Out of Africa: Juvenile Dispersal of Black-Shouldered Kites in the Emerging European Population
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. The Study System
2.2. Field Procedures
2.3. Dispersal Measurements
2.4. Statistical Analyses
3. Results
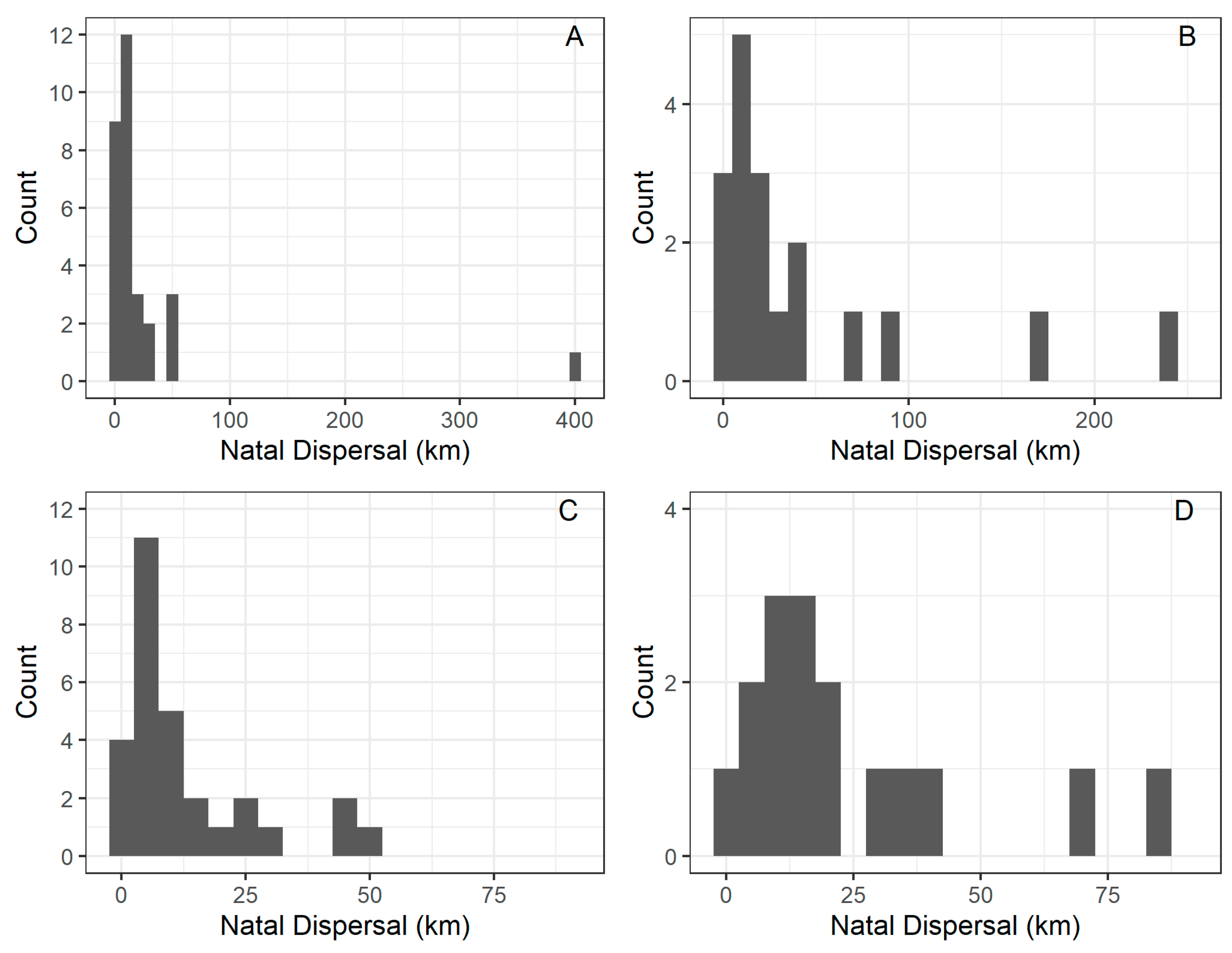
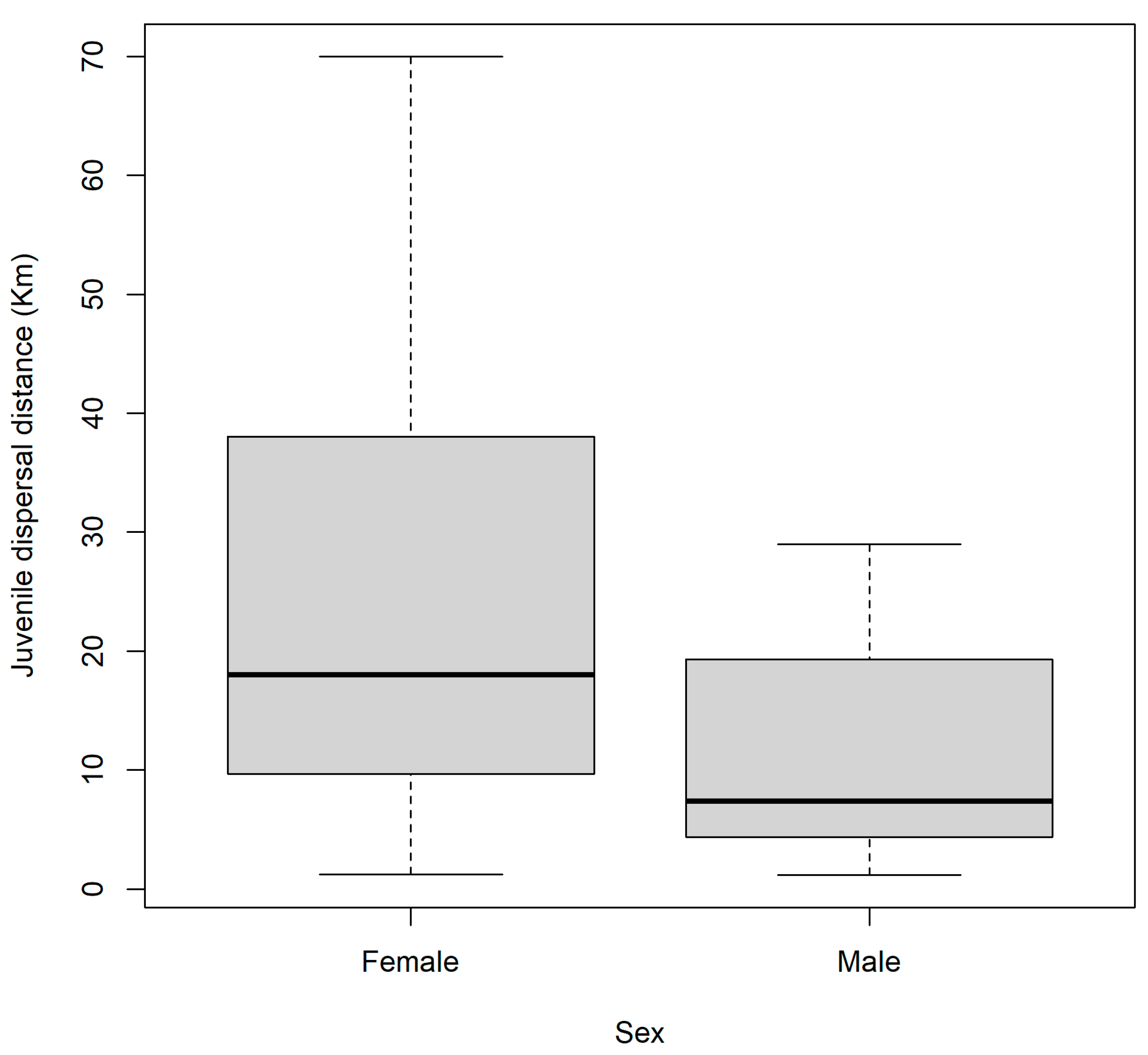
3.1. Juvenile Dispersal
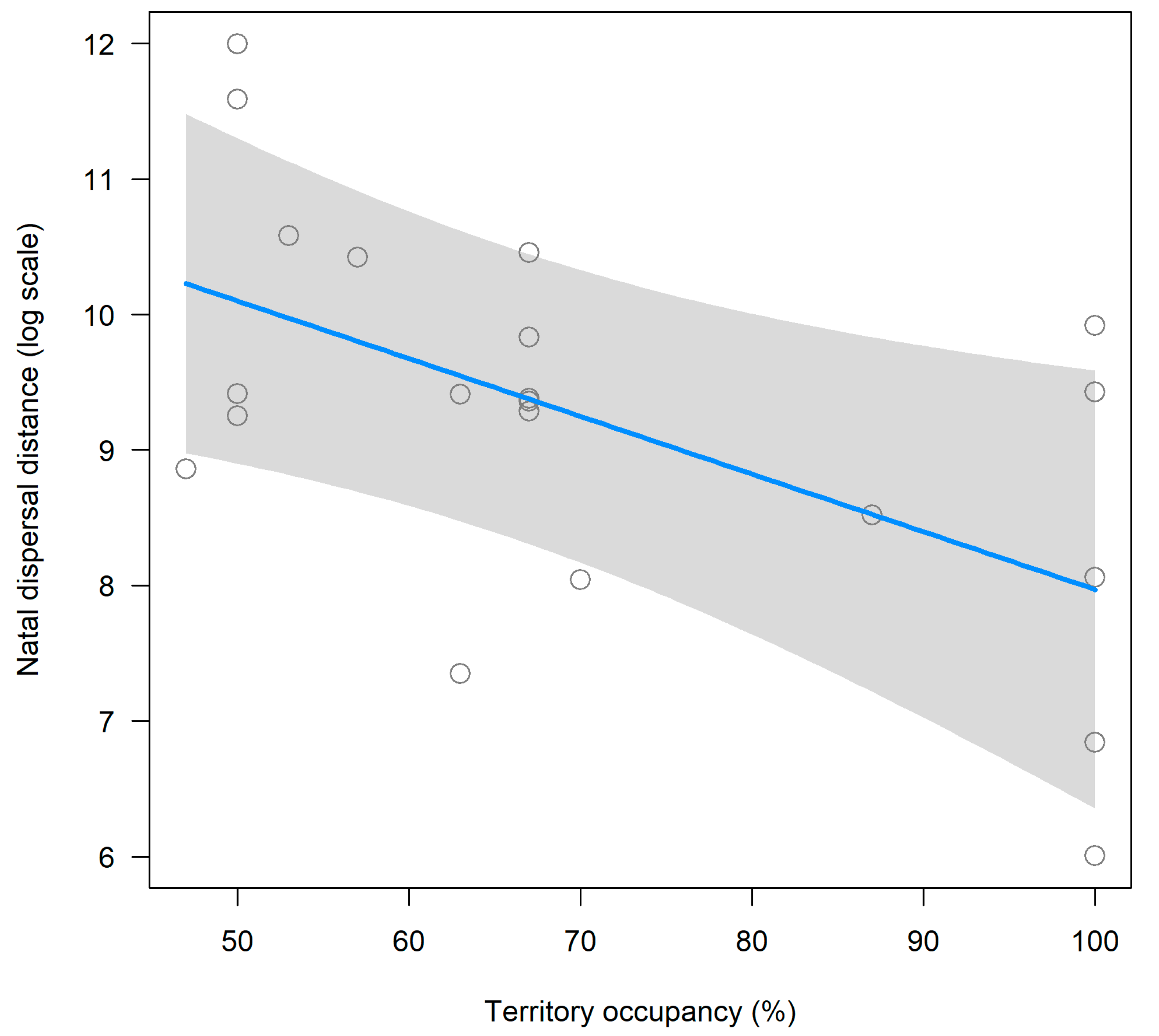
3.2. Natal Dispersal
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clobert, J.; Danchin, E.; Dhondt, A.A.; Nichols, J.D. Dispersal; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Garant, D.; Kruuk, L.E.; Wilkin, T.A.; McCleery, R.H.; Sheldon, B.C. Evolution driven by differential dispersal within a wild bird population. Nature 2005, 433, 60–65. [Google Scholar] [CrossRef]
- Greenwood, P.J.; Harvey, P.H. The natal and breeding dispersal of birds. Annu. Rev. Ecol. Syst. 1982, 13, 1–21. [Google Scholar] [CrossRef]
- Cresswell, W. Migratory connectivity of Palaearctic–African migratory birds and their responses to environmental change, the serial residency hypothesis. IBIS 2014, 156, 493–510. [Google Scholar] [CrossRef]
- Berthold, P. Bird Migration: A General Survey; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Valkama, J.; Saurola, P.; Lehikoinen, A.; Lehikoinen, E.; Piha, M.V.; Sola, P.; Velmala, W. Somen Rengastusatlas. Osa II; Luonnontieteellinen Keskusmuseo: Helsinki, Finland, 2014. [Google Scholar]
- Whitfield, D.P.; Duffy, K.; McLeod, D.R.A.; Evans, R.J.; MacLennan, A.M.; Reid, R.; Sexton, D.; Wilson, J.D.; Douse, A. Juvenile dispersal of white-tailed eagles in Western Scotland. J. Raptor Res. 2009, 43, 110–120. [Google Scholar]
- Engler, M.; Krone, O. Movement patterns of the White-tailed Sea Eagle (Haliaeetus albicilla): Post-fledging behaviour, natal dispersal onset and the role of the natal environment. IBIS 2022, 164, 188–201. [Google Scholar] [CrossRef]
- Koenig, W.D.; Van Vuren, D.; Hooge, P.N. Detectability philopatry and the distribution of dispersal distances in vertebrates. Trends Ecol. Evol. 1996, 11, 514–517. [Google Scholar] [CrossRef]
- McGuire, B.; Getz, L.L.; Hofmann, J.E.; Pizzuto, T.; Frase, B. Natal dispersal and philopatry in prairie voles Microtus ochrogaster in relation to population density season and natal social environment. Behav. Ecol. Soc. 1993, 32, 293–302. [Google Scholar] [CrossRef]
- Hendry, A.P.; Berg, O.K.; Quinn, T.P. Breeding location choice in salmon, causes habitat competition body size energy stores and consequences life span energy stores. Oikos 2001, 93, 407–418. [Google Scholar] [CrossRef]
- Enfjall, K.; Leimar, O. Density-dependent dispersal in the Glanville fritillary Melitaea cinxia. Oikos 2005, 108, 465–472. [Google Scholar] [CrossRef]
- Matthysen, E. Density-dependent dispersal in birds and mammals. Ecography 2005, 283, 403–416. [Google Scholar] [CrossRef]
- Cote, J.; Clobert, J. Social personalities influence natal dispersal in a lizard. Proc. Biol. Sci. 2007, 274, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Rouquette, J.R.; Thompson, D.J. Patterns of movement and dispersal in an endangered damselfly and the consequences for its management. J. Appl. Ecol. 2007, 44, 692–701. [Google Scholar] [CrossRef]
- Buechner, M. A geometric model of vertebrate dispersal, test and implications. Ecology 1987, 68, 310–318. [Google Scholar] [CrossRef]
- Miller, G.L.; Carroll, B.W. Modelling vertebrate dispersal distances, alternatives to geometric distribution. Ecology 1989, 70, 977–986. [Google Scholar] [CrossRef]
- Ferrer, M. Ontogeny of dispersal distances in young Spanish imperial eagles. Behav. Ecol. Sociobiol. 1993, 32, 259–263. [Google Scholar] [CrossRef]
- Van Houtan, K.S.; Pimm, S.L.; Halley, J.M.; Bierregaard, R.O.; Lovejoy, T.E. Dispersal of Amazonian birds in continuous and fragmented forest. Ecol. Lett. 2007, 10, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Ceresa, F.; Belda, E.J.; Monrós, J.S. Similar dispersal patterns between two closely related birds with contrasting migration strategies. Popul. Ecol. 2016, 58, 421–427. [Google Scholar] [CrossRef]
- Rushing, C.S.; Ryder, T.B.; Valente, J.J.; Sillett, T.S.; Marra, P.P. Empirical tests of habitat selection theory reveal that conspecific density and patch quality; but not habitat amount; drive long-distance immigration in a wild bird. Ecol. Lett. 2021, 24, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Clobert, J.; Baguette, M.; Benton, T.G.; Bullock, J.M. Dispersal Ecology and Evolution; Oxford University Press: Oxford, UK, 2012; p. 496. [Google Scholar]
- Muriel, R.; Morandini, V.; Ferrer, M.; Balbontín, J.; Morlanes, V. Juvenile dispersal behaviour and conspecific attraction, An alternative approach with translocated Spanish imperial eagles. Anim. Behav. 2016, 116, 17–29. [Google Scholar] [CrossRef]
- Murray, B.G. Dispersal in vertebrates. Ecology 1967, 48, 975–978. [Google Scholar] [CrossRef]
- Serrano, D. Dispersal in raptors. In Birds of Prey: Biology and Conservation in the XXI Century; Sarasola, J.H., Grande, J.M., Negro, J.J., Eds.; Springer International Publishing: Seville, Spain, 2018; pp. 95–121. [Google Scholar]
- Serrano, D.; Cortés-Avizanda, A.; Zuberogoitia, I.; Blanco, G.; Benítez, J.R.; Ponchon, C.; Grande, J.M.; Ceballos, O.; Morant, J.; Arrondo, E.; et al. Phenotypic and environmental correlates of natal dispersal in a long-lived territorial vulture. Sci. Rep. 2021, 11, 5424. [Google Scholar] [CrossRef]
- Waser, P.M. Does competition drive dispersal? Ecology 1985, 66, 1171–1175. [Google Scholar] [CrossRef]
- Pärt, T. Natal dispersal in the collared flycatcher, possible causes and reproductive consequences. Ornis. Scand. 1990, 21, 83–88. [Google Scholar] [CrossRef]
- Negro, J.J.; Hiraldo, F.; Donázar, J.A. Causes of natal dispersal in the lesser kestrel, inbreeding avoidance or resource competition? J. Anim. Ecol. 1997, 66, 640–648. [Google Scholar] [CrossRef]
- Verhulst, S.; Perrins, C.M.; Riddington, R. Natal dispersal of great tits in a patchy environment. Ecology 1997, 78, 864–872. [Google Scholar] [CrossRef]
- Ferrer, M.; Morandini, V. Better nutritional condition changes the distribution of juvenile dispersal distances, an experiment with Spanish imperial eagle. J. Avian Biol. 2017, 48, 1342–1347. [Google Scholar] [CrossRef]
- Greenwood, P.J. Mating systems philopatry and dispersal in birds and Mammals. Anim. Behav. 1980, 28, 1140–1162. [Google Scholar] [CrossRef]
- Trochet, A.; Courtois, E.A.; Stevens, V.M.; Baguette, M.; Chaine, A.; Schmeller, D.S.; Clobert, J. Evolution of sex-biased dispersal. Q. Rev. Biol. 2016, 913, 297–320. [Google Scholar] [CrossRef] [PubMed]
- Végvári, Z.; Katona, G.; Vági, B.; Freckleton, R.P.; Gaillard, J.-M.; Székely, T.; Liker, A. Sex-biased breeding dispersal is predicted by social environment in birds. Ecol. Evol. 2018, 8, 6483–6491. [Google Scholar] [CrossRef]
- Arlt, D.; Pärt, T. Sex-biased dispersal, a result of a sex difference in breeding site availability. Am. Nat. 2008, 171, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.L.; Saether, B.E.; Roskaft, E. Sex biases in avian dispersal, a reappraisal. Oikos 1997, 79, 429–438. [Google Scholar] [CrossRef]
- Sutherland, G.D.; Harestad, A.S.; Price, K.; Lertzman, K.P. Scaling of natal dispersal distances in terrestrial birds and mammals. Conserv. Ecol. 2000, 4, 16. [Google Scholar] [CrossRef]
- Mabry, K.E.; Shelley, E.L.; Davis, K.E.; Blumstein, D.T.; Van Vuren, D.H. Social mating system and sex-biased dispersal in mammals and birds, a phylogenetic analysis. PLoS ONE 2013, 8, e57980. [Google Scholar] [CrossRef] [PubMed]
- Bernis, F. Dos capturas de Elanus caeruleus en el Centro de España. Ardeola 1961, 7, 255–256. [Google Scholar]
- England, D.M. Observations on the Black-winged Kite in Portugal with preliminary notes on its status. Br. Birds 1963, 56, 444–456. [Google Scholar]
- Kemp, A.C.; Kirwan, G.M.; Marks, J.S.; Motis, A.; Garcia, E.F.J. Black-winged Kite Elanus caeruleus en. In Handbook of the Birds of the World Alive; del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A., de Juana, E., Eds.; Lynx Edicions: Barcelona, Spain, 2019. [Google Scholar]
- Mendelsohn, J.M. Social behaviour and dispersion of the black-shouldered kite. Ostrich 1983, 54, 1–18. [Google Scholar] [CrossRef]
- Brown, L.; Amadon, D. Eagles Hawks and Falcons of the World; Country Life Books: London, UK, 1968. [Google Scholar]
- Tyrberg, T. Pleistocene Birds of the Palearctic; Nuttall Ornithological Club: Cambridge, MA, USA, 1998; Number 27. [Google Scholar]
- Tyrberg, T. Online Supplement to Pleistocene Birds of the Palearctic a Catalogue; Publication of the Nuttall Ornithological Club: Cambridge, MA, USA, 2008; Number 27; Last updated 24 February 2008. [Google Scholar]
- Mañosa, S.; Montes, G.; Bota, G.; Bonfil, J. Black-shouldered Kite Elanus caeruleus diet in an area recently colonized in the north-east of the Iberian Peninsula. Rev. Catalana D’ornitologia 2005, 211, 11–16. [Google Scholar]
- Logeais, J.M. Première nidification de l’Élanion blanc en Maine-et-Loire. Crex 2015, 13, 45–50. [Google Scholar]
- Ferguson-Lees, J.; Christie, D.A. Raptors of the World; Christopher Helm: London, UK, 2001. [Google Scholar]
- Rivera, D.; Pérez Gil, S.; Casas, J.M.; Balbontín, J.; Negro, J.J.; Abad-Gómez, J.M. Elanio Común—Elanus caeruleus en. Enciclopedia Virtual de los Vertebrados Españoles; López, P., Martín, J., Zuberogoitia, I., Eds.; Museo Nacional de Ciencias Naturales: Madrid, Spain, 2019; Available online: http://www.vertebradosibericos.org/ (accessed on 1 June 2022).
- Rivera, D.; Negro, J.J.; Balbontín, J.; Casas, J.M.; Ferrero, J.J.; Sarasola, J.H. Seguimiento de una población extremeña de elanio azul Elanus caeruleus. Quercus 2006, 239, 10–15. [Google Scholar]
- Bustamante, J. The post-fledging dependence period of the black-shouldered Kite. J. Raptor Res. 1993, 27, 185–190. [Google Scholar]
- Parejo, D.; Avilés, J.M.; Ferrero, J.J.; Rivera, D.; Casas, J.M. Communal roosting and diet of black shouldered kites Elanus caeruleus wintering in southwestern Spain. J. Raptor Res. 2001, 352, 162–164. [Google Scholar]
- Maldonado, M.R. Variación Estacional en la Dieta del Elanio Azul Elanus caeruleus en Extremadura Suroeste de España. Bachelor’s Thesis, Facultad de Biología, Universidad de Sevilla, Seville, Spain, 2015. [Google Scholar]
- Balbontín, J.; Negro, J.J.; Sarasola, J.H.; Ferrero, J.J.; Rivera, D. Land-use changes may explain the recent range expansion of the Black-shouldered Kite Elanus caeruleus in southern Europe. IBIS 2008, 150, 707–716. [Google Scholar] [CrossRef]
- Fridolfsson, A.-K.; Ellegren, H. A simple and universal method for molecular sexing of non-ratite birds. J. Avian Biol. 1999, 30, 116–121. [Google Scholar] [CrossRef]
- QGIS.org. QGIS Geographic Information System QGIS Association. 2021. Available online: http://www.qgis.org (accessed on 1 May 2021).
- Sergio, F.; Newton, I. Occupancy as a measure of territory quality. J. Anim. Ecol. 2003, 72, 857–865. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 1 May 2021).
- Höglund, J. Evolutionary Conservation Genetics; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Newton, I.; Marquiss, M. Dispersal of sparrowhawks between birthplace and breeding place. J. Anim. Ecol. 1983, 52, 463–477. [Google Scholar] [CrossRef]
- Limiñana, R.; García, J.T.; González, J.M.; Guerrero, A.; Lavedán, J.; Moreno, J.D.; Román-Muñoz, A.; Palomares, L.E.; Pinilla, A.; Ros, G.; et al. Philopatry and natal dispersal of Montagu’s harriers Circus pygargus breeding in Spain, a review of existing data. Eur. J. Wildl. Res. 2012, 58, 549–555. [Google Scholar] [CrossRef]
- BirdLife International. European Red List of Birds; Office for Official Publications of the European Communities: Luxembourg, 2015. [Google Scholar]
- Clobert, J.; Ims, R.A.; Rousset, F. Causes mechanisms and con sequences of dispersal. In Ecology Genetics and Evolution of Metapopulations; Hanski, I., Gaggiotti, O., Eds.; Academic Press: London, UK, 2004; pp. 307–335. [Google Scholar]
- Martínez-Padilla, J.; Vergara, P.; Fargallo, J.A. Increased lifetime reproductive success of first-hatched siblings in Common Kestrels Falcotinnunculus. IBIS 2017, 1594, 803–811. [Google Scholar] [CrossRef]
- Velando, A. The importance of hatching date for dominance in young shags. Anim. Behav. 2000, 60, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Cam, E.; Monnat, J.Y.; Hines, J.E. Long-term fitness consequences of early conditions in the kittiwake. J. Anim. Ecol. 2003, 723, 411–424. [Google Scholar] [CrossRef]
- Becker, P.H.; Wink, M. Influences of sex sex composition of brood and hatching order on mass growth in Common Terns Sterna hirundo. Behav. Ecol. Sociobiol. 2003, 54, 136–146. [Google Scholar] [CrossRef]
- Saino, N.; Romano, M.; Ambrosini, R.; Rubolini, D.; Boncoraglio, G.; Caprioli, M.; Romano, A. Longevity and lifetime reproductive success of Barn Swallow offspring are predicted by their hatching date and phenotypic quality. J. Anim. Ecol. 2012, 81, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Newton, I.; Marquiss, M. Seasonal trend in the breeding performance of Sparrowhawks. J. Anim. Ecol. 1984, 53, 809–829. [Google Scholar] [CrossRef]
- Verboven, N.; Visser, M.E. Seasonal variation in local recruitment of Great Tits, the importance of being early. Oikos 1998, 81, 511–524. [Google Scholar] [CrossRef]
- Bêty, J.; Gauthier, G.; Giroux, J.-F. Body condition migration and timing of reproduction in snow geese, a test of the condition dependent model of optimal clutch size. Am. Nat. 2003, 162, 110–121. [Google Scholar] [CrossRef]
- Verhulst, S.; Nilsson, J.-Å. The timing of birds’ breeding seasons, a review of experiments that manipulated timing of breeding. Philos. Trans. R. Soc. B 2008, 363, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Bowler, D.E.; Benton, T.G. Causes and consequences of animal dispersal strategies, Relating individual behaviour to spatial dynamics. Biol. Rev. 2005, 80, 205–225. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, A.R.; Muñoz, A.; Onrubia, A.; de la Cruz, A.; Cuenca, D.; González, J.M.; Arroyo, G.M. Spring movements of Rüppell’s Vulture Gyps rueppellii across the Strait of Gibraltar. Ostrich 2011, 82, 71–73. [Google Scholar] [CrossRef]
- Ávila, A.; Guirado, M.A.; Navarrete, J. Ratonero Moro Noticiario Ornitológico. Ardeola 2004, 51, 548. [Google Scholar]
- Elorriaga, J.; Muñoz, A.R. First breeding record of North African Long-legged Buzzard Buteo Rufinus cirtensis in continental Europe. Br. Birds. 2010, 103, 399–401. [Google Scholar]
- Negro, J.J.; Pertoldi, C.; Randi, E.; Ferrero, J.J.; Lopez Caballero, J.M.; Rivera, D.; Korpimaki, E. Convergent Evolution of Elanus Kites and The Owls. J. Raptor Res. 2006, 403, 222–225. [Google Scholar] [CrossRef]
- Mindell, D.P.; Fuchs, J.; Johnson, J.A. Phylogeny Taxonomy and Geographic Diversity of Diurnal Raptors, Falconiformes Accipitriformes and Cathartiformes. In Birds of Prey Biology and Conservation in the XXI Century; Sarasola, J.H., Grande, J.M., Negro, J.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 3–32. [Google Scholar]


| Predictor | Estimate | SE | t-Value | p-Value |
|---|---|---|---|---|
| Intercept | 9.078 | 0.463 | 19.58 | <0.001 |
| Brood Order (Second) | 1.356 | 0.717 | 1.89 | 0.07 |
| Brood Order (Third) | 0.955 | 0.770 | 1.24 | 0.23 |
| Territory Occupancy (%) | −1.467 | 0.653 | −2.24 | 0.038 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera, D.; Balbontín, J.; Pérez Gil, S.; Abad Gómez-Pantoja, J.M.; Negro, J.J. Out of Africa: Juvenile Dispersal of Black-Shouldered Kites in the Emerging European Population. Animals 2022, 12, 2070. https://doi.org/10.3390/ani12162070
Rivera D, Balbontín J, Pérez Gil S, Abad Gómez-Pantoja JM, Negro JJ. Out of Africa: Juvenile Dispersal of Black-Shouldered Kites in the Emerging European Population. Animals. 2022; 12(16):2070. https://doi.org/10.3390/ani12162070
Chicago/Turabian StyleRivera, Domingo, Javier Balbontín, Sergio Pérez Gil, José María Abad Gómez-Pantoja, and Juan José Negro. 2022. "Out of Africa: Juvenile Dispersal of Black-Shouldered Kites in the Emerging European Population" Animals 12, no. 16: 2070. https://doi.org/10.3390/ani12162070






