The Effects of Bacillus licheniformis—Fermented Products on the Microbiota and Clinical Presentation of Cats with Chronic Diarrhea
Abstract
:Simple Summary
Abstract
1. Background
2. Methods
2.1. Animals and Treatments
2.2. Stool Scoring
2.3. Feline Chronic Enteropathy Activity Index (FCEAI)
2.4. Fecal Collection
2.5. DNA Extraction
2.6. Next-Generation Sequencing
2.7. Library Construction and Sequencing
2.8. Statistical Analyses
3. Results
3.1. Animals and Grouping
3.2. Stool Scoring
3.3. Feline Chronic Enteropathy Activity Index (FCEAI)
3.4. Next Generation Sequencing (NGS)
3.4.1. Sequence Analysis and Rarefaction
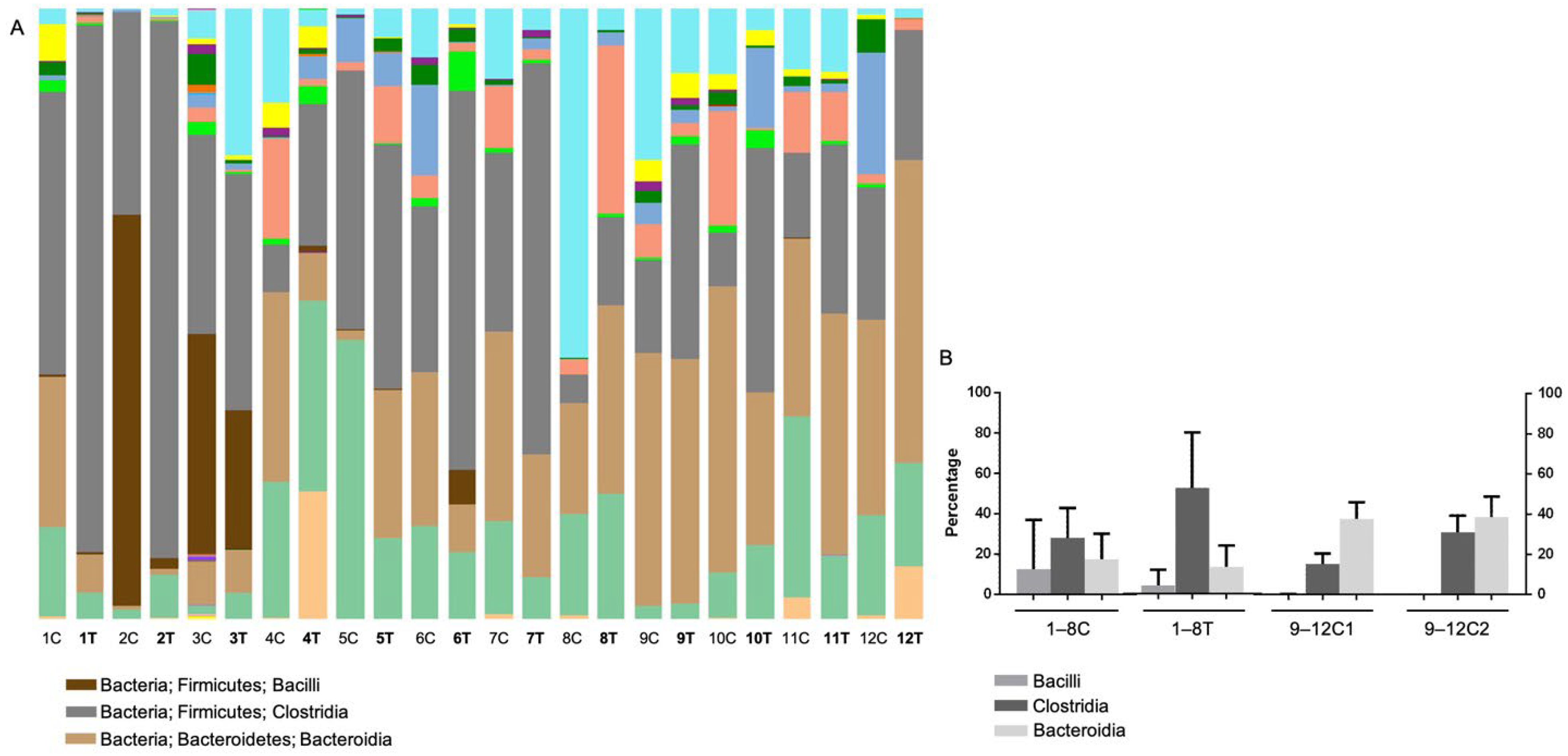
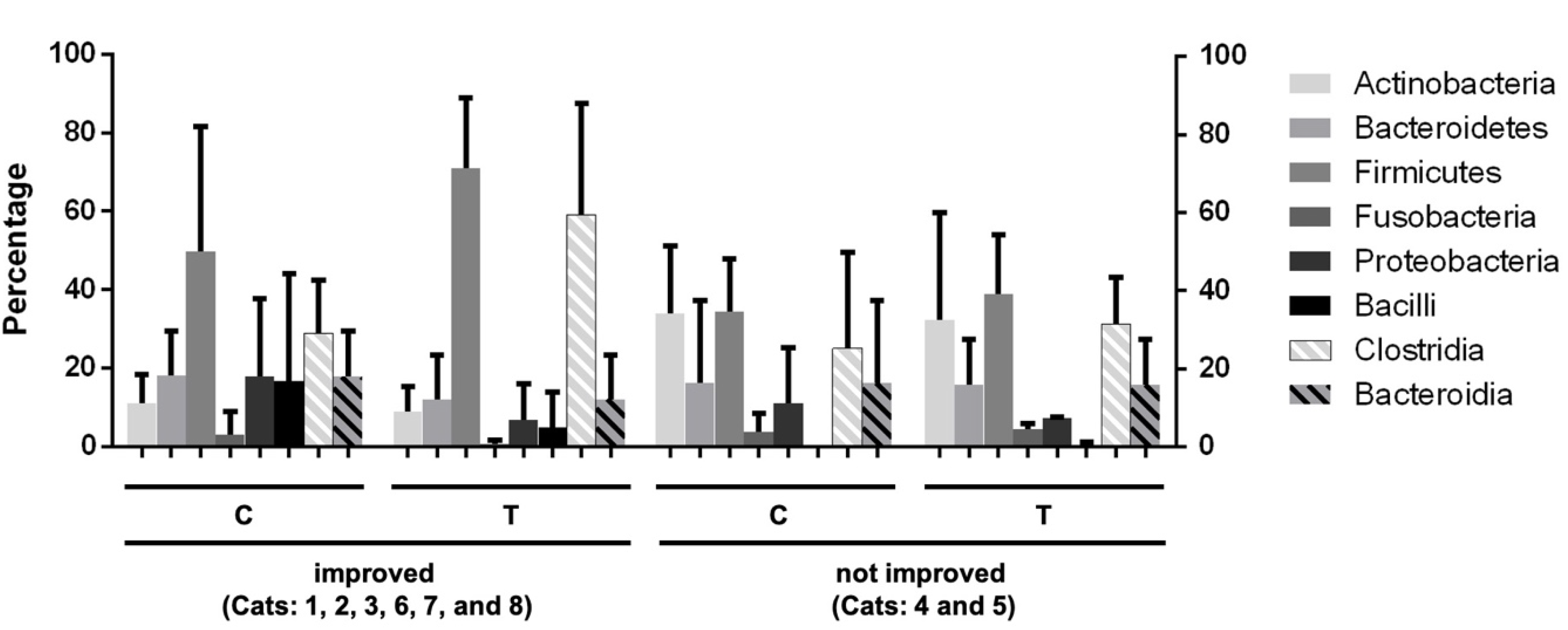
3.4.2. Operational Taxonomic Unit (OTU)-Based Analysis
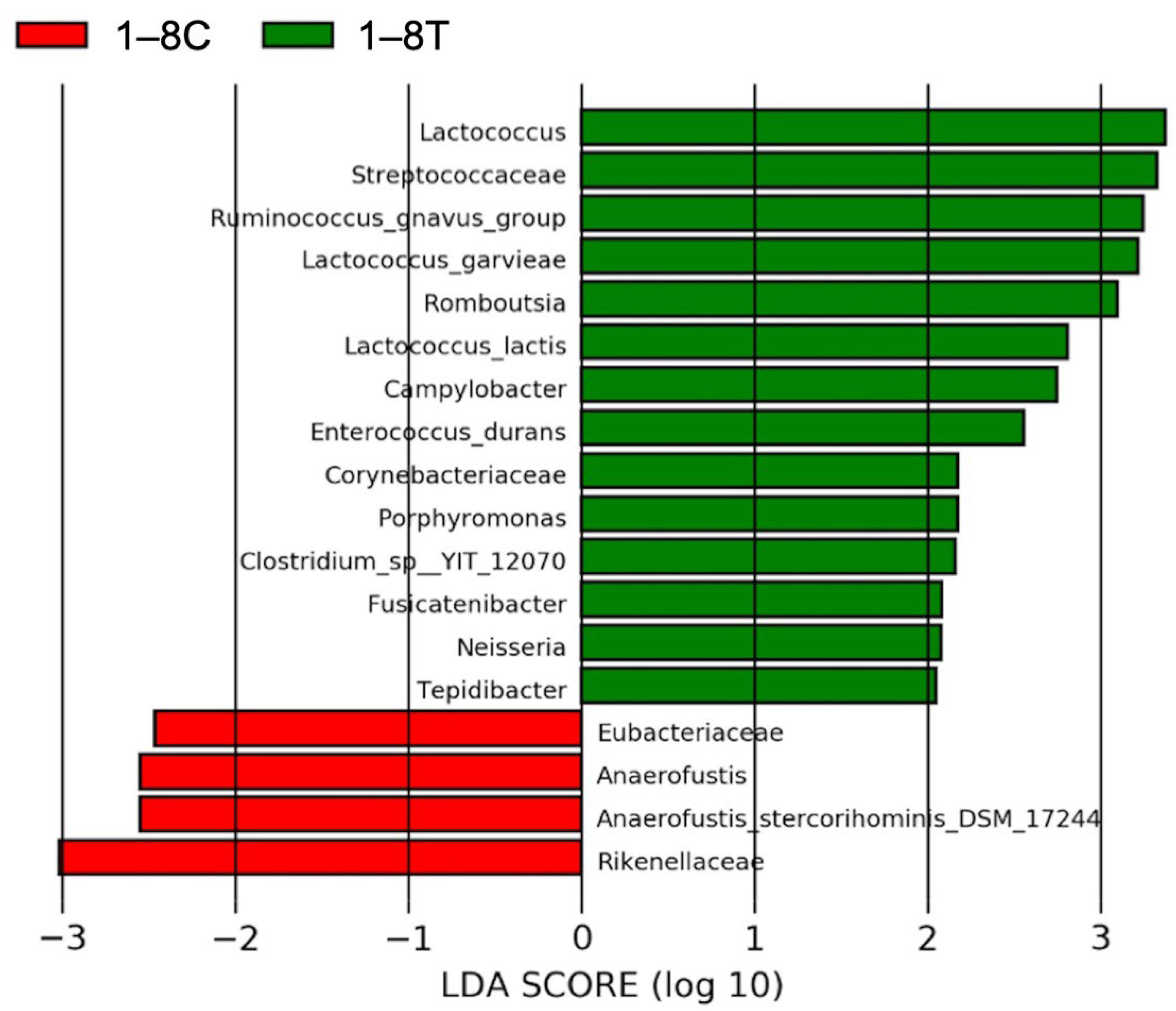
3.4.3. Results of Linear Discriminant Analysis Effective Size (LEfSe)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO/WHO. Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Acid Bacteria. In Proceedings of the Report of a Joint FAO/WHO Expert Consultation, Córdoba, Argentina, 1–4 October 2001. [Google Scholar]
- Ezema, C. Probiotics in animal production: A review. J. Vet. Med. Anim. Health 2013, 5, 308–316. [Google Scholar] [CrossRef]
- Weese, J.S.; Sharif, S.; Rodriguez-Palacios, A. Therapeutic Microbiology: Probiotics and Related Strategies, 1st ed.; Versalovic, J., Wilson, M., Eds.; ASM Press: Washington, DC, USA, 2008. [Google Scholar]
- Bybee, S.N.; Scorza, A.V.; Lappin, M.R. Effect of the probiotic Enterococcus faecium SF68 on presence of diarrhea in cats and dogs housed in an animal shelter. J. Vet. Intern. Med. 2011, 25, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Kelley, R.L.; Minikhiem, D.; Kiely, B.; O’Mahony, L.; O’Sullivan, D.; Boileau, T.; Park, J.S. Clinical benefits of probiotic canine-derived Bifidobacterium animalis strain AHC7 in dogs with acute idiopathic diarrhea. Vet. Ther. 2009, 10, 121–130. [Google Scholar] [PubMed]
- Martin, R.; Olivares, M.; Perez, M.; Xaus, J.; Torre, C.; Fernandez, L.; Rodriguez, J.M. Identification and evaluation of the probiotic potential of lactobacilli isolated from canine milk. Vet. J. 2010, 185, 193–198. [Google Scholar] [CrossRef]
- Sorokulova, I.B.; Pinchuk, I.V.; Denayrolles, M.; Osipova, I.G.; Huang, J.M.; Cutting, S.M.; Urdaci, M.C. The safety of two Bacillus probiotic strains for human use. Dig. Dis. Sci. 2008, 53, 954–963. [Google Scholar] [CrossRef]
- Cutting, S.M. Bacillus probiotics. Food Microbiol 2011, 28, 214–220. [Google Scholar] [CrossRef]
- Lin, E.-R.; Cheng, Y.-H.; Hsiao, F.S.-H.; Proskura, W.S.; Dybus, A.; Yu, Y.-H. Optimization of solid-state fermentation conditions of Bacillus licheniformis and its effects on Clostridium perfringens-induced necrotic enteritis in broilers. Rev. Bras. Zootec. 2019, 48. [Google Scholar] [CrossRef]
- Chen, Y.C.; Yu, Y.H. Bacillus licheniformis-fermented products improve growth performance and the fecal microbiota community in broilers. Poult. Sci. 2020, 99, 1432–1443. [Google Scholar] [CrossRef]
- Peng, J.Y.; Horng, Y.B.; Wu, C.H.; Chang, C.Y.; Chang, Y.C.; Tsai, P.S.; Jeng, C.R.; Cheng, Y.H.; Chang, H.W. Evaluation of antiviral activity of Bacillus licheniformis-fermented products against porcine epidemic diarrhea virus. AMB Express 2019, 9, 191. [Google Scholar] [CrossRef]
- Dethlefsen, L.; Eckburg, P.B.; Bik, E.M.; Relman, D.A. Assembly of the human intestinal microbiota. Trends Ecol. Evol. 2006, 21, 517–523. [Google Scholar] [CrossRef]
- Lee, W.J.; Hase, K. Gut microbiota-generated metabolites in animal health and disease. Nat. Chem. Biol. 2014, 10, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Grzeskowiak, L.; Endo, A.; Beasley, S.; Salminen, S. Microbiota and probiotics in canine and feline welfare. Anaerobe 2015, 34, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Marsilio, S.; Pilla, R.; Sarawichitr, B.; Chow, B.; Hill, S.L.; Ackermann, M.R.; Estep, J.S.; Lidbury, J.A.; Steiner, J.M.; Suchodolski, J.S. Characterization of the fecal microbiome in cats with inflammatory bowel disease or alimentary small cell lymphoma. Sci. Rep. 2019, 9, 19208. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Lepage, P.; Häsler, R.; Spehlmann, M.E.; Rehman, A.; Zvirbliene, A.; Begun, A.; Ott, S.; Kupcinskas, L.; Doré, J.; Raedler, A. Twin study indicates loss of interaction between microbiota and mucosa of patients with ulcerative colitis. Gastroenterology 2011, 141, 227–236. [Google Scholar] [CrossRef]
- Manichanh, C.; Rigottier-Gois, L.; Bonnaud, E.; Gloux, K.; Pelletier, E.; Frangeul, L.; Nalin, R.; Jarrin, C.; Chardon, P.; Marteau, P.; et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut 2006, 55, 205–211. [Google Scholar] [CrossRef]
- Louis, P.; Hold, G.L.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [Google Scholar] [CrossRef]
- Minamoto, Y.; Hooda, S.; Swanson, K.S.; Suchodolski, J.S. Feline gastrointestinal microbiota. Anim. Health Res. Rev. 2012, 13, 64–77. [Google Scholar] [CrossRef]
- Suchodolski, J.S.; Foster, M.L.; Sohail, M.U.; Leutenegger, C.; Queen, E.V.; Steiner, J.M.; Marks, S.L. The fecal microbiome in cats with diarrhea. PLoS ONE 2015, 10, e0127378. [Google Scholar] [CrossRef]
- Honneffer, J.B.; Minamoto, Y.; Suchodolski, J.S. Microbiota alterations in acute and chronic gastrointestinal inflammation of cats and dogs. World J. Gastroenterol. 2014, 20, 16489–16497. [Google Scholar] [CrossRef]
- O’Donnell, L.; Virjee, J.; Heaton, K.W. Detection of pseudodiarrhoea by simple clinical assessment of intestinal transit rate. Br. Med. J. 1990, 300, 439. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.M.; Jenkins, T.P.; Latrofa, M.S.; Giannelli, A.; Papadopoulos, E.; de Carvalho, L.M.; Nolan, M.J.; Otranto, D.; Cantacessi, C. Helminth infections and gut microbiota—A feline perspective. Parasites Vectors 2016, 9, 625. [Google Scholar] [CrossRef] [PubMed]
- Jergens, A.E.; Crandell, J.M.; Evans, R.; Ackermann, M.; Miles, K.G.; Wang, C. A clinical index for disease activity in cats with chronic enteropathy. J. Vet. Intern. Med. 2010, 24, 1027–1033. [Google Scholar] [CrossRef]
- Barbosa, T.M.; Serra, C.R.; La Ragione, R.M.; Woodward, M.J.; Henriques, A.O. Screening for bacillus isolates in the broiler gastrointestinal tract. Appl. Environ. Microbiol. 2005, 71, 968–978. [Google Scholar] [CrossRef] [PubMed]
- Thaniyavarn, J.; Roongsawang, N.; Kameyama, T.; Haruki, M.; Imanaka, T.; Morikawa, M.; Kanaya, S. Production and characterization of biosurfactants from Bacillus licheniformis F2.2. Biosci. Biotechnol. Biochem. 2003, 67, 1239–1244. [Google Scholar] [CrossRef]
- Wang, X.; Hu, W.; Zhu, L.; Yang, Q. Bacillus subtilis and surfactin inhibit the transmissible gastroenteritis virus from entering the intestinal epithelial cells. Biosci. Rep. 2017, 37, BSR20170082. [Google Scholar] [CrossRef]
- Ritchie, L.E.; Steiner, J.M.; Suchodolski, J.S. Assessment of microbial diversity along the feline intestinal tract using 16S rRNA gene analysis. FEMS Microbiol. Ecol. 2008, 66, 590–598. [Google Scholar] [CrossRef]
- Honneffer, J.B.; Steiner, J.M.; Lidbury, J.A.; Suchodolski, J.S. Variation of the microbiota and metabolome along the canine gastrointestinal tract. Metabolomics 2017, 13, 26. [Google Scholar] [CrossRef]
- Deng, P.; Swanson, K.S. Gut microbiota of humans, dogs and cats: Current knowledge and future opportunities and challenges. Br. J. Nutr. 2015, 113, S6–S17. [Google Scholar] [CrossRef]
- Inness, V.; McCartney, A.; Khoo, C.; Gross, K.; Gibson, G. Molecular characterisation of the gut microflora of healthy and inflammatory bowel disease cats using fluorescence In Situ hybridisation with special reference to Desulfovibrio spp. J. Anim. Physiol. Anim. Nutr. 2007, 91, 48–53. [Google Scholar] [CrossRef]
- Chaitman, J.; Ziese, A.-L.; Pilla, R.; Minamoto, Y.; Blake, A.B.; Guard, B.C.; Isaiah, A.; Lidbury, J.A.; Steiner, J.M.; Unterer, S. Fecal microbial and metabolic profiles in dogs with acute diarrhea receiving either fecal microbiota transplantation or oral metronidazole. Front. Vet. Sci. 2020, 7, 192. [Google Scholar] [CrossRef] [PubMed]
- Packey, C.D.; Sartor, R.B. Commensal bacteria, traditional and opportunistic pathogens, dysbiosis and bacterial killing in inflammatory bowel diseases. Curr. Opin. Infect. Dis. 2009, 22, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Baeza, Y.; Hyde, E.R.; Suchodolski, J.S.; Knight, R. Dog and human inflammatory bowel disease rely on overlapping yet distinct dysbiosis networks. Nat. Microbiol. 2016, 1, 16177. [Google Scholar] [CrossRef]
- Bermingham, E.N.; Young, W.; Butowski, C.F.; Moon, C.D.; Maclean, P.H.; Rosendale, D.; Cave, N.J.; Thomas, D.G. The fecal microbiota in the domestic cat (Felis catus) is influenced by interactions between age and diet; a five year longitudinal study. Front. Microbiol. 2018, 9, 1231. [Google Scholar] [CrossRef] [PubMed]
- Weese, J.S.; Arroyo, L. Bacteriological evaluation of dog and cat diets that claim to contain probiotics. Can. Vet. J. 2003, 44, 212–216. [Google Scholar] [PubMed]
- Fang, S.; Qin, T.; Yu, T.; Zhang, G. Improvement of the Gut Microbiota In Vivo by a Short-Chain Fatty Acids-Producing Strain Lactococcus garvieae CF11. Processes 2022, 10, 604. [Google Scholar] [CrossRef]
- Henke, M.T.; Kenny, D.J.; Cassilly, C.D.; Vlamakis, H.; Xavier, R.J.; Clardy, J. Ruminococcus gnavus, a member of the human gut microbiome associated with Crohn’s disease, produces an inflammatory polysaccharide. Proc. Natl. Acad. Sci. USA 2019, 116, 12672–12677. [Google Scholar] [CrossRef]
- Yu, D.; Xia, Y.; Ge, L.; Tan, B.; Chen, S. Effects of Lactococcus lactis on the Intestinal Functions in Weaning Piglets. Front. Nutr. 2021, 8, 713256. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Chun, W.K.; Kim, A.; Kim, N.; Roh, H.J.; Lee, Y.; Yi, M.; Kim, S.; Park, C.I.; Kim, D.H. Dietary Probiotic Effect of Lactococcus lactis WFLU12 on Low-Molecular-Weight Metabolites and Growth of Olive Flounder (Paralichythys olivaceus). Front. Microbiol. 2018, 9, 2059. [Google Scholar] [CrossRef]
- Zhang, Z.; Xing, R.; Lv, Z.; Shao, Y.; Zhang, W.; Zhao, X.; Li, C. Analysis of gut microbiota revealed Lactococcus garviaeae could be an indicative of skin ulceration syndrome in farmed sea cucumber Apostichopus japonicus. Fish Shellfish Immunol. 2018, 80, 148–154. [Google Scholar] [CrossRef]
- Kajiura, T.; Takeda, T.; Sakata, S.; Sakamoto, M.; Hashimoto, M.; Suzuki, H.; Suzuki, M.; Benno, Y. Change of intestinal microbiota with elemental diet and its impact on therapeutic effects in a murine model of chronic colitis. Dig. Dis. Sci. 2009, 54, 1892–1900. [Google Scholar] [CrossRef]
- Bell, J.A.; Kopper, J.J.; Turnbull, J.A.; Barbu, N.I.; Murphy, A.J.; Mansfield, L.S. Ecological characterization of the colonic microbiota of normal and diarrheic dogs. Interdiscip. Perspect. Infect. Dis. 2008, 2008, 149694. [Google Scholar] [CrossRef] [PubMed]
- Suchodolski, J.S.; Markel, M.E.; Garcia-Mazcorro, J.F.; Unterer, S.; Heilmann, R.M.; Dowd, S.E.; Kachroo, P.; Ivanov, I.; Minamoto, Y.; Dillman, E.M.; et al. The fecal microbiome in dogs with acute diarrhea and idiopathic inflammatory bowel disease. PLoS ONE 2012, 7, e51907. [Google Scholar] [CrossRef] [PubMed]
- Markel, M.; Berghoff, N.; Unterer, S.; Oliveira-Barros, L.; Grellet, A.; Allenspach, K.; Toresson, L.; Barr, J.; Heilmann, R.; Garcia-Mazcorro, J. Characterization of fecal dysbiosis in dogs with chronic enteropathies and acute hemeorrhagic diarrhea. J. Vet. Intern. Med. 2012, 26, 765–766. [Google Scholar]
- Schoster, A.; Kokotovic, B.; Permin, A.; Pedersen, P.D.; Dal Bello, F.; Guardabassi, L. In Vitro inhibition of Clostridium difficile and Clostridium perfringens by commercial probiotic strains. Anaerobe 2013, 20, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Busch, K.; Suchodolski, J.; Kühner, K.; Minamoto, Y.; Steiner, J.; Mueller, R.S.; Hartmann, K.; Unterer, S. Clostridium perfringens enterotoxin and Clostridium difficile toxin A/B do not play a role in acute haemorrhagic diarrhoea syndrome in dogs. Vet. Rec. 2015, 176, 253. [Google Scholar] [CrossRef]
- Prideaux, L.; Kang, S.; Wagner, J.; Buckley, M.; Mahar, J.E.; De Cruz, P.; Wen, Z.; Chen, L.; Xia, B.; van Langenberg, D.R.; et al. Impact of ethnicity, geography, and disease on the microbiota in health and inflammatory bowel disease. Inflamm. Bowel Dis. 2013, 19, 2906–2918. [Google Scholar] [CrossRef]



| Number | Breed | Age | Gender | Stool Score | FCEAI | ||
|---|---|---|---|---|---|---|---|
| Before | After | Before | After | ||||
| 1 | DSH | 12 | Mc | 5 | 2.5 | 2 | 1 |
| 2 | DSH | 12 | Fsp | 5 | 2.5 | 5 | 2 |
| 3 | DSH | 3 | Mc | 5.5 | 5 | 2 | 2 |
| 4 | DSH | 7 | Mc | 5.5 | 5.5 | 4 | 4 |
| 5 | DSH | 5 | Mc | 5 | 5 | 1 | 1 |
| 6 | DSH | 9 | Fsp | 7 | 6.5 | 5 | 3 |
| 7 | DSH | 3 | Mc | 5.5 | 5 | 2 | 2 |
| 8 | Scottish fold | 3 | Fsp | 7 | 6.5 | 4 | 4 |
| 9 | DSH | 3 | Fsp | 3 | 3 | 1 | 2 |
| 10 | DSH | 5 | Mc | 4 | 4 | 4 | 5 |
| 11 | DSH | 3 | Mc | 3 | 3 | 2 | 3 |
| 12 | American short hair | 15 | Fsp | 2.5 | 2.5 | 1 | 1 |
| OTU | Phylum | Class | Order | Family | Genus | p Value | Fold Change |
|---|---|---|---|---|---|---|---|
| 204 | Firmicutes | Bacilli | Lactobacillales | Streptococcaceae | Lactococcus | 0.00359 | 188.630666 |
| 200 | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | Ruminococcus_gnavus_group | 0.0058 | 9.36044619 |
| 24 | Firmicutes | Clostridia | Clostridiales | Clostridiaceae_1 | Clostridium_sensu_stricto_1 | 0.01115 | 238.432716 |
| 158 | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | Blautia | 0.01427 | 7.98145294 |
| 3 | Firmicutes | Clostridia | Clostridiales | Peptostreptococcaceae | Paeniclostridium | 0.01847 | 14.0464061 |
| 163 | Firmicutes | Clostridia | Clostridiales | Clostridiaceae_1 | Clostridium_sensu_stricto_1 | 0.02056 | 1.60162436 |
| 92 | Firmicutes | Negativicutes | Selenomonadales | Acidaminococcaceae | Acidaminococcus | 0.02345 | −3.434832 |
| 626 | Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | Butyricicoccus | 0.02598 | −10.838548 |
| 81 | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | Ruminococcus_torques_group | 0.03359 | 5.76459407 |
| 260 | Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | Anaerotruncus | 0.034 | 22.2240326 |
| 13 | Bacteroidetes | Bacteroidia | Bacteroidales | Bacteroidaceae | Bacteroides | 0.03848 | −11.225547 |
| 411 | Firmicutes | Bacilli | Lactobacillales | Enterococcaceae | Enterococcus | 0.04221 | 13.6340283 |
| 135 | Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | Ruminococcaceae_UCG-014 | 0.04417 | 46.5310365 |
| 636 | Firmicutes | Clostridia | Clostridiales | Clostridiaceae_1 | Clostridium_sensu_stricto_1 | 0.04948 | −211.89874 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, T.-W.; Chao, T.-Y.; Chang, H.-W.; Cheng, Y.-H.; Wu, C.-H.; Chang, Y.-C. The Effects of Bacillus licheniformis—Fermented Products on the Microbiota and Clinical Presentation of Cats with Chronic Diarrhea. Animals 2022, 12, 2187. https://doi.org/10.3390/ani12172187
Lee T-W, Chao T-Y, Chang H-W, Cheng Y-H, Wu C-H, Chang Y-C. The Effects of Bacillus licheniformis—Fermented Products on the Microbiota and Clinical Presentation of Cats with Chronic Diarrhea. Animals. 2022; 12(17):2187. https://doi.org/10.3390/ani12172187
Chicago/Turabian StyleLee, Ting-Wei, Tzu-Yi Chao, Hui-Wen Chang, Yeong-Hsiang Cheng, Ching-Ho Wu, and Yen-Chen Chang. 2022. "The Effects of Bacillus licheniformis—Fermented Products on the Microbiota and Clinical Presentation of Cats with Chronic Diarrhea" Animals 12, no. 17: 2187. https://doi.org/10.3390/ani12172187
APA StyleLee, T.-W., Chao, T.-Y., Chang, H.-W., Cheng, Y.-H., Wu, C.-H., & Chang, Y.-C. (2022). The Effects of Bacillus licheniformis—Fermented Products on the Microbiota and Clinical Presentation of Cats with Chronic Diarrhea. Animals, 12(17), 2187. https://doi.org/10.3390/ani12172187






