. Profiling the Venom Gland Transcriptomes of Costa Rican Snakes by 454 Pyrosequencing. BMC Genom. 2011, 12, 259. [Google Scholar] [CrossRef]
- Francischetti, I.M.B.; My-Pham, V.; Harrison, J.; Garfield, M.K.; Ribeiro, J.M.C. Bitis Gabonica (Gaboon viper) Snake Venom Gland: Toward a Catalog for the Full-Length Transcripts (CDNA) and Proteins. Gene 2004, 337, 55–69. [Google Scholar] [CrossRef]
- Cardoso, K.C.; Da Silva, M.J.; Costa, G.G.; Torres, T.T.; Del Bem, L.E.V.; Vidal, R.O.; Menossi, M.; Hyslop, S. A Transcriptomic Analysis of Gene Expression in the Venom Gland of the Snake Bothrops Alternatus (Urutu). BMC Genom. 2010, 11, 605. [Google Scholar] [CrossRef]
- Neiva, M.; Arraes, F.B.M.; de Souza, J.V.; Rádis-Baptista, G.; Prieto da Silva, Á.R.B.; Walter, M.E.M.T.; Brigido, M.d.M.; Yamane, T.; López-Lozano, J.L.; Astolfi-Filho, S. Transcriptome Analysis of the Amazonian Viper Bothrops Atrox Venom Gland Using Expressed Sequence Tags (ESTs). Toxicon 2009, 53, 427–436. [Google Scholar] [CrossRef]
- Cidade, D.A.P.; Simão, T.A.; Dávila, A.M.R.; Wagner, G.; de L.M. Junqueira-de-Azevedo, I.; Lee Ho, P.; Bon, C.; Zingali, R.B.; Albano, R.M. Bothrops Jararaca Venom Gland Transcriptome: Analysis of the Gene Expression Pattern. Toxicon 2006, 48, 437–461. [Google Scholar] [CrossRef]
- Zelanis, A.; Andrade-Silva, D.; Rocha, M.M.; Furtado, M.F.; Serrano, S.M.T.; Junqueira-de-Azevedo, I.L.M.; Ho, P.L. A Transcriptomic View of the Proteome Variability of Newborn and Adult Bothrops Jararaca Snake Venoms. PLoS Negl. Trop. Dis. 2012, 6, e1554. [Google Scholar] [CrossRef]
- Rokyta, D.R.; Lemmon, A.R.; Margres, M.J.; Aronow, K. The Venom-Gland Transcriptome of the Eastern Diamondback Rattlesnake (Crotalus adamanteus). BMC Genom. 2012, 13, 312. [Google Scholar] [CrossRef] [PubMed]
- Boldrini-França, J.; Rodrigues, R.S.; Fonseca, F.P.P.; Menaldo, D.L.; Ferreira, F.B.; Henrique-Silva, F.; Soares, A.M.; Hamaguchi, A.; Rodrigues, V.M.; Otaviano, A.R.; et al. Crotalus Durissus Collilineatus Venom Gland Transcriptome: Analysis of Gene Expression Profile. Biochimie 2009, 91, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Wiezel, G.A.; Shibao, P.Y.T.; Cologna, C.T.; Morandi Filho, R.; Ueira-Vieira, C.; De Pauw, E.; Quinton, L.; Arantes, E.C. In-Depth Venome of the Brazilian Rattlesnake Crotalus Durissus Terrificus: An Integrative Approach Combining Its Venom Gland Transcriptome and Venom Proteome. J. Proteome Res. 2018, 17, 3941–3958. [Google Scholar] [CrossRef] [PubMed]
- Durban, J.; Pérez, A.; Sanz, L.; Gómez, A.; Bonilla, F.; Rodríguez, S.; Chacón, D.; Sasa, M.; Angulo, Y.; Gutiérrez, J.M.; et al. Integrated “Omics” Profiling Indicates That MiRNAs Are Modulators of the Ontogenetic Venom Composition Shift in the Central American Rattlesnake, Crotalus Simus Simus. BMC Genom. 2013, 14, 234. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, Q.; Yin, W.; Zhang, X.; Huang, Y.; Luo, Y.; Qiu, P.; Su, X.; Yu, J.; Hu, S.; et al. Transcriptome Analysis of Deinagkistrodon Acutus Venomous Gland Focusing on Cellular Structure and Functional Aspects Using Expressed Sequence Tags. BMC Genom. 2006, 7, 152. [Google Scholar] [CrossRef]
- Casewell, N.R.; Harrison, R.A.; Wüster, W.; Wagstaff, S.C. Comparative Venom Gland Transcriptome Surveys of the Saw-Scaled Vipers (Viperidae: Echis) Reveal Substantial Intra-Family Gene Diversity and Novel Venom Transcripts. BMC Genom. 2009, 10, 564. [Google Scholar] [CrossRef]
- Wagstaff, S.C.; Sanz, L.; Juárez, P.; Harrison, R.A.; Calvete, J.J. Combined Snake Venomics and Venom Gland Transcriptomic Analysis of the Ocellated Carpet Viper, Echis Ocellatus. J. Proteom. 2009, 71, 609–623. [Google Scholar] [CrossRef]
- Wagstaff, S.C.; Harrison, R.A. Venom Gland EST Analysis of the Saw-Scaled Viper, Echis Ocellatus, Reveals Novel A9β1 Integrin-Binding Motifs in Venom Metalloproteinases and a New Group of Putative Toxins, Renin-like Aspartic Proteases. Gene 2006, 377, 21–32. [Google Scholar] [CrossRef]
- Junqueira-de-Azevedo, I.L.M.; Ching, A.T.C.; Carvalho, E.; Faria, F.; Nishiyama, M.Y.; Ho, P.L.; Diniz, M.R.V. Lachesis Muta (Viperidae) CDNAs Reveal Diverging Pit Viper Molecules and Scaffolds Typical of Cobra (Elapidae) Venoms: Implications for Snake Toxin Repertoire Evolution. Genetics 2006, 173, 877–889. [Google Scholar] [CrossRef]
- Pahari, S.; Mackessy, S.P.; Kini, R.M. The Venom Gland Transcriptome of the Desert Massasauga Rattlesnake (Sistrurus catenatus edwardsii): Towards an Understanding of Venom Composition among Advanced Snakes (Superfamily Colubroidea). BMC Mol. Biol. 2007, 8, 115. [Google Scholar] [CrossRef]
- Leonardi, A.; Sajevic, T.; Pungerčar, J.; Križaj, I. Comprehensive Study of the Proteome and Transcriptome of the Venom of the Most Venomous European Viper: Discovery of a New Subclass of Ancestral Snake Venom Metalloproteinase Precursor-Derived Proteins. J. Proteome Res. 2019, 18, 2287–2309. [Google Scholar] [CrossRef]
- Kashima, S.; Roberto, P.G.; Soares, A.M.; Astolfi-Filho, S.; Pereira, J.O.; Giuliati, S.; Faria, M., Jr.; Xavier, M.A.S.; Fontes, M.R.M.; Giglio, J.R.; et al. Analysis of Bothrops Jararacussu Venomous Gland Transcriptome Focusing on Structural and Functional Aspects: I—Gene Expression Profile of Highly Expressed Phospholipases A2. Biochimie 2004, 86, 211–219. [Google Scholar] [CrossRef]
- Rajagopalan, N.; Pung, Y.F.; Zhu, Y.Z.; Wong, P.T.H.; Kumar, P.P.; Kini, R.M. Β-Cardiotoxin: A New Three-finger Toxin from Ophiophagus Hannah (King Cobra) Venom with Beta-blocker Activity. FASEB J. 2007, 21, 3685–3695. [Google Scholar] [CrossRef]
- Calvete, J.J. Antivenomics and Venom Phenotyping: A Marriage of Convenience to Address the Performance and Range of Clinical Use of Antivenoms. Toxicon 2010, 56, 1284–1291. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; León, G.; Burnouf, T. Antivenoms for the Treatment of Snakebite Envenomings: The Road Ahead. Biologicals 2011, 39, 129–142. [Google Scholar] [CrossRef]
- Kini, R.; Sidhu, S.; Laustsen, A. Biosynthetic Oligoclonal Antivenom (BOA) for Snakebite and Next-Generation Treatments for Snakebite Victims. Toxins 2018, 10, 534. [Google Scholar] [CrossRef]
- Laustsen, A.H.; Engmark, M.; Milbo, C.; Johannesen, J.; Lomonte, B.; Gutiérrez, J.M.; Lohse, B. From Fangs to Pharmacology: The Future of Snakebite Envenoming Therapy. Curr. Pharm. Des. 2016, 22, 5270–5293. [Google Scholar] [CrossRef]
- Boulain, J.-C.; Mdnez, A.; Couderc, J.; Faure, G.; Liacopoulos, P.; Fromageot, P. Neutralizing Monoclonal Antibody Specific for Naja Nigricollis Toxin Alpha: Preparation, Characterization, and Localization of the Antigenic Binding Site. Biochemistry 1982, 21, 2910–2915. [Google Scholar] [CrossRef]
- Morine, N.; Matsuda, S.; Terada, K.; Eto, A.; Ishida, I.; Oku, H. Neutralization of Hemorrhagic Snake Venom Metalloproteinase HR1a from Protobothrops Flavoviridis by Human Monoclonal Antibody. Toxicon 2008, 51, 345–352. [Google Scholar] [CrossRef]
- Laustsen, A.H.; Karatt-Vellatt, A.; Masters, E.W.; Arias, A.S.; Pus, U.; Knudsen, C.; Oscoz, S.; Slavny, P.; Griffiths, D.T.; Luther, A.M.; et al. In Vivo Neutralization of Dendrotoxin-Mediated Neurotoxicity of Black Mamba Venom by Oligoclonal Human IgG Antibodies. Nat. Commun. 2018, 9, 3928. [Google Scholar] [CrossRef]
- Lafaye, P.; Choumet, V.; Demangel, C.; Bon, C.; Mazié, J.-C. Biologically Active Human Anti-Crotoxin ScFv Isolated from a Semi-Synthetic Phage Library. Immunotechnology 1997, 3, 117–125. [Google Scholar] [CrossRef]
- Tamarozzi, M.B.; Soares, S.G.; Marcussi, S.; Giglio, J.R.; Barbosa, J.E. Expression of Recombinant Human Antibody Fragments Capable of Inhibiting the Phospholipase and Myotoxic Activities of Bothrops Jararacussu Venom. Biochim. Biophys. Acta Gen. Subj. 2006, 1760, 1450–1457. [Google Scholar] [CrossRef]
- Kulkeaw, K.; Sakolvaree, Y.; Srimanote, P.; Tongtawe, P.; Maneewatch, S.; Sookrung, N.; Tungtrongchitr, A.; Tapchaisri, P.; Kurazono, H.; Chaicumpa, W. Human Monoclonal ScFv Neutralize Lethal Thai Cobra, Naja Kaouthia, Neurotoxin. J. Proteom. 2009, 72, 270–282. [Google Scholar] [CrossRef]
- Silva, L.C.; Pucca, M.B.; Pessenda, G.; Campos, L.B.; Martinez, E.Z.; Cerni, F.A.; Barbosa, J.E. Discovery of Human ScFvs That Cross-Neutralize the Toxic Effects of B. Jararacussu and C. d. Terrificus Venoms. Acta Trop. 2018, 177, 66–73. [Google Scholar] [CrossRef]
- Lewin, M.; Samuel, S.; Merkel, J.; Bickler, P. Varespladib (LY315920) Appears to Be a Potent, Broad-Spectrum, Inhibitor of Snake Venom Phospholipase A2 and a Possible Pre-Referral Treatment for Envenomation. Toxins 2016, 8, 248. [Google Scholar] [CrossRef]
- Lewin, M.; Gutiérrez, J.; Samuel, S.; Herrera, M.; Bryan-Quirós, W.; Lomonte, B.; Bickler, P.; Bulfone, T.; Williams, D. Delayed Oral LY333013 Rescues Mice from Highly Neurotoxic, Lethal Doses of Papuan Taipan (Oxyuranus scutellatus) Venom. Toxins 2018, 10, 380. [Google Scholar] [CrossRef] [PubMed]
- Arias, A.S.; Rucavado, A.; Gutiérrez, J.M. Peptidomimetic Hydroxamate Metalloproteinase Inhibitors Abrogate Local and Systemic Toxicity Induced by Echis Ocellatus (Saw-Scaled) Snake Venom. Toxicon 2017, 132, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, J.; Zhang, D.; Xiao, H.; Xiong, S.; Huang, C. Exploration of the Inhibitory Potential of Varespladib for Snakebite Envenomation. Molecules 2018, 23, 391. [Google Scholar] [CrossRef] [PubMed]
- Escalante, T.; Franceschi, A.; Rucavado, A.; Gutiérrez, J.M. Effectiveness of Batimastat, a Synthetic Inhibitor of Matrix Metalloproteinases, in Neutralizing Local Tissue Damage Induced by BaP1, a Hemorrhagic Metalloproteinase from the Venom of the Snake Bothrops Asper. Biochem. Pharm. 2000, 60, 269–274. [Google Scholar] [CrossRef]
- Ferreira, F.B.; Pereira, T.M.; Souza, D.L.N.; Lopes, D.S.; Freitas, V. Structure-Based Discovery of Thiosemicarbazone Metalloproteinase Inhibitors for Hemorrhage Treatment in Snakebites. ACS Med. Chem. Lett. 2017, 8, 1136–1141. [Google Scholar] [CrossRef]
- Karain, B.D.; Lee, M.K.H.; Hayes, W.K. C60 Fullerenes as a Novel Treatment for Poisoning and Envenomation: A Proof-of-Concept Study for Snakebite. J. Nanosci. Nanotechnol. 2016, 16, 7764–7771. [Google Scholar] [CrossRef]
- Richard, G.; Meyers, A.J.; McLean, M.D.; Arbabi-Ghahroudi, M.; MacKenzie, R.; Hall, J.C. In Vivo Neutralization of α-Cobratoxin with High-Affinity Llama Single-Domain Antibodies (VHHs) and a VHH-Fc Antibody. PLoS ONE 2013, 8, e69495. [Google Scholar] [CrossRef]
- Chavanayarn, C.; Thanongsaksrikul, J.; Thueng-in, K.; Bangphoomi, K.; Sookrung, N.; Chaicumpa, W. Humanized-Single Domain Antibodies (VH/VHH) That Bound Specifically to Naja Kaouthia Phospholipase A2 and Neutralized the Enzymatic Activity. Toxins 2012, 4, 554–567. [Google Scholar] [CrossRef]
- Luiz, M.; Pereira, S.; Prado, N.; Gonçalves, N.; Kayano, A.; Moreira-Dill, L.; Sobrinho, J.; Zanchi, F.; Fuly, A.; Fernandes, C.; et al. Camelid Single-Domain Antibodies (VHHs) against Crotoxin: A Basis for Developing Modular Building Blocks for the Enhancement of Treatment or Diagnosis of Crotalic Envenoming. Toxins 2018, 10, 142. [Google Scholar] [CrossRef]
- Castro, J.M.A.; Oliveira, T.S.; Silveira, C.R.F.; Caporrino, M.C.; Rodriguez, D.; Moura-da-Silva, A.M.; Ramos, O.H.P.; Rucavado, A.; Gutiérrez, J.M.; Magalhães, G.S.; et al. A Neutralizing Recombinant Single Chain Antibody, ScFv, against BaP1, A P-I Hemorrhagic Metalloproteinase from Bothrops Asper Snake Venom. Toxicon 2014, 87, 81–91. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, Y.; Liu, M.; Chen, X.; Xiang, Q.; Tian, J. Functional Recombinant Single-chain Variable Fragment Antibody against Agkistrodon Acutus Venom. Exp. Med. 2019, 17, 3768–3774. [Google Scholar] [CrossRef]
- Al-Jabi, S.W. Arab World’s Growing Contribution to Global Leishmaniasis Research (1998–2017): A Bibliometric Study. BMC Public Health 2019, 19, 625. [Google Scholar] [CrossRef]
- Sweileh, W.M.; Al-Jabi, S.W.; Sawalha, A.F.; AbuTaha, A.S.; Zyoud, S.H. Bibliometric Analysis of Worldwide Publications on Antimalarial Drug Resistance (2006–2015). Malar. Res. Treat. 2017, 2017, 1–13. [Google Scholar] [CrossRef]

| SCR a | Country | No. of Documents (%) | No. of Collaborating Countries b |
|---|---|---|---|
| 1 | Brazil | 729 (24.3) | 18 |
| 2 | United States | 548 (18.2) | 24 |
| 3 | Australia | 240 (8.00) | 21 |
| 4 | Costa Rica | 235 (7.83) | 18 |
| 5 | United Kingdom | 208 (6.93) | 21 |
| 6 | Japan | 202 (6.73) | 11 |
| 7 | China | 182 (6.06) | 15 |
| 8 | India | 180 (6.00) | 13 |
| 9 | Taiwan, China | 103 (3.43) | 6 |
| 10 | Germany | 100 (3.33) | 21 |
| SCR a | Journal Title | No. of Documents (%) | Impact Factor b |
|---|---|---|---|
| 1 | Toxicon | 682 (22.74) | 2.74 |
| 2 | Toxins | 115 (3.83) | 4.086 |
| 3 | Journal of Venomous Animals and Toxins Including Tropical Diseases | 47 (1.56) | 2.71 |
| 4 | Journal of Proteomics | 40 (1.33) | 4.044 |
| 5 | Biochimie | 36 (1.20) | 4.079 |
| 6 | Journal of Biological Chemistry | 35 (1.16) | 5.157 |
| 7 | International Journal of Biological Macromolecules | 34 (1.13) | 6.953 |
| 8 | Archives of Biochemistry and Biophysics | 33 (1.10) | 4.013 |
| 8 | Biochemical and Biophysical Research Communications | 33 (1.10) | 3.575 |
| 10 | Biochemistry | 27 (0.90) | 3.162 |
| SCR a | Authors | Title | Article Type | Year | Journal Title | No. of Citations |
|---|---|---|---|---|---|---|
| 1 | Bode et al. [101] | Astacins, serralysins, snake venom and matrix metalloproteinases exhibit identical zinc-binding environments (HEXXHXXGXXH and Met-turn) and topologies and should be grouped into a common family, the ‘metzincins’ | Article | 1993 | FEBS Letters | 630 |
| 2 | Markland [102] | Snake venoms and the hemostatic system | Review | 1998 | Toxicon | 546 |
| 3 | Bjarnason and Fox [103] | Hemorrhagic metalloproteinases from snake venoms | Review | 1994 | Pharmacology and Therapeutics | 483 |
| 4 | Theakston and Reid [104] | Development of simple standard assay procedures for the characterization of snake venoms | Article | 1983 | Bulletin of the World Health Organization | 482 |
| 5 | Daltry et al. [105] | Diet and snake venom evolution | Article | 1996 | Nature | 477 |
| 6 | Fry et al. [106] | Early evolution of the venom system in lizards and snakes | Article | 2006 | Nature | 423 |
| 7 | Gutiérrez and Lomonte [107] | Phospholipase A2 myotoxins from Bothrops snake venoms | Review | 1995 | Toxicon | 422 |
| 8 | Gutiérrez and Rucavado [27] | Snake venom metalloproteinases: Their role in the pathogenesis of local tissue damage | Review | 2000 | Biochimie | 416 |
| 9 | Fox and Serrano [108] | Structural considerations of the snake venom metalloproteinases, key members of the M12 reprolysin family of metalloproteinases | Article | 2005 | Toxicon | 406 |
| 10 | Matsui et al. [109] | Snake venom proteases affecting hemostasis and thrombosis | Review | 2000 | Biochimica et Biophysica Acta | 359 |
| SCR a | Institution | Country | No. of Documents (%) |
|---|---|---|---|
| 1 | Universidad de Costa Rica | Costa Rica | 240 (8.00) |
| 2 | Instituto Butantan | Brazil | 228 (7.60) |
| 3 | Universidade de São Paulo | Brazil | 213 (7.10) |
| 4 | Universidade Estadual de Campinas | Brazil | 95 (3.16) |
| 5 | National University of Singapore | Singapore | 77 (2.56) |
| 6 | Universidade Estadual Paulista Júlio de Mesquita Filho | Brazil | 76 (2.53) |
| 7 | Liverpool School of Tropical Medicine | United Kingdom | 73 (2.43) |
| 8 | Fundação Oswaldo Cruz | Brazil | 72 (2.40) |
| 9 | National Taiwan University | Taiwan, China | 59 (1.96) |
| 9 | Universidade Federal de Uberlândia | Brazil | 59 (1.96) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sofyantoro, F.; Yudha, D.S.; Lischer, K.; Nuringtyas, T.R.; Putri, W.A.; Kusuma, W.A.; Purwestri, Y.A.; Swasono, R.T. Bibliometric Analysis of Literature in Snake Venom-Related Research Worldwide (1933–2022). Animals 2022, 12, 2058. https://doi.org/10.3390/ani12162058
Sofyantoro F, Yudha DS, Lischer K, Nuringtyas TR, Putri WA, Kusuma WA, Purwestri YA, Swasono RT. Bibliometric Analysis of Literature in Snake Venom-Related Research Worldwide (1933–2022). Animals. 2022; 12(16):2058. https://doi.org/10.3390/ani12162058
Chicago/Turabian StyleSofyantoro, Fajar, Donan Satria Yudha, Kenny Lischer, Tri Rini Nuringtyas, Wahyu Aristyaning Putri, Wisnu Ananta Kusuma, Yekti Asih Purwestri, and Respati Tri Swasono. 2022. "Bibliometric Analysis of Literature in Snake Venom-Related Research Worldwide (1933–2022)" Animals 12, no. 16: 2058. https://doi.org/10.3390/ani12162058
APA StyleSofyantoro, F., Yudha, D. S., Lischer, K., Nuringtyas, T. R., Putri, W. A., Kusuma, W. A., Purwestri, Y. A., & Swasono, R. T. (2022). Bibliometric Analysis of Literature in Snake Venom-Related Research Worldwide (1933–2022). Animals, 12(16), 2058. https://doi.org/10.3390/ani12162058






