Yeast β-Glucans as Fish Immunomodulators: A Review
Abstract
:Simple Summary
Abstract
1. Introduction
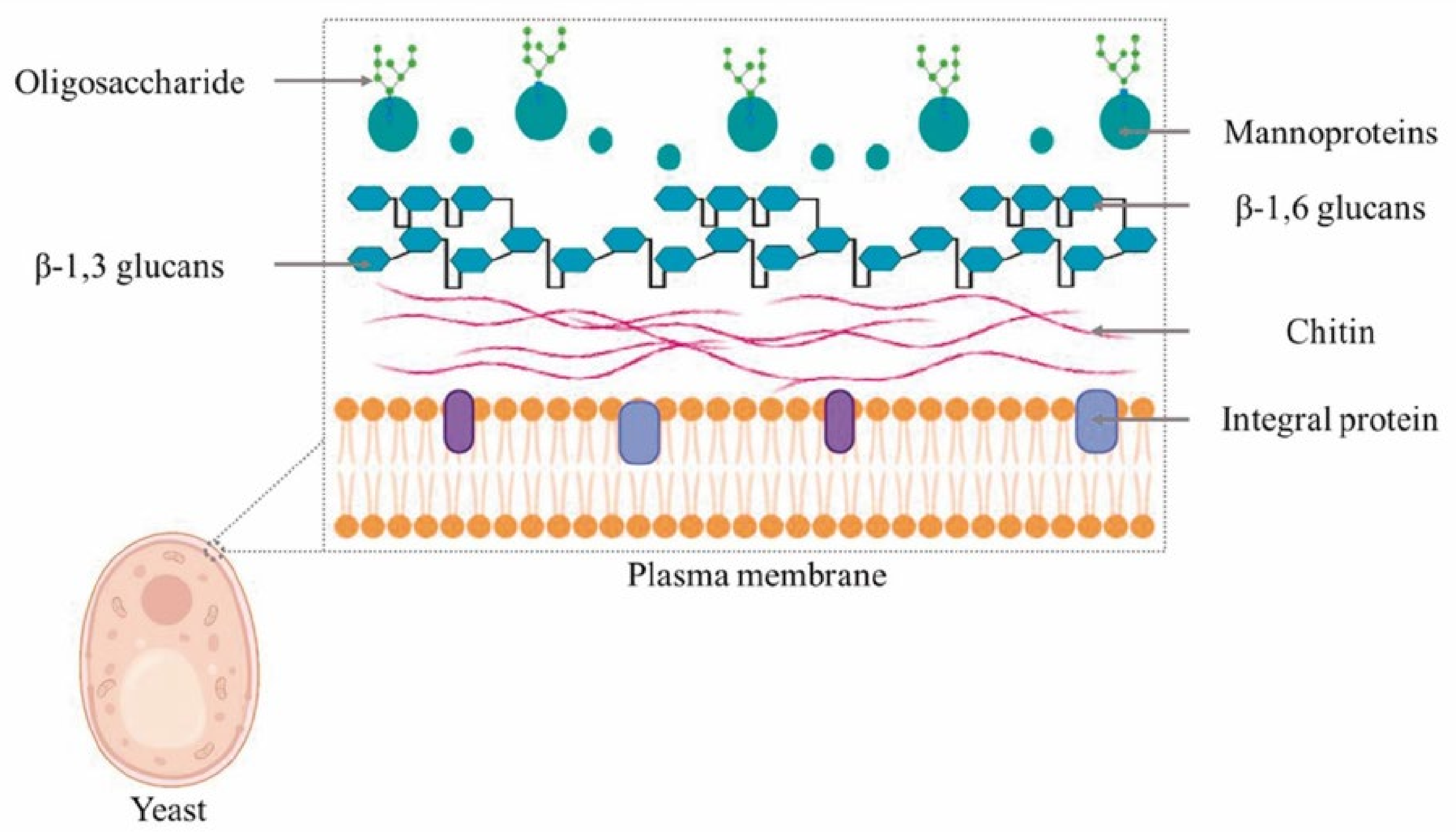
2. Yeast Cell Wall and β-Glucan Composition
2.1. Yeast Cell Wall
2.2. Yeast β-Glucans
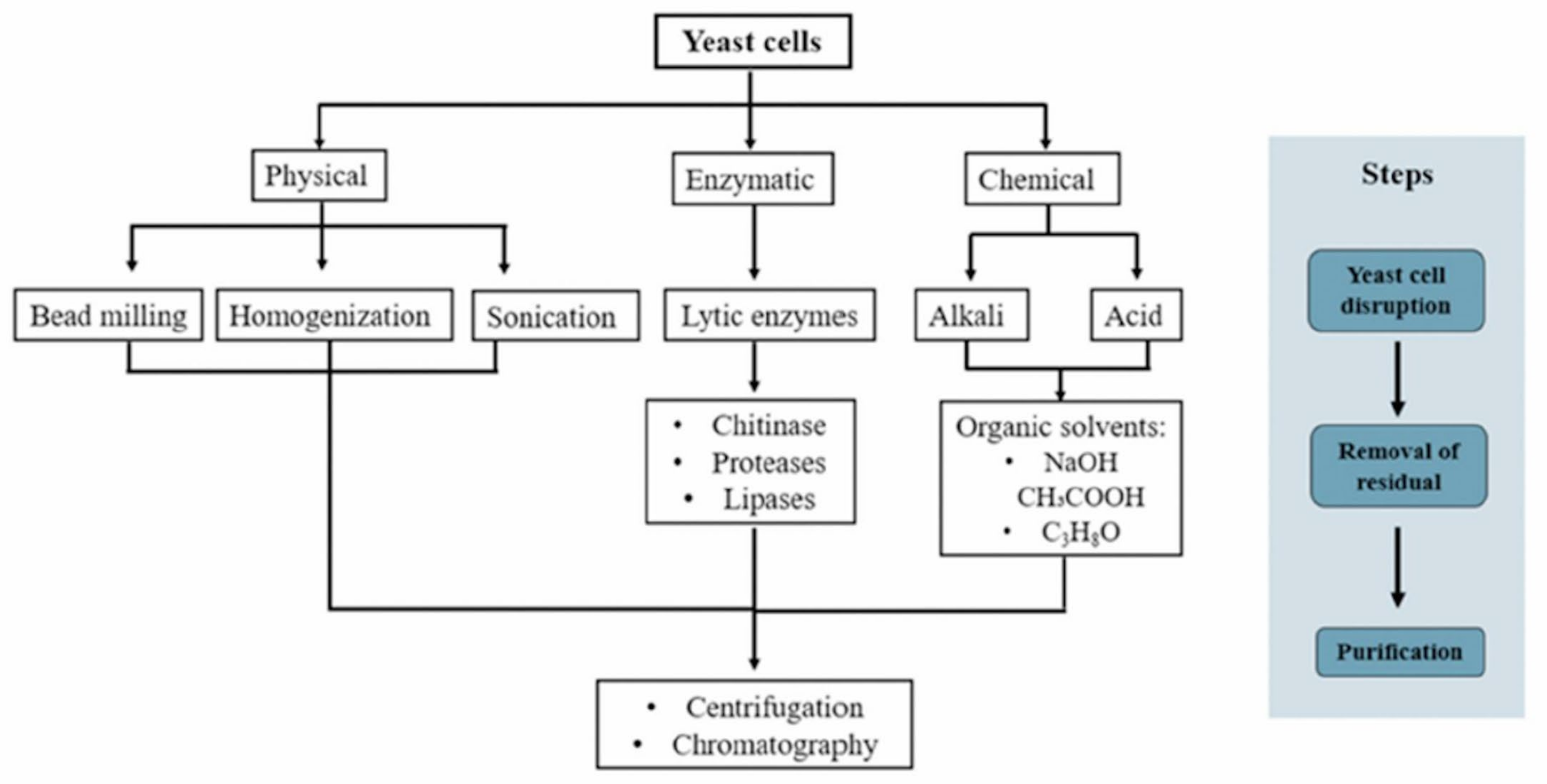
3. Yeast β-Glucan Extraction
3.1. Physical Method
3.2. Chemical Method
3.3. Enzymatic Method
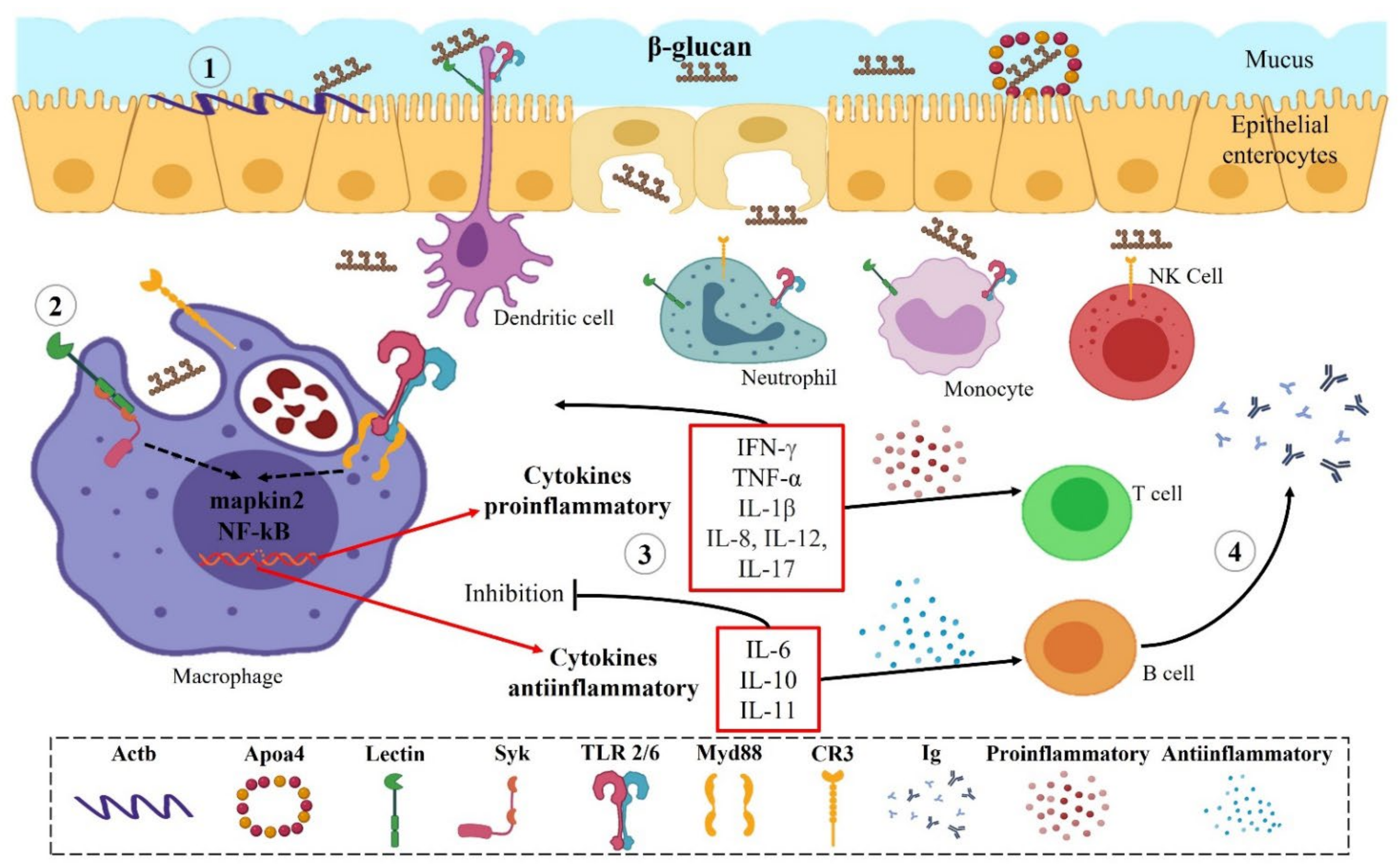
4. Effects of Yeast β-Glucans on Fish Immune System
4.1. Freshwater Fish
4.2. Marine Fish
5. Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Øverland, M.; Skrede, A. Yeast derived from lignocellulosic biomass as a sustainable feed resource for use in aquaculture: Yeast from lignocellulosic biomass as a feed in aquaculture. J. Sci. Food Agric. 2016, 97, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Gianni, L. The fascinating and secret wild life of the budding yeast S. cerevisiae. eLife 2015, 4, e05835. [Google Scholar] [CrossRef]
- Sagot, I.; Laporte, D. The cell biology of quiescent yeast—A diversity of individual scenarios. J. Cell Sci. 2019, 132, jcs213025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, A.M.; Goddard, S.; Bemibster, P. Production of Candida utilis biomass as aquaculture feed. J. Sci. Food Agric. 1993, 61, 363–370. [Google Scholar] [CrossRef]
- Reveco-Urzua, F.E.; Hofossæter, M.; Kovi, M.R.; Mydland, L.T.; Ånestad, R.; Sørby, R.; Press, C.M.; Lagos, L.; Øverland, M. Candida utilis yeast as a functional protein source for Atlantic salmon (Salmo salar L.): Local intestinal tissue and plasma proteome responses. PLoS ONE 2019, 14, e0218360. [Google Scholar] [CrossRef] [Green Version]
- Dimitroglou, A.; Merrifield, D.L.; Spring, P.; Sweetman, J.; Moate, R.; Davies, S.J. Effects of mannan oligosaccharide (MOS) supplementation on growth performance, feed utilisation, intestinal histology and gut microbiota of gilthead sea bream (Sparus aurata). Aquaculture 2010, 300, 182–188. [Google Scholar] [CrossRef]
- Navarrete, P.; Tovar-Ramírez, D. Use of Yeasts as Probiotics in Fish Aquaculture. Sustain. Aquac. Tech. 2014, 1, 135–172. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, A.; Gallardo-Escárate, C. Microbiome dynamic modulation through functional diets based on pre- and probiotics (mannan-oligosaccharides and Saccharomyces cerevisiae) in juvenile rainbow trout (Oncorhynchus mykiss). J. Appl. Microbiol. 2017, 122, 1333–1347. [Google Scholar] [CrossRef]
- Yuan, X.-Y.; Liu, W.-B.; Liang, C.; Sun, C.-X.; Xue, Y.-F.; Wan, Z.-D.; Jiang, G.-Z. Effects of partial replacement of fish meal by yeast hydrolysate on complement system and stress resistance in juvenile Jian carp (Cyprinus carpio var. Jian). Fish Shellfish Immunol. 2017, 67, 312–321. [Google Scholar] [CrossRef]
- Boonanuntanasarn, S.; Ditthab, K.; Jangprai, A.; Nakharuthai, C. Effects of Microencapsulated Saccharomyces cerevisiae on Growth, Hematological Indices, Blood Chemical, and Immune Parameters and Intestinal Morphology in Striped Catfish, Pangasianodon hypophthalmus. Probiotics Antimicrob. Proteins 2018, 11, 427–437. [Google Scholar] [CrossRef]
- Voloski, A.P.D.S.; Soveral, L.D.F.; Dazzi, C.C.; Sutili, F.; Frandoloso, R.; Kreutz, L.C. β-Glucan improves wound healing in silver catfish (Rhamdia quelen). Fish Shellfish Immunol. 2019, 93, 575–579. [Google Scholar] [CrossRef]
- Divya, M.; Gopi, N.; Iswarya, A.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Almanaa, T.N.; Vaseeharan, B. β-glucan extracted from eukaryotic single-celled microorganism Saccharomyces cerevisiae: Dietary supplementation and enhanced ammonia stress tolerance on Oreochromis mossambicus. Microb. Pathog. 2020, 139, 103917. [Google Scholar] [CrossRef]
- Ji, L.; Fu, S.; Ji, R.; Li, X.; Liu, Y. β-glucan mitigated trinitrobenzene sulfonic acid-induced enteritis in the rainbow trout (Oncorhynchus mykiss). Aquaculture 2019, 513, 734393. [Google Scholar] [CrossRef]
- Orlean, P. Architecture and Biosynthesis of the Saccharomyces cerevisiae Cell Wall. Genetics 2012, 192, 775–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aon, J.C.; Sun, J.; Leighton, J.M.; Appelbaum, E.R. Hypoxia-elicited impairment of cell wall integrity, glycosylation precursor synthesis, and growth in scaled-up high-cell density fed-batch cultures of Saccharomyces cerevisiae. Microb. Cell Factories 2016, 15, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lesage, G.; Bussey, H. Cell Wall Assembly in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2006, 70, 317–343. [Google Scholar] [CrossRef] [Green Version]
- Ozhovan, S.M.; Knorre, D.A.; Severin, F.F.; Bakeeva, L.E. Ultrastructure of yeast cell Saccharomyces cerevisiae after amiodarone treatment. Cell Tissue Biol. 2010, 4, 90–95. [Google Scholar] [CrossRef]
- Reyes-Becerril, M.; Guardiola, F.A.; Sanchez, V.; Maldonado, M.; Angulo, C. Sterigmatomyces halophilus β-glucan improves the immune response and bacterial resistance in Pacific red snapper (Lutjanus peru) peripheral blood leucocytes: In vitro study. Fish Shellfish Immunol. 2018, 78, 392–403. [Google Scholar] [CrossRef]
- Guzmán-Villanueva, L.T.; Ascencio-Valle, F.; Macías-Rodríguez, M.E.; Tovar-Ramírez, D. Effects of dietary β-1,3/1,6-glucan on the antioxidant and digestive enzyme activities of Pacific red snapper (Lutjanus peru) after exposure to lipopolysaccharides. Fish Physiol. Biochem. 2013, 40, 827–837. [Google Scholar] [CrossRef]
- Elder, M.J.; Webster, S.J.; Chee, R.; Williams, D.L.; Gaston, J.S.H.; Goodall, J.C. β-Glucan Size Controls Dectin-1-Mediated Immune Responses in Human Dendritic Cells by Regulating IL-1β Production. Front. Immunol. 2017, 8, 791. [Google Scholar] [CrossRef] [Green Version]
- Petit, J.; Wiegertjes, G.F. Long-lived effects of administering β-glucans: Indications for trained immunity in fish. Dev. Comp. Immunol. 2016, 64, 93–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyes-Becerril, M.; Angulo, M.; Sanchez, V.; Machuca, C.; Méndez-Martínez, Y.; Angulo, C. β-Glucan bioactivities from Cystobasidium benthicum in Totoaba macdonaldi thymus cells. Fish Shellfish Immunol. 2021, 119, 542–553. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Gani, A.; Masoodi, F.; Amin, F.; Wani, I.A.; Khanday, F.A.; Gani, A. Structural, thermal, functional, antioxidant & antimicrobial properties of β- d -glucan extracted from baker’s yeast (Saccharomyces cereviseae)—Effect of γ-irradiation. Carbohydr. Polym. 2016, 140, 442–450. [Google Scholar] [CrossRef]
- Araújo, V.B.D.S.; De Melo, A.N.F.; De Souza, N.T.; Da Silva, V.M.B.; Castro-Gomez, R.H.; Silva, A.S.; De Souza, E.L.; Magnani, M. Oral Intake of Carboxymethyl-Glucan (CM-G) from Yeast (Saccharomyces uvarum) Reduces Malondialdehyde Levels in Healthy Men. Molecules 2015, 20, 14950–14958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, H.; Lan, P.; He, Y.; Li, C.; Ma, X. Effect of the Modifications on the Physicochemical and Biological Properties of β-Glucan—A Critical Review. Molecules 2019, 25, 57. [Google Scholar] [CrossRef] [Green Version]
- Reyes-Becerril, M.; Angulo, M.; Sanchez, V.; Guluarte, C.; Angulo, C. β-D-glucan from marine yeast Debaryomyces hansenii BCS004 enhanced intestinal health and glucan-expressed receptor genes in Pacific red snapper Lutjanus peru. Microb. Pathog. 2020, 143, 104141. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Fleet, G.H.; Rogers, P.L. Composition of the cell walls of several yeast species. Appl. Microbiol. Biotechnol. 1998, 50, 206–212. [Google Scholar] [CrossRef]
- Leger-Silvestre, I.; Gas, N. The Nucleolus. In The Nucleolar Ultrastructure in Yeast; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2004; pp. 21–28. [Google Scholar]
- Zhu, F.; Du, B.; Bian, Z.; Xu, B. Beta-glucans from edible and medicinal mushrooms: Characteristics, physicochemical and biological activities. J. Food Compos. Anal. 2015, 41, 165–173. [Google Scholar] [CrossRef]
- Bohn, J.A.; BeMiller, J.N. (1→3)-β-d-Glucans as biological response modifiers: A review of structure-functional activity relationships. Carbohydr. Polym. 1995, 28, 3–14. [Google Scholar] [CrossRef]
- Gopalakannan, A.; Arul, V. Enhancement of the innate immune system and disease-resistant activity in Cyprinus carpio by oral administration of β-glucan and whole cell yeast. Aquac. Res. 2009, 41, 884–892. [Google Scholar] [CrossRef]
- Adams, E.L.; Rice, P.J.; Graves, B.; Ensley, H.E.; Yu, H.; Brown, G.D.; Gordon, S.; Monteiro, M.A.; Papp-Szabo, E.; Lowman, D.W.; et al. Differential High-Affinity Interaction of Dectin-1 with Natural or Synthetic Glucans Is Dependent upon Primary Structure and Is Influenced by Polymer Chain Length and Side-Chain Branching. J. Pharmacol. Exp. Ther. 2008, 325, 115–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Tang, Q.; Zhang, J.; Xia, Y.; Yang, Y.; Wu, D.; Fan, H.; Cui, S.W. Triple helix conformation of β-d-glucan from Ganoderma lucidum and effect of molecular weight on its immunostimulatory activity. Int. J. Biol. Macromol. 2018, 114, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Benito-Román, Ó.; Alonso, E.; Cocero, M.J. Ultrasound-assisted extraction of β-glucans from barley. LWT-Food Sci. Technol. 2013, 50, 57–63. [Google Scholar] [CrossRef]
- Bystryak, S.; Santockyte, R.; Peshkovsky, A.S. Cell disruption of S. cerevisiae by scalable high-intensity ultrasound. Biochem. Eng. J. 2015, 99, 99–106. [Google Scholar] [CrossRef]
- Sourki, A.H.; Koocheki, A.; Elahi, M. Ultrasound-assisted extraction of β-d-glucan from hull-less barley: Assessment of physicochemical and functional properties. Int. J. Biol. Macromol. 2017, 95, 462–475. [Google Scholar] [CrossRef]
- Ahmad, A.; Anjum, F.M.; Zahoor, T.; Nawaz, H.; Ahmed, Z. Extraction and characterization of β-d-glucan from oat for industrial utilization. Int. J. Biol. Macromol. 2010, 46, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Aryasomayajula, A.; Selvaganapathy, P.R. A Review on Macroscale and Microscale Cell Lysis Methods. Micromachines 2017, 8, 83. [Google Scholar] [CrossRef]
- Varelas, V.; Liouni, M.; Calokerinos, A.C.; Nerantzis, E.T. An evaluation study of different methods for the production of β-D-glucan from yeast biomass. Drug Test. Anal. 2015, 8, 46–55. [Google Scholar] [CrossRef]
- Javmen, A.; Grigiskis, S.; Gliebutė, R. β-glucan extraction from Saccharomyces cerevisiae yeast using Actinomyces rutgersensis 88 yeast lyzing enzymatic complex. Biologija 2012, 58, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Dallies, N.; François, J.; Paquet, V. A new method for quantitative determination of polysaccharides in the yeast cell wall. Application to the cell wall defective mutants of Saccharomyces cerevisiae. Yeast 1998, 14, 1297–1306. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Xu, B. A critical review on production and industrial applications of beta-glucans. Food Hydrocoll. 2016, 52, 275–288. [Google Scholar] [CrossRef]
- Robinson, P.K. Enzymes: Principles and biotechnological applications. Essays Biochem. 2015, 59, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Pionnier, N.; Falco, A.; Miest, J.J.; Shrive, A.K.; Hoole, D. Feeding common carp Cyprinus carpio with β-glucan supplemented diet stimulates C-reactive protein and complement immune acute phase responses following PAMPs injection. Fish Shellfish Immunol. 2014, 39, 285–295. [Google Scholar] [CrossRef] [Green Version]
- Kiron, V.; Kulkarni, A.; Dahle, D.; Vasanth, G.; Lokesh, J.; Elvebo, O. Recognition of purified beta 1,3/1,6 glucan and molecular signalling in the intestine of Atlantic salmon. Dev. Comp. Immunol. 2016, 56, 57–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauriano, E.; Pergolizzi, S.; Capillo, G.; Kuciel, M.; Alesci, A.; Faggio, C. Immunohistochemical characterization of Toll-like receptor 2 in gut epithelial cells and macrophages of goldfish Carassius auratus fed with a high-cholesterol diet. Fish Shellfish Immunol. 2016, 59, 250–255. [Google Scholar] [CrossRef]
- Pietretti, D.; Vera-Jimenez, N.; Hoole, D.; Wiegertjes, G. Oxidative burst and nitric oxide responses in carp macrophages induced by zymosan, MacroGard® and selective dectin-1 agonists suggest recognition by multiple pattern recognition receptors. Fish Shellfish Immunol. 2013, 35, 847–857. [Google Scholar] [CrossRef]
- Angulo, C.; Sanchez, V.; Delgado, K.; Reyes-Becerril, M. C-type lectin 17A and macrophage-expressed receptor genes are magnified by fungal β-glucan after Vibrio parahaemolyticus infection in Totoaba macdonaldi cells. Immunobiology 2018, 224, 102–109. [Google Scholar] [CrossRef]
- Ji, L.; Sun, G.; Li, X.; Liu, Y. Comparative transcriptome analysis reveals the mechanism of β-glucan in protecting rainbow trout (Oncorhynchus mykiss) from Aeromonas salmonicida infection. Fish Shellfish Immunol. 2019, 98, 87–99. [Google Scholar] [CrossRef]
- Mikrou, A.; Marioli, D.; Papanastasiou, A.D.; Zarkadis, I.K. CR3 complement receptor: Cloning and characterization in rainbow trout. Fish Shellfish Immunol. 2009, 26, 19–28. [Google Scholar] [CrossRef]
- Zhang, C.; Li, C.; Jia, X.; Wang, K.; Tu, Y.; Wang, R.; Liu, K.; Lu, T.; He, C. In Vitro and In Vivo Anti-Inflammatory Effects of Polyphyllin VII through Downregulating MAPK and NF-κB Pathways. Molecules 2019, 24, 875. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Li, B.; Wu, L.; Yin, X.; Zhong, X.; Li, Y.; Wang, Y.; Guo, Z.; Ye, J. Interleukin-6 gets involved in response to bacterial infection and promotes antibody production in Nile tilapia (Oreochromis niloticus). Dev. Comp. Immunol. 2018, 89, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Huo, H.J.; Chen, S.N.; Li, L.; Nie, P. Functional characterization of IL-10 and its receptor subunits in a perciform fish, the mandarin fish, Siniperca chuatsi. Dev. Comp. Immunol. 2019, 97, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Fan, Z.-J.; Cai, S.-X.; Yao, C.-L. Molecular and immunological characterizations of interleukin-11 in large yellow croaker (Larimichthys crocea). Fish Shellfish Immunol. 2020, 100, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Piazzon, M.C.; Galindo-Villegas, J.; Pereiro, P.; Estensoro, I.; Calduch-Giner, J.A.; Gómez-Casado, E.; Novoa, B.; Mulero, V.; Sitjà-Bobadilla, A.; Pérez-Sánchez, J. Differential Modulation of IgT and IgM upon Parasitic, Bacterial, Viral, and Dietary Challenges in a Perciform Fish. Front. Immunol. 2016, 7, 637. [Google Scholar] [CrossRef] [Green Version]
- Danilova, N.; Bussmann, J.; Jekosch, K.; Steiner, L.A. The immunoglobulin heavy-chain locus in zebrafish: Identification and expression of a previously unknown isotype, immunoglobulin Z. Nat. Immunol. 2005, 6, 295–302. [Google Scholar] [CrossRef]
- Hansen, J.D.; Landis, E.D.; Phillips, R.B. Discovery of a unique Ig heavy-chain isotype (IgT) in rainbow trout: Implications for a distinctive B cell developmental pathway in teleost fish. Proc. Natl. Acad. Sci. USA 2005, 102, 6919–6924. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-A.; Salinas, I.; Li, J.; Parra, D.; Bjork, S.; Xu, Z.; La Patra, S.E.; Bartholomew, J.; Sunyer, J.O. IgT, a primitive immunoglobulin class specialized in mucosal immunity. Nat. Immunol. 2010, 11, 827–835. [Google Scholar] [CrossRef]
- Salah, A.S.; El Nahas, A.F.; Mahmoud, S. Modulatory effect of different doses of β-1,3/1,6-glucan on the expression of antioxidant, inflammatory, stress and immune-related genes of Oreochromis niloticus challenged with Streptococcus iniae. Fish Shellfish Immunol. 2017, 70, 204–213. [Google Scholar] [CrossRef]
- Angulo, C.; Alamillo, E.; Ascencio, F.; Reyes-Becerril, M. Characterization of nuclear factor of activated T-cells-c3 (NFATc3) and gene expression of upstream-downstream signaling molecules in response to immunostimulants in Pacific red snapper cells. Dev. Comp. Immunol. 2018, 78, 149–159. [Google Scholar] [CrossRef]
- Alamillo, E.; Reyes-Becerril, M.; Cuesta, A.; Angulo, C. Marine yeast Yarrowia lipolytica improves the immune responses in Pacific red snapper (Lutjanus peru) leukocytes. Fish Shellfish Immunol. 2017, 70, 48–56. [Google Scholar] [CrossRef]
- El-Boshy, M.E.; El-Ashram, A.M.; Abdelhamid, F.M.; Gadalla, H.A. Immunomodulatory effect of dietary Saccharomyces cerevisiae, β-glucan and laminaran in mercuric chloride treated Nile tilapia (Oreochromis niloticus) and experimentally infected with Aeromonas hydrophila. Fish Shellfish Immunol. 2010, 28, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Siwicki, A.K.; Zakęś, Z.; Terech-Majewska, E.; Kazun, K.; Lepa, A.; Głąbski, E. Dietary Macrogard reduces Aeromonas hydrophila mortality in tench (Tinca tinca) through the activation of cellular and humoral defence mechanisms. Rev. Fish Biol. Fish. 2009, 20, 435–439. [Google Scholar] [CrossRef]
- Welker, T.L.; Klesius, P.H.; Yildirim-Aksoy, M.; Lim, C. Effect of short-term feeding duration of diets containing commercial whole-cell yeast or yeast subcomponents on immune function and disease resistance in channel catfish, Ictalurus punctatus. J. Anim. Physiol. Anim. Nutr. 2011, 96, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Jamal, I.N.; Tumbol, R.A.; Mangindaan, R.E. The use of β-glucan extracted from baker’s yeast (Saccharomyces cerevisiae) to increase non-specific immune system and resistence of tilapia (Oreochromis niloticus) to Aeromonas hydrophila. Aquat. Sci. Manag. 2013, 1, 92–98. [Google Scholar] [CrossRef] [Green Version]
- Talpur, A.D.; Munir, M.B.; Mary, A.; Hashim, R. Dietary probiotics and prebiotics improved food acceptability, growth performance, haematology and immunological parameters and disease resistance against Aeromonas hydrophila in snakehead (Channa striata) fingerlings. Aquaculture 2014, 426–427, 14–20. [Google Scholar] [CrossRef]
- Kühlwein, H.; Merrifield, D.; Rawling, M.; Foey, A.; Davies, S. Effects of dietary β-(1, 3)(1, 6)-D-glucan supplementation on growth performance, intestinal morphology and haemato-immunological profile of mirror carp (C yprinus carpio L.). J. Anim. Physiol. Anim. Nutr. 2014, 98, 279–289. [Google Scholar] [CrossRef]
- Ghaedi, G.; Keyvanshokooh, S.; Azarm, H.M.; Akhlaghi, M. Effects of dietary β-glucan on maternal immunity and fry quality of rainbow trout (Oncorhynchus mykiss). Aquaculture 2015, 441, 78–83. [Google Scholar] [CrossRef]
- Sirimanapong, W.; Adams, A.; Ooi, E.L.; Green, D.M.; Nguyen, D.K.; Browdy, C.L.; Collet, B.; Thompson, K.D. The effects of feeding immunostimulant β-glucan on the immune response of Pangasianodon hypophthalmus. Fish Shellfish Immunol. 2015, 45, 357–366. [Google Scholar] [CrossRef]
- Sirimanapong, W.; Thompson, K.; Ooi, E.L.; Bekaert, M.; Collet, B.; Taggart, J.; Bron, J.; Green, D.; Shinn, A.P.; Adams, A.; et al. The effects of feeding β-glucan to Pangasianodon hypophthalmus on immune gene expression and resistance to Edwardsiella ictaluri. Fish Shellfish Immunol. 2015, 47, 595–605. [Google Scholar] [CrossRef]
- Montoya, L.N.F.; Martins, T.P.; Gimbo, R.; Zanuzzo, F.S.; Urbinati, E.C. β-Glucan-induced cortisol levels improve the early immune response in matrinxã (Brycon amazonicus). Fish Shellfish Immunol. 2017, 60, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Munir, M.B.; Hashim, R.; Nor, S.A.M.; Marsh, T.L. Effect of dietary prebiotics and probiotics on snakehead (Channa striata) health: Haematology and disease resistance parameters against Aeromonas hydrophila. Fish Shellfish Immunol. 2018, 75, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Medina-Gali, R.M.; Ortega-Villaizan, M.D.M.; Mercado, L.; Novoa, B.; Coll, J.; Perez, L. Beta-glucan enhances the response to SVCV infection in zebrafish. Dev. Comp. Immunol. 2018, 84, 307–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chirapongsatonkul, N.; Mueangkan, N.; Wattitum, S.; U-Taynapun, K. Comparative evaluation of the immune responses and disease resistance of Nile tilapia (Oreochromis niloticus) induced by yeast β-glucan and crude glucan derived from mycelium in the spent mushroom substrate of Schizophyllum commune. Aquac. Rep. 2019, 15, 100205. [Google Scholar] [CrossRef]
- Cao, H.; Yu, R.; Zhang, Y.; Hu, B.; Jian, S.; Wen, C.; Kajbaf, K.; Kumar, V.; Yang, G. Effects of dietary supplementation with β-glucan and Bacillus subtilis on growth, fillet quality, immune capacity, and antioxidant status of Pengze crucian carp (Carassius auratus var. Pengze). Aquaculture 2019, 508, 106–112. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Mandiki, S.N.; Tran, T.N.T.; Larondelle, Y.; Mellery, J.; Mignolet, E.; Cornet, V.; Flamion, E.; Kestemont, P. Growth performance and immune status in common carp Cyprinus carpio as affected by plant oil-based diets complemented with β-glucan. Fish Shellfish Immunol. 2019, 92, 288–299. [Google Scholar] [CrossRef] [PubMed]
- de Mello, M.M.M.; Faria, C.D.F.P.D.; Zanuzzo, F.S.; Urbinati, E.C. β-glucan modulates cortisol levels in stressed pacu (Piaractus mesopotamicus) inoculated with heat-killed Aeromonas hydrophila. Fish Shellfish Immunol. 2019, 93, 1076–1083. [Google Scholar] [CrossRef]
- Sabioni, R.E.; Zanuzzo, F.S.; Gimbo, R.Y.; Urbinati, E.C. β-Glucan enhances respiratory activity of leukocytes suppressed by stress and modulates blood glucose levels in pacu (Piaractus mesopotamicus). Fish Physiol. Biochem. 2019, 46, 629–640. [Google Scholar] [CrossRef]
- Kazuń, B.; Małaczewska, J.; Kazuń, K.; Kamiński, R.; Adamek-Urbańska, D.; Urban, J. Dietary administration of β-1,3/1,6-glucan and Lactobacillus plantarum improves innate immune response and increases the number of intestine immune cells in roach (Rutilus rutilus). BMC Veter Res. 2020, 16, 1–10. [Google Scholar] [CrossRef]
- Koch, J.F.A.; de Oliveira, C.A.F.; Zanuzzo, F.S. Dietary β-glucan (MacroGard®) improves innate immune responses and disease resistance in Nile tilapia regardless of the administration period. Fish Shellfish Immunol. 2021, 112, 56–63. [Google Scholar] [CrossRef]
- Cornet, V.; Khuyen, T.D.; Mandiki, S.N.M.; Betoulle, S.; Bossier, P.; Reyes-López, F.E.; Tort, L.; Kestemont, P. GAS1: A New β-Glucan Immunostimulant Candidate to Increase Rainbow Trout (Oncorhynchus mykiss) Resistance to Bacterial Infections with Aeromonas salmonicida achromogenes. Front. Immunol. 2021, 12, 693613. [Google Scholar] [CrossRef]
- Akhtar, M.; Tripathi, P.H.; Pandey, A.; Ciji, A. β-glucan modulates non-specific immune gene expression, thermal tolerance and elicits disease resistance in endangered Tor putitora fry challenged with Aeromonas salmonicida. Fish Shellfish Immunol. 2021, 119, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Lokesh, J.; Fernandes, J.M.; Korsnes, K.; Bergh, Ø.; Brinchmann, M.F.; Kiron, V. Transcriptional regulation of cytokines in the intestine of Atlantic cod fed yeast derived mannan oligosaccharide or β-Glucan and challenged with Vibrio anguillarum. Fish Shellfish Immunol. 2012, 33, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Aramli, M.S.; Kamangar, B.; Nazari, R.M. Effects of dietary β-glucan on the growth and innate immune response of juvenile Persian sturgeon, Acipenser persicus. Fish Shellfish Immunol. 2015, 47, 606–610. [Google Scholar] [CrossRef] [PubMed]
- Miest, J.J.; Arndt, C.; Adamek, M.; Steinhagen, D.; Reusch, T.B. Dietary β-glucan (MacroGard®) enhances survival of first feeding turbot (Scophthalmus maximus) larvae by altering immunity, metabolism and microbiota. Fish Shellfish Immunol. 2016, 48, 94–104. [Google Scholar] [CrossRef]
- Carballo, C.; Mateus, A.P.; Maya, C.; Mantecón, L.; Power, D.M.; Manchado, M. Microalgal extracts induce larval programming and modify growth and the immune response to bioactive treatments and LCDV in Senegalese sole post-larvae. Fish Shellfish Immunol. 2020, 106, 263–272. [Google Scholar] [CrossRef]
- Soto, E.; Coleman, D.; Yazdi, Z.; Purcell, S.L.; Camus, A.; Fast, M.D. Analysis of the white sturgeon (Acipenser transmontanus) immune response during immunostimulation and Veronaea botryosa infection. Comp. Biochem. Physiol. Part D Genom. Proteom. 2021, 40, 100879. [Google Scholar] [CrossRef]
- Caipang, C.M.A.; Lazado, C.C.; Brinchmann, M.F.; Kiron, V. Transcription of selected immune-related genes in spleen cells of cod, Gadus morhua following incubation with alginic acid and β-glucan. J. Exp. Mar. Biol. Ecol. 2012, 416, 202–207. [Google Scholar] [CrossRef]
- Velazquez-Carriles, C.; Macías-Rodríguez, M.E.; Carbajal-Arizaga, G.G.; Silva-Jara, J.; Angulo, C.; Reyes-Becerril, M. Immobilizing yeast β-glucan on zinc-layered hydroxide nanoparticle improves innate immune response in fish leukocytes. Fish Shellfish Immunol. 2018, 82, 504–513. [Google Scholar] [CrossRef]
- Amphan, S.; Unajak, S.; Printrakoon, C.; Areechon, N. Feeding-regimen of β-glucan to enhance innate immunity and disease resistance of Nile tilapia, Oreochromis niloticus Linn., against Aeromonas hydrophila and Flavobacterium columnare. Fish Shellfish Immunol. 2018, 87, 120–128. [Google Scholar] [CrossRef]
- Douxfils, J.; Fierro-Castro, C.; Mandiki, S.; Emile, W.; Tort, L.; Kestemont, P. Dietary β-glucans differentially modulate immune and stress-related gene expression in lymphoid organs from healthy and Aeromonas hydrophila-infected rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2017, 63, 285–296. [Google Scholar] [CrossRef]
- Lin, S.; Pan, Y.; Luo, L. Effects of dietary β-1,3-glucan, chitosan or raffinose on the growth, innate immunity and resistance of koi (Cyprinus carpio koi). Fish Shellfish Immunol. 2011, 31, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Carballo, C.; Pinto, P.I.; Mateus, A.P.; Berbel, C.; Guerreiro, C.C.; Martinez-Blanch, J.F.; Codoñer, F.M.; Mantecon, L.; Power, D.M.; Manchado, M. Yeast β-glucans and microalgal extracts modulate the immune response and gut microbiome in Senegalese sole (Solea senegalensis). Fish Shellfish Immunol. 2019, 92, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-H.; Zhao, L.-Q.; Liu, J.-F.; Wang, H.; Xiao, S. Effect of potential probiotic Rhodotorula benthica D30 on the growth performance, digestive enzyme activity and immunity in juvenile sea cucumber Apostichopus japonicus. Fish Shellfish Immunol. 2015, 43, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Zullo, B.; Ciafardini, G. Evaluation of physiological properties of yeast strains isolated from olive oil and their in vitro probiotic trait. Food Microbiol. 2018, 78, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Lara-Hidalgo, C.E.; Dorantes-Álvarez, L.; Hernández-Sánchez, H.; Santoyo-Tepole, F.; Martínez-Torres, A.; Villa-Tanaca, L.; Hernández-Rodríguez, C. Isolation of Yeasts from Guajillo Pepper (Capsicum annuum L.) Fermentation and Study of Some Probiotic Characteristics. Probiotics Antimicrob. Proteins 2018, 11, 748–764. [Google Scholar] [CrossRef]
- Sokół, I.; Gaweł, A.; Bobrek, K. The Prevalence of Yeast and Characteristics of the Isolates from the Digestive Tract of Clinically Healthy Turkeys. Avian Dis. 2018, 62, 286–290. [Google Scholar] [CrossRef]
- Yang, S.-P.; Wu, Z.-H.; Jian, J.-C. Distribution of Marine Red Yeasts in Shrimps and the Environments of Shrimp Culture. Curr. Microbiol. 2011, 62, 1638–1642. [Google Scholar] [CrossRef]
- Caruffo, M.; Navarrete, N.; Salgado, O.; Díaz, A.; Lopez, P.; García, K.; Feijoo, C.G.; Navarrete, P. Potential probiotic yeasts isolated from the fish gut protect zebrafish (Danio rerio) from a Vibrio anguillarum challenge. Front. Microbiol. 2015, 6, 1093. [Google Scholar] [CrossRef] [Green Version]
- Kourelis, A.; Kotzamanidis, C.; Litopoulou-Tzanetaki, E.; Papaconstantinou, J.; Tzanetakis, N.; Yiangou, M. Immunostimulatory activity of potential probiotic yeast strains in the dorsal air pouch system and the gut mucosa. J. Appl. Microbiol. 2010, 109, 260–271. [Google Scholar] [CrossRef]
- Coutinho, J.O.P.A.; Peixoto, T.S.; de Menezes, G.C.A.; Carvalho, C.R.; Ogaki, M.B.; Gomes, E.C.Q.; Rosa, C.A.; Rosa, L.H.; Arantes, R.M.E.; Nicoli, J.R.; et al. In Vitro and In Vivo Evaluation of the Probiotic Potential of Antarctic Yeasts. Probiotics Antimicrob. Proteins 2021, 13, 1338–1354. [Google Scholar] [CrossRef]
- Pulschen, A.A.; Rodrigues, F.; Duarte, R.T.D.; Araujo, G.G.; Santiago, I.F.; Paulino-Lima, I.G.; Rosa, C.A.; Kato, M.J.; Pellizari, V.H.; Galante, D. UV-resistant yeasts isolated from a high-altitude volcanic area on the Atacama Desert as eukaryotic models for astrobiology. MicrobiologyOpen 2015, 4, 574–588. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Liu, N.-N.; Liu, G.-L.; Chi, Z.; Wang, J.-M.; Zhang, L.-L.; Chi, Z.-M. Melanin production by a yeast strain XJ5-1 of Aureobasidium melanogenum isolated from the Taklimakan desert and its role in the yeast survival in stress environments. Extremophiles 2016, 20, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Hu, X.; He, H.; Xia, F.; Ma, Y.; Qi, J.; Dong, X.; Zhao, W.; Lu, Y.; Wu, W. Tracking translocation of glucan microparticles targeting M cells: Implications for oral drug delivery. J. Mater. Chem. B 2016, 4, 2864–2873. [Google Scholar] [CrossRef] [PubMed]
- Novak, M.; Vetvicka, V. Glucans as biological response modifiers. Endocr. Metab. Immune Disord. Drug Targets 2009, 9, 67–75. [Google Scholar] [CrossRef]
- Williams, D.; Al-Tuwaijri, A.; Di Luzio, N. Influence of glucan on experimental infections in mice. Int. J. Immunopharmacol. 1980, 2, 189. [Google Scholar] [CrossRef]
- Przybylska-Diaz, D.; Schmidt, J.; Vera-Jiménez, N.; Steinhagen, D.; Nielsen, M. β-glucan enriched bath directly stimulates the wound healing process in common carp (Cyprinus carpio L.). Fish Shellfish Immunol. 2013, 35, 998–1006. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, J.; Andersen, E.; Ersbøll, B.; Nielsen, M. Muscle wound healing in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2015, 48, 273–284. [Google Scholar] [CrossRef] [Green Version]
- Huu, H.D.; Sang, H.M.; Thuy, N.T.T. Dietary β-glucan improved growth performance, Vibrio counts, haematological parameters and stress resistance of pompano fish, Trachinotus ovatus Linnaeus, 1758. Fish Shellfish Immunol. 2016, 54, 402–410. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, D.; Huang, Q.; Liu, Z.; Luo, X.; Xiong, S.; Yin, T. Adsorption kinetics and thermodynamics of yeast β-glucan for off-odor compounds in silver carp mince. Food Chem. 2020, 319, 126232. [Google Scholar] [CrossRef]
- Anusuya, S.; Sathiyabama, M. Preparation of β-d-glucan nanoparticles and its antifungal activity. Int. J. Biol. Macromol. 2014, 70, 440–443. [Google Scholar] [CrossRef]
- Kodama, A.; Nakagawa, A.; Nonoguchi, Y.; Sakurai, H.; Yano, C.; Suzuki, T.; Koumoto, K. Solubilization of poorly water-soluble bioactive molecules in neutral aqueous media by complexation with renatured β-1,3-1,6-glucan nanoparticles. Biopolymers 2020, 111, e23349. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Lee, K.; Gilad, A.A.; Choi, J. Synthesis of Beta-glucan Nanoparticles for the Delivery of Single Strand DNA. Biotechnol. Bioprocess Eng. 2018, 23, 144–149. [Google Scholar] [CrossRef]
- Colaço, M.; Marques, A.P.; Jesus, S.; Duarte, A.; Borges, O. Safe-by-Design of Glucan Nanoparticles: Size Matters When Assessing the Immunotoxicity. Chem. Res. Toxicol. 2020, 33, 915–932. [Google Scholar] [CrossRef] [PubMed]
| Species | Mw * | Reference |
|---|---|---|
| Cystobasidium benthicum | 2.32 kDa | [22] |
| Saccharomyces cerevisiae (bakery) | 175 kDa | [23] |
| Saccharomyces uvarum | 220 kDa | [24] |
| Saccharomyces cerevisiae (brewery) | 240 kDa | [25] |
| Debaryomyces hansenii (BCS004) | 689.35 kDa | [26] |
| Yeast Species (Origin) | Β-Glucan Type | Administration Dose and Route | Fish | Pathogen Challenge (Name, Dose, Route and Challenge Day) | Outcomes | Ref. |
|---|---|---|---|---|---|---|
| (Relative Survival Upon Challenge and Increased Immune Parameters) | ||||||
| Saccharomyces uvarum (β-glucan and whole cells) | β-1,3 y β-1,6 | 10 g Kg−1 Diet | Cyprinus carpio | Aeromonas hydrophila 1.5 × 106 CFU mL−1 | Survival: 77.8% and 71.6% | [31] |
| S. cerevisiae (bakery, Hang Zhou) | β-1,3 y β-1,6 | 60 days | Oreochromis niloticus | Intramuscularly 30 and 60 days | Significant increase in white blood cells, NBT, and serum lysozyme activity. | [62] |
| 21 days | Intra-peritoneal 21 day | Increase in cellular immunological parameters (neutrophil adhesion, macrophage oxidative oxide, lymphocyte transformation index, and phagocytic activity), and humoral parameters (bactericidal activity in serum, lysozyme and NO) | ||||
| S. cerevisiae (MacroGard®) | β-1,3 y β-1,6 | 0, 0.5, 1 and 2 g Kg−1 Diet | Tinca tinca | Aeromonas hydrophila | The 2 g Kg−1 dose had the lowest mortality after infection | [63] |
| 30 days | 1 × 107 CFU mL−1 Intraperitoneal at day 30 | Additionally, increased respiratory burst activity in spleen macrophages, lysozyme activity, and total serum Ig levels | ||||
| S. cerevisiae (MacroGard® and Betagard A®) | β-1,3 y β-1,6 | 1 g Kg−1 and 0.1 g Kg−1 Diet | Ictalurus punctatus | Edwardsiella ictalurid 9.5 × 106 CFU mL−1 | Survival: 56.7% and 46.4% | [64] |
| 7 and 14 days | Immersion 7 and 14 days | Increase in hematological parameters (% hematocrit, hemoglobin, TCC, RBC, WBC) and immunological parameters (SH50, lysozyme, total plasma protein) | ||||
| S. cerevisiae (bakery) | β-1,3 y β-1,6 | 10 mg Kg−1 fish Intraperitoneal | Oreochromis niloticus | Aeromonas hydrophila 1 × 106 CFU mL−1 | RPS: 83.3% | [65] |
| S. cerevisiae (MacroGard®) | β-1,3 y β-1,6 | Nine days (injection every three days) | Cyprinus carpio | Intraperitoneal nine day | - | [44] |
| 15 days | Intraperitoneal Sampling 7 day | Increase in total leukocytes and phagocytic activity. Induced expression in CRP (crp1, crp2) and ACP (c1r/s, bf/c2, c3 and masp2) | ||||
| S. cerevisiae (MacroGard®) | β-1,3 y β-1,6 | 1 g Kg−1 Diet | Channa striata | Aeromonas hydrophila 1 × 107 CFU mL−1 | RPS: 61.54% | [66] |
| 84 days | Intraperitoneal 56 and 84 days 14 days mortality record | Increase of hematological parameters RBC, WBC, PCV, Hb%, VSG, serum protein, and immunological Ig and lysozyme activity | ||||
| S. cerevisiae (MacroGard®) | β-1,3 y β-1,6 | 10 and 20 g Kg−1 Diet | Cyprinus carpio L. | - | [67] | |
| 56 days | - | Significant increase in localized infiltration of intestinal leukocytes, monocytes, and hematocrit value | ||||
| S. cerevisiae (MacroGard®) | β-1,3 y β-1,6 | 1–2 g Kg−1 Diet | Oncorhynchus mykiss | Yersinia ruckeri 2 × 108 cells mL−1 | RPS: Breeding females diet 2 g Kg−1 (42.2%) and fry diet 1 g/Kg (35.6%) | [68] |
| 90 days breeding females and 60 days fry | Immersion Sampling 25 days | Increased WBC, ACH-50, lysozyme activity, Ig, IgM | ||||
| S. cerevisiae (bakery) | β-1,3 y β-1,6 | 1 g Kg−1 Diet | Pangasianodon hypophthalmus | Edwardsiella ictalurid 8 × 104 CFU mL−1 | RPS: 37.7%. | [69] |
| 28 days | Immersion 28 day 14 days mortality record | Increased phagocytic activity, total IgM, | ||||
| S. cerevisiae (bakery) | β-1,3 y β-1,6 | 1 g Kg−1 Diet | Pangasianodon hypophthalmus | Edwardsiella ictaluri 1 × 106 CFU mL−1 | RPS: 83%. | [70] |
| 14 days | Immersion 14 day 24 h of infection | Overall expression of immune genes in the liver, kidney, and spleen | ||||
| S. cerevisiae (MacroGard®) | β-1,3 y β-1,6 | 1–2 g Kg−1 Diet | Oreochromis niloticus | Streptoccus iniae 2 × 107 CFU mL−1 | - | [59] |
| 21 days | Intraperitoneal Sampling one, three, and seven days | 1 g kg−1: induced greater expression of the hsp-70, cxc chemokine, mhc-ii β and mx genes. Presented expression of hsp-70, mhc-ii β, and tlr 7 in the challenged group. 1 g Kg−1: induced expression of vtg, cas, igm-h, gst, il8, tnf-α in the unchallenged and challenged groups. More significant expression of hsp, cxc, and mhc-ii β in the challenged group. | ||||
| S. cerevisiae (MacroGard®) | β-1,3 y β-1,6 | 1 g Kg−1 Diet | Brycon amazonicus | Aeromonas hydrophila 3.8 × 108 CFU mL−1 | - | [71] |
| 15 days | Sampling 30 min and 24 h | Increased levels of cortisol, serum lysozyme, and complement system | ||||
| S. cerevisiae (MacroGard®) | β-1,3 y β-1,6 | 2 g Kg−1 Diet | Channa striata | Aeromonas hydrophila 2 × 106 CFU mL−1 | Resistance to bacterial infection. | [72] |
| 112 days immunization 56 days intake | Intraperitoneal 56, 120 and 168 days | Increase in hematological parameters (RBC, WBC, %PCV, Hb) and immunological parameters (Ig, lysozyme). | ||||
| S. cerevisiae (Zymosan) | β-1,3 y β-1,6 | In vitro: 10 µg mL−1 ZF4 cells. In vivo: 5 µg fish Intraperitoneal | Danio rerio | Spring viremia of carp virus In vitro: 1 × 10−3 MOI In vivo: 104 PFU mL−1 | RPS: 59.7% | [73] |
| In vitro:24 h In vivo:14 days | Immersion 14 day 17 days mortality record | Immunized and challenged + immunized fish showed increased expression of genes il-1b, il-6, il-8, il-10, and tnf-α | ||||
| S. cerevisiae (bakery, Sigma) | β-1,3 y β-1,6 | 10 μg fish Intraperitoneal injection | Oreochromis niloticus | Aeromonas veronii 1 × 106 CFU mL−1 | Relative survival 25% | [74] |
| 6, 12 and 24 h | Intraperitoneal 10 days mortality record | Increased hematological parameters. Cellular activity: lymphocytes, monocytes. Humoral activity: Total Ig, bactericidal activity, lysozyme, trypsin inhibition. Gene expression: tlr2, jak-1, nf-kb, il-1β, and tnf-1α. | ||||
| S. cerevisiae (bakery, BettcanTM) | β-1,3 y β-1,6 | 1 g Kg−1 Diet | Carassius auratus var. Pengze | - | - | [75] |
| 70 days | - | Enhanced immunity and antioxidant capacity, increased acid phosphatase, alkaline phosphatase, glutathione peroxidase, reduced glutathione, catalase, and superoxide dismutase activities | ||||
| S. cerevisiae (MacroGard®) | β-1,3 y β-1,6 | 0.25 g Kg−1 Diet | Cyprinus carpio | Aeromonas hydrophila 5.01 × 108 CFU mL−1 | Survival > 50% | [76] |
| 63 days | Intraperitoneal 64 day 10 days mortality record | Increased lysozyme activity, complements and improves expression of immune genes (nk, lys, and il-8) | ||||
| S. cerevisiae (MacroGard®) | β-1,3 y β-1,6 | 1 g Kg−1 Diet | Piaractus mesopotamicus | Aeromonas hydrophila 1.5 × 108 CFU mL−1 Inactivated at 50 °C | - | [77] |
| 15 days | Intraperitoneal Sampling 3 and 24 h | Increased plasma levels of cortisol, complement activity, and reduced numbers of monocytes and lymphocytes in peripheral blood | ||||
| S. cerevisiae (MacroGard®) | β-1,3 y β-1,6 | 5 g Kg−1 Diet | Piaractus mesopotamicus | Aeromonas hydrophila 1 × 102 CFU mL−1 | Increased cortisol, glucose, and CR3 y lysozyme by manipulation and bacterial inoculation. | [78] |
| 10 days | Intraperitoneal | Promoted inflammatory response in lymphocytes and neutrophils. | ||||
| S. cerevisiae (brewery Leiber® Beta-S | β-1,3 y β-1,6 | 10 g Kg−1 Diet + Lactobacillus plantarum (1 × 108 CFU cells mL−1) | Rutilus rutilus | - | Increased nonspecific humoral immunity parameters (lysozyme and total Ig) | [79] |
| 28 days | - | Cellular (pinocytic activity of phagocytes, respiratory burst) | ||||
| S. cerevisiae (bakery, BettcanTM) | β-1,3 y β-1,6 | 2 g Kg−1 Diet | Oncorhynchus mykiss | Aeromonas salmonicida 3 × 105 CFU mL−1 | - | [49] |
| 42 days | Intraperitoneal Sampling four and six days | Differential expression of genes involved in immune or metabolic signaling pathways (fgg, fgb, f5, c9, c3, c5, tlr5, and myd88) | ||||
| S. cerevisiae (MacroGard®) | β-1,3 y β-1,6 | 0,1 g kg−1 | Oreochromis niloticus | Aeromonas sobria and Streptococcus agalactiae | 100% survival in immunized fish for 45 days | [80] |
| 15, 30 and 45 days | 2 × 108 and 1 × 108 CFU mL−1 Intramuscular at day 10 | Longer periods of administration of β-glucans increased growth, innate immune activity, and bacterial resistance | ||||
| S. cerevisiae [BY 4741 strain (G), MacroGard® (M) and wild-type (W)] | β-1,3 y β-1,6 | 2 and 5 g Kg−1 | Oncorhynchus mykiss | Aeromonas salmonicida achromogenes | G (2 and 5 g Kg−1) had the best survival rate | [81] |
| 15, 30 and 45 days | 3.1 × 107 UFC/100 g fish Intraperitoneal day 37 | The G represented the best immunostimulant by increasing lysozyme activity, total Ig, and some immune genes (mcsfra, hepcidin) in the short and mid-term | ||||
| S. cerevisiae M/s Kuber | β-1,3 y β-1,6 | 5, 10 and 15 g kg−1 | Tor putitora | Aeromonas salmonicida | RPS: 20% with diet 10 g kg−1 | [82] |
| 56 days | 2.5 × 107 CFU mL−1 Immersion 56 day for 12 h 10 days mortality record | Total antioxidant levels increased, expression of cytokines such as tnf-α, il-1β, defensin1, c3 pre-post-challenge, and antiprotease activity increased only post-challenge |
| Yeast Species (Origin) | β-Glucan Type | Administration Dose and Route | Fish | Pathogen Challenge (Name, Challenge Day, Dose and Route) | Outcomes | Ref. |
|---|---|---|---|---|---|---|
| (Survival Upon Challenge and Increased Immune Parameters) | ||||||
| S. cerevisiae (MacroGard®) | β-1,3 y β-1,6 | 1 g Kg−1 Diet | Gadus morhua L. | Vibrio anguillarum strain HI610 2.6 × 107 CFU mL−1 | - | [83] |
| 35 days | Immersion 36 day | Increased expression of anti-inflammatory genes (il-10 and ifn-γ). Active inflammation due to expression of pro-inflammatory cytokines (il1- β and il-8) post-challenge | ||||
| S. cerevisiae (bakery Fibosel ®) | β-1,3 y β-1,6 | 1 g Kg−1 Diet | Lutjanus peru | LPS 3 mg Kg−1 | - | [19] |
| 42 days | Intraperitoneal | Improved growth, effectiveness in antioxidant enzymes (SOD and CAT) before and after exposure to LPS, activity of digestive enzymes (include trypsin, aminopeptidase, and chymotrypsin) | ||||
| S. cerevisiae (MacroGard®) | β-1,3 y β-1,6 | 1, 2 and 3 g Kg−1 Diet | Acipenser persicus | - | - | [84] |
| 42 days | - | Higher doses induced increases in WBC, %lymphocytes, and lysozyme and ACH-50 immune activity | ||||
| S. cerevisiae (MacroGard®) | β-1,3 y β-1,6 | 15 mg Kg−1 of fish | Atlantic salmon | - | - | [45] |
| Sampling 1 and 7 days | - | Expression of β-glucan receptors sclra, sclrb, sclrc, and cr3; Syk, mapkin2, il1b, and mip2a target genes; apoa4 protein involved in carbohydrate metabolism; tagln, actb sensors | ||||
| S. cerevisiae (MacroGard®) | β-1,3 y β-1,6 | 0.5 g L−1 (incubated rotifers B. plicatilis) | Scophthalmus maximus | - | - | [85] |
| 10 days | - | Increase in chymotrypsin and trypsin activity. Complemented c3 activity and anti-inflammatory effect of hsp-70, tnf-α, and il-1β | ||||
| S. cerevisiae brewery (Yestimun®) | β-1,3 y β-1,6 | 1 mg/fish in PBS Oral intubation | Solea senegalensis | - | - | [86] |
| sampling at 3, 24, 48 h and 7 days | - | Expression: il-1 β, clec, and irf7 | ||||
| Debaryomyces hansenii BCS004 | β-1,3 y β-1,6 | 500 mg Kg−1 Diet | Lutjanus peru | - | [26] | |
| 28 days | - | Did not show pathological damages, edema, or inflammation in the intestine. Increased regulation of receptors (tlr2, dectin-2, c-type lectin-4, mmr-1) | ||||
| S. cerevisiae (MacroGard®) | β-1,3 y β-1,6 | 1 and 3 g Kg−1 | Acipenser transmontanus | Veronaea botryose | RPS: 30% with diet 30 g Kg−1 | [87] |
| 21 days | 7.25 × 105 spores mL−1 intramuscular | Increased expression of genes such as haptoglobin, serotransferrin, SAA, cathelicidin, and il-17, irf8 post-challenge |
| Yeast Species (Origin) | β-Glucan Type | Dose | Fish | Pathogen Challenge (Name, Dose and Route) | Outcomes | Ref. |
|---|---|---|---|---|---|---|
| (Post-Challenge and Increased Immune Parameters) | ||||||
| S. cerevisiae (bakery) | β-1,3 y β-1,6 | 100 μg/mL | Gadus morhua | - | - | [88] |
| - | Increased antibacterial genes BPI/LBP and g-type lysozyme, pro-inflammatory cytokines il-1β and il-8, and antioxidants CAT and Cu/Zn-SOD | |||||
| S. cerevisiae (MacroGard® and Zymosan) | β-1,3 y β-1,6 | 10–100 μg/mL | Cyprinus carpio carpio | - | [47] | |
| - | Increased production of reactive radicals (oxygen and nitrogen), expression of cytokine genes (il-1 β, il-6 and il-11) | |||||
| S. cerevisiae (Zymosan) | β-1,3 y β-1,6 | 50 μg/mL | Lutjanus peru | Vibrio parahaemolyticus | - | [60] |
| 1 × 108 cell mL−1 | Stimulated the expression upstream of ilf2, ilf3, can, and downstream of cd3, tcrβ, il-6, il-12 | |||||
| Yarrowia lipolytica N6 (marine) | β-1,3 y β-1,6 | 200 μg/mL | Lutjanus peru | Vibrio parahaemolyticus | Immunized and challenged leukocytes | [89] |
| 1 × 108 cell mL−1 | Increased ON, SOD, CAT, PO. Regulated pro-inflammatory (il-1β, il-8, il-12, il-17) and anti-inflammatory (il-6, il-10) cytokines | |||||
| Sterigmatomyces halophilus (marine) | β-1,3 y β-1,6 | 200 μg/mL | Lutjanus peru | Aeromonas hydrophila | Immunized and challenged leukocytes | [18] |
| 1 × 108 cell mL−1 | Increased phagocytic activity, NBT, NO, PO, SOD, CAT. Genetic experimentation in cytokines il-1β, il-10, and il-17 | |||||
| Debaryomyces hansenii BCS004 | β-1,3 y β-1,6 | 100 μg/mL | Lutjanus peru | - | Increased cell viability with doses of 50, 100 and 500 μg/mL | [26] |
| - | - | |||||
| Cystobasidium benthicum | β-1,3 y β-1,6 | 50, 100 and 200 μg/mL | Totoaba macdonaldi | - | Increased phagocytic activity, MPO, production of intracellular-mitochondrial ROS, NO, SOD, and gene expression of tlr2, clec17a, mmr, il-β, and 1csf1r2 | [22] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machuca, C.; Méndez-Martínez, Y.; Reyes-Becerril, M.; Angulo, C. Yeast β-Glucans as Fish Immunomodulators: A Review. Animals 2022, 12, 2154. https://doi.org/10.3390/ani12162154
Machuca C, Méndez-Martínez Y, Reyes-Becerril M, Angulo C. Yeast β-Glucans as Fish Immunomodulators: A Review. Animals. 2022; 12(16):2154. https://doi.org/10.3390/ani12162154
Chicago/Turabian StyleMachuca, Cristian, Yuniel Méndez-Martínez, Martha Reyes-Becerril, and Carlos Angulo. 2022. "Yeast β-Glucans as Fish Immunomodulators: A Review" Animals 12, no. 16: 2154. https://doi.org/10.3390/ani12162154







