Phenol-Rich Botanicals Modulate Oxidative Stress and Epithelial Integrity in Intestinal Epithelial Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Intestinal Caco-2 Culture Conditions
2.3. Viability Assay on Caco-2
2.4. Measurement of Reactive Oxygen Species (ROS) Level
2.5. Dose Response on Caco-2 Cells
2.6. Gene Expression Analysis
2.7. Statistical Analysis
3. Results
3.1. Ginger Essential Oil
3.1.1. Viability and Dose Response
3.1.2. Antioxidant Activity
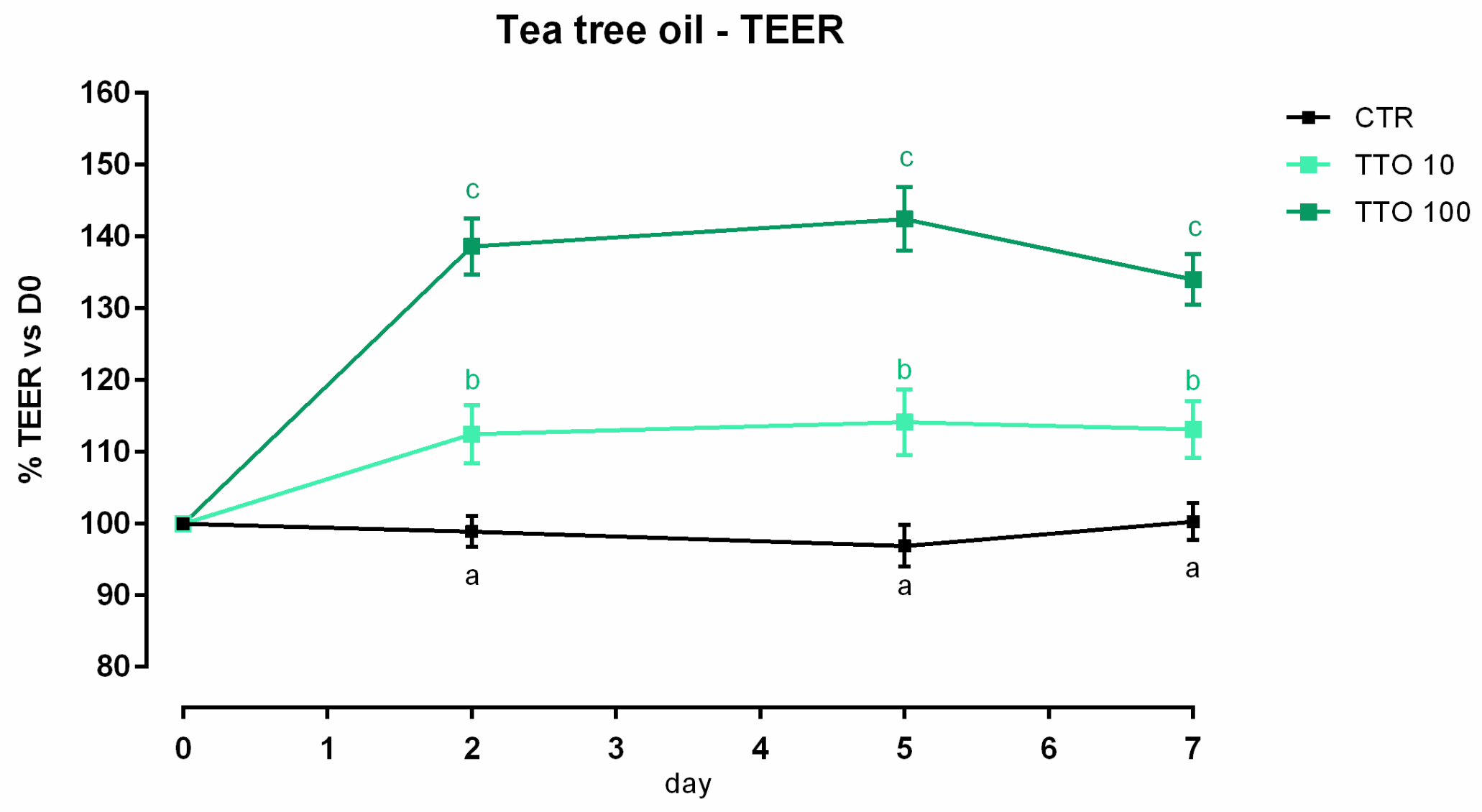
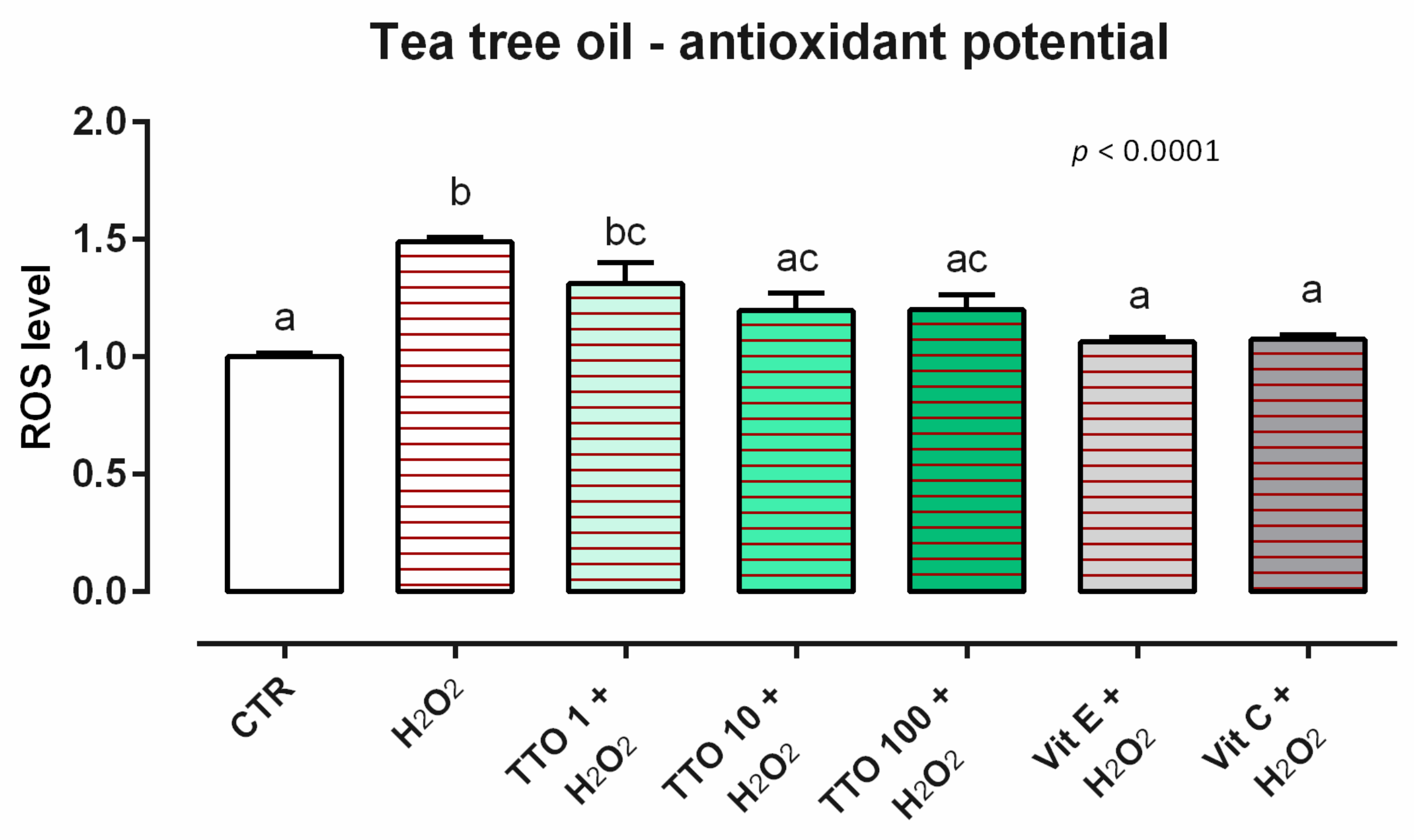
3.2. Tea Tree Oil
3.2.1. Viability and Dose Response
3.2.2. Antioxidant Activity
3.3. Grape Seed Extract
3.3.1. Viability and Dose Response
3.3.2. Antioxidant Activity
3.4. Green Tea Extract
3.4.1. Viability and Dose Response
3.4.2. Antioxidant Activity
3.5. Olive Extract
3.5.1. Viability and Dose Response
3.5.2. Antioxidant Activity
3.6. Pomegranate Extract
3.6.1. Viability and Dose Response
3.6.2. Antioxidant Activity
3.7. Chestnut Extract
3.7.1. Viability and Dose Response
3.7.2. Antioxidant Activity
3.8. Thyme Essential Oil
3.8.1. Viability and Dose Response
3.8.2. Antioxidant Activity
3.9. Capsicum Oleoresin
3.9.1. Viability and Dose Response
3.9.2. Antioxidant Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors Affecting Secondary Metabolite Production in Plants: Volatile Components and Essential Oils. Flavour Frag. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Eslahi, H.; Fahimi, N.; Sardarian, A.R. Chemical Composition of Essential Oils. In Essential Oils in Food Processing; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 119–171. ISBN 978-1-119-14939-2. [Google Scholar]
- Rossi, B.; Toschi, A.; Piva, A.; Grilli, E. Single Components of Botanicals and Nature-Identical Compounds as a Non-Antibiotic Strategy to Ameliorate Health Status and Improve Performance in Poultry and Pigs. Nutr. Res. Rev. 2020, 33, 218–234. [Google Scholar] [CrossRef]
- Windisch, W.; Schedle, K.; Plitzner, C.; Kroismayr, A. Use of Phytogenic Products as Feed Additives for Swine and Poultry. J. Anim. Sci. 2008, 86, E140–E148. [Google Scholar] [CrossRef]
- Ribnicky, D.M.; Poulev, A.; Schmidt, B.; Cefalu, W.T.; Raskin, I. Evaluation of Botanicals for Improving Human Health. Am. J. Clin. Nutr. 2008, 87, 472S–475S. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhang, S.; Wang, H.; Piao, X. Essential Oil and Aromatic Plants as Feed Additives in Non-Ruminant Nutrition: A Review. J. Anim. Sci. Biotechnol. 2015, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Kraimi, N.; Dawkins, M.; Gebhardt-Henrich, S.G.; Velge, P.; Rychlik, I.; Volf, J.; Creach, P.; Smith, A.; Colles, F.; Leterrier, C. Influence of the Microbiota-Gut-Brain Axis on Behavior and Welfare in Farm Animals: A Review. Physiol. Behav. 2019, 210, 112658. [Google Scholar] [CrossRef]
- Pham, V.H.; Kan, L.; Huang, J.; Geng, Y.; Zhen, W.; Guo, Y.; Abbas, W.; Wang, Z. Dietary Encapsulated Essential Oils and Organic Acids Mixture Improves Gut Health in Broiler Chickens Challenged with Necrotic Enteritis. J. Anim. Sci. Biotechnol. 2020, 11, 1–18. [Google Scholar] [CrossRef]
- Beecher, G.R. Overview of Dietary Flavonoids: Nomenclature, Occurrence and Intake. J. Nutr. 2003, 133, 3248S–3254S. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [Green Version]
- Serra, V.; Salvatori, G.; Pastorelli, G. Dietary Polyphenol Supplementation in Food Producing Animals: Effects on the Quality of Derived Products. Animals 2021, 11, 401. [Google Scholar] [CrossRef] [PubMed]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial Activity of Polyphenols and Alkaloids in Middle Eastern Plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef]
- Mallo, N.; Lamas, J.; Sueiro, R.A.; Leiro, J.M. Molecular Targets Implicated in the Antiparasitic and Anti-Inflammatory Activity of the Phytochemical Curcumin in Trichomoniasis. Molecules 2020, 25, 5321. [Google Scholar] [CrossRef] [PubMed]
- Toschi, A.; Rossi, B.; Tugnoli, B.; Piva, A.; Grilli, E. Nature-Identical Compounds and Organic Acids Ameliorate and Prevent the Damages Induced by an Inflammatory Challenge in Caco-2 Cell Culture. Molecules 2020, 25, 4296. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER Measurement Techniques for In Vitro Barrier Model Systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2–ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Park, H.-Y.; Kunitake, Y.; Hirasaki, N.; Tanaka, M.; Matsui, T. Theaflavins Enhance Intestinal Barrier of Caco-2 Cell Monolayers through the Expression of AMP-Activated Protein Kinase-Mediated Occludin, Claudin-1, and ZO-1. Biosci. Biotechnol. Biochem. 2015, 79, 130–137. [Google Scholar] [CrossRef]
- Hichino, A.; Okamoto, M.; Taga, S.; Akizuki, R.; Endo, S.; Matsunaga, T.; Ikari, A. Down-Regulation of Claudin-2 Expression and Proliferation by Epigenetic Inhibitors in Human Lung Adenocarcinoma A549 Cells. J. Biol. Chem. 2017, 292, 2411–2421. [Google Scholar] [CrossRef]
- Tatsuta, M.; Kan-o, K.; Ishii, Y.; Yamamoto, N.; Ogawa, T.; Fukuyama, S.; Ogawa, A.; Fujita, A.; Nakanishi, Y.; Matsumoto, K. Effects of Cigarette Smoke on Barrier Function and Tight Junction Proteins in the Bronchial Epithelium: Protective Role of Cathelicidin LL-37. Respir. Res. 2019, 20, 251. [Google Scholar] [CrossRef]
- Dydensborg, A.B.; Herring, E.; Auclair, J.; Tremblay, E.; Beaulieu, J.-F. Normalizing Genes for Quantitative RT-PCR in Differentiating Human Intestinal Epithelial Cells and Adenocarcinomas of the Colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G1067–G1074. [Google Scholar] [CrossRef] [Green Version]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate Normalization of Real-Time Quantitative RT-PCR Data by Geometric Averaging of Multiple Internal Control Genes. Genome Biol. 2002, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Aldars-García, L.; Marin, A.C.; Chaparro, M.; Gisbert, J.P. The Interplay between Immune System and Microbiota in Inflammatory Bowel Disease: A Narrative Review. Int. J. Mol. Sci. 2021, 22, 3076. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EC) No 1831/2003; Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on Additives for Use in Animal Nutrition (Text with EEA Relevance). European Parliament: Strasbourg, France, 2003; Volume 268.
- Veterinary Feed Directive. Available online: https://www.federalregister.gov/documents/2015/06/03/2015-13393/veterinary-feed-directive (accessed on 27 June 2018).
- Aziz, Z.A.A.; Ahmad, A.; Setapar, S.H.M.; Karakucuk, A.; Azim, M.M.; Lokhat, D.; Rafatullah, M.; Ganash, M.; Kamal, M.A.; Ashraf, G.M. Essential Oils: Extraction Techniques, Pharmaceutical And Therapeutic Potential—A Review. Curr. Drug Metab. 2018, 19, 1100–1110. [Google Scholar] [CrossRef] [PubMed]
- Galassi, G.; Battelli, M.; Verdile, N.; Rapetti, L.; Zanchi, R.; Arcuri, S.; Petrera, F.; Abeni, F.; Crovetto, G.M. Effect of a Polyphenol-Based Additive in Pig Diets in the Early Stages of Growth. Animals 2021, 11, 3241. [Google Scholar] [CrossRef]
- Mahfuz, S.; Shang, Q.; Piao, X. Phenolic Compounds as Natural Feed Additives in Poultry and Swine Diets: A Review. J. Anim. Sci. Biotechnol. 2021, 12, 48. [Google Scholar] [CrossRef]
- Lipiński, K.; Antoszkiewicz, Z.; Mazur-Kuśnirek, M.; Korniewicz, D.; Kotlarczyk, S. The Effect of Polyphenols on the Performance and Antioxidant Status of Sows and Piglets. Ital. J. Anim. Sci. 2019, 18, 174–181. [Google Scholar] [CrossRef]
- Herve, T.; Raphaël, K.J.; Ferdinand, N.; Victor Herman, N.; Willy Marvel, N.M.; Cyril D’Alex, T.; Laurine Vitrice, F.T. Effects of Ginger (Zingiber Officinale, Roscoe) Essential Oil on Growth and Laying Performances, Serum Metabolites, and Egg Yolk Antioxidant and Cholesterol Status in Laying Japanese Quail. J. Vet. Med. 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- Libera, J.; Latoch, A.; Wójciak, K.M. Utilization of Grape Seed Extract as a Natural Antioxidant in the Technology of Meat Products Inoculated with a Probiotic Strain of LAB. Foods 2020, 9, 103. [Google Scholar] [CrossRef]
- Puvača, N.; Čabarkapa, I.; Petrović, A.; Bursić, V.; Prodanović, R.; Soleša, D.; Lević, J. Tea Tree (Melaleuca Alternifolia) and Its Essential Oil: Antimicrobial, Antioxidant and Acaricidal Effects in Poultry Production. Worlds Poult. Sci. J. 2019, 75, 235–246. [Google Scholar] [CrossRef]
- Weseler, A.R.; Bast, A. Masquelier’s Grape Seed Extract: From Basic Flavonoid Research to a Well-Characterized Food Supplement with Health Benefits. Nutr. J. 2017, 16, 5. [Google Scholar] [CrossRef] [Green Version]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Aviello, G.; Knaus, U. ROS in Gastrointestinal Inflammation: Rescue Or Sabotage? Br. J. Pharmacol. 2017, 174, 1704–1718. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, A.; Parker, R.D.; Abdollahi, M. Oxidative Stress and Pathogenesis of Inflammatory Bowel Disease: An Epiphenomenon or the Cause? Dig. Dis. Sci. 2007, 52, 2015–2021. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.S.; Steinauer, K.K.; Hornung, B.; Irish, J.M.; Lecane, P.; Birrell, G.W.; Peehl, D.M.; Knox, S.J. Role of Glutathione Depletion and Reactive Oxygen Species Generation in Apoptotic Signaling in a Human B Lymphoma Cell Line. Cell Death Differ. 2002, 9, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Dietary Polyphenols: Good, Bad, or Indifferent for Your Health? Cardiovasc. Res. 2007, 73, 341–347. [Google Scholar] [CrossRef]
- Afshari, A.T.; Shirpoor, A.; Farshid, A.; Saadatian, R.; Rasmi, Y.; Saboory, E.; Ilkhanizadeh, B.; Allameh, A. The Effect of Ginger on Diabetic Nephropathy, Plasma Antioxidant Capacity and Lipid Peroxidation in Rats. Food Chem. 2007, 101, 148–153. [Google Scholar] [CrossRef]
- Hamed, M.A.; Ali, S.A.; Saba El-Rigal, N. Therapeutic Potential of Ginger against Renal Injury Induced by Carbon Tetrachloride in Rats. Available online: https://www.hindawi.com/journals/tswj/2012/840421/ (accessed on 20 April 2020).
- Hosseinzadeh, A.; Bahrampour Juybari, K.; Fatemi, M.J.; Kamarul, T.; Bagheri, A.; Tekiyehmaroof, N.; Sharifi, A.M. Protective Effect of Ginger (Zingiber Officinale Roscoe) Extract against Oxidative Stress and Mitochondrial Apoptosis Induced by Interleukin-1β in Cultured Chondrocytes. Cells Tissues Organs. 2017, 204, 241–250. [Google Scholar] [CrossRef]
- Mao, Q.-Q.; Xu, X.-Y.; Cao, S.-Y.; Gan, R.-Y.; Corke, H.; Beta, T.; Li, H.-B. Bioactive Compounds and Bioactivities of Ginger (Zingiber Officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef]
- Li, Y.; Xu, B.; Xu, M.; Chen, D.; Xiong, Y.; Lian, M.; Sun, Y.; Tang, Z.; Wang, L.; Jiang, C.; et al. 6-Gingerol Protects Intestinal Barrier from Ischemia/Reperfusion-Induced Damage via Inhibition of P38 MAPK to NF-ΚB Signalling. Pharmacol. Res. 2017, 119, 137–148. [Google Scholar] [CrossRef]
- Pinent, M.; Castell-Auví, A.; Genovese, M.I.; Serrano, J.; Casanova, A.; Blay, M.; Ardévol, A. Antioxidant Effects of Proanthocyanidin-Rich Natural Extracts from Grape Seed and Cupuassu on Gastrointestinal Mucosa. J. Sci. Food Agric. 2016, 96, 178–182. [Google Scholar] [CrossRef]
- Jeong, W.-S.; Kong, A.-N.T. Biological Properties of Monomeric and Polymeric Catechins: Green Tea Catechins and Procyanidins. Pharm. Biol. 2004, 42, 84–93. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, C.; García-Villanova, B.; Guerra-Hernández, E.; Verardo, V. Grape Seeds Proanthocyanidins: An Overview of In Vivo Bioactivity in Animal Models. Nutrients 2019, 11, 2435. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.; Slade, D. Oligomeric Proanthocyanidins: Naturally Occurring O-Heterocycles. Nat. Prod. Rep. 2002, 19, 517–541. [Google Scholar] [CrossRef] [PubMed]
- Hassen, I.; Casabianca, H.; Hosni, K. Biological Activities of the Natural Antioxidant Oleuropein: Exceeding the Expectation—A Mini-Review. J. Funct. Foods 2015, 18, 926–940. [Google Scholar] [CrossRef]
- de la Puerta, R.; Domínguez, M.E.M.; Ruíz-Gutíerrez, V.; Flavill, J.A.; Hoult, J.R.S. Effects of Virgin Olive Oil Phenolics on Scavenging of Reactive Nitrogen Species and upon Nitrergic Neurotransmission. Life Sci. 2001, 69, 1213–1222. [Google Scholar] [CrossRef]
- Smulikowska, S.; Pastuszewska, B.; Święch, E.; Ochtabińska, A.; Mieczkowska, A.; Nguyen, V.C.; Buraczewska, L. Tannin Content Affects Negatively Nutritive Value of Pea for Monogastrics. J. Anim. Feed Sci. 2001, 10, 511–523. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Antioxidant Activity and Phenolic Compounds in Selected Herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef]
- Zarfeshany, A.; Asgary, S.; Javanmard, S.H. Potent Health Effects of Pomegranate. Adv. Biomed. Res. 2014, 3, 100. [Google Scholar] [CrossRef]
- Nagoor Meeran, M.F.; Javed, H.; Al Taee, H.; Azimullah, S.; Ojha, S.K. Pharmacological Properties and Molecular Mechanisms of Thymol: Prospects for Its Therapeutic Potential and Pharmaceutical Development. Front. Pharmacol. 2017, 8, 380. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Zhang, X.; Lu, Y.; Chen, H. New Insights in Intestinal Oxidative Stress Damage and the Health Intervention Effects of Nutrients: A Review. J. Funct. Foods 2020, 75, 104248. [Google Scholar] [CrossRef]
- Zucco, F.; Batto, A.-F.; Bises, G.; Chambaz, J.; Chiusolo, A.; Consalvo, R.; Cross, H.; Dal Negro, G.; de Angelis, I.; Fabre, G.; et al. An Inter-Laboratory Study to Evaluate the Effects of Medium Composition on the Differentiation and Barrier Function of Caco-2 Cell Lines. Altern. Lab. Anim. 2005, 33, 603–618. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Van Itallie, C.M. Physiology and Function of the Tight Junction. Cold Spring Harb. Perspect. Biol. 2009, 1, a002584. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.C.; Shukla, R.; Ali, J.; Sharma, S.; Bajpai, P.; Pathak, N. Phytochemical Evaluation, Antioxidant Assay, Antibacterial Activity and Determination of Cell Viability (J774 and THP1 Alpha Cell Lines) of P. Sylvestris Leaf Crude and Methanol Purified Fractions. EXCLI J. 2016, 15, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Tanabe, S.; Hara, H. Kaempferol Enhances Intestinal Barrier Function through the Cytoskeletal Association and Expression of Tight Junction Proteins in Caco-2 Cells. J. Nutr. 2011, 141, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, R.; Milatz, S.; Krug, S.M.; Oelrich, B.; Schulzke, J.-D.; Amasheh, S.; Günzel, D.; Fromm, M. Claudin-2, a Component of the Tight Junction, Forms a Paracellular Water Channel. J. Cell Sci. 2010, 123, 1913–1921. [Google Scholar] [CrossRef]
- Deli, M.A. Potential Use of Tight Junction Modulators to Reversibly Open Membranous Barriers and Improve Drug Delivery. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2009, 1788, 892–910. [Google Scholar] [CrossRef]
- Singh, G.; Kapoor, I.P.S.; Singh, P.; de Heluani, C.S.; de Lampasona, M.P.; Catalan, C.A.N. Chemistry, Antioxidant and Antimicrobial Investigations on Essential Oil and Oleoresins of Zingiber Officinale. Food Chem. Toxicol. 2008, 46, 3295–3302. [Google Scholar] [CrossRef]
- Atki, Y.E.; Aouam, I. Antibacterial Efficacy of Thymol, Carvacrol, Eugenol and Menthol as Alternative Agents to Control the Growth of Nosocomial Infection-Bacteria. J. Pharm. Sci. 2019, 11, 4. [Google Scholar]
- Luettig, J.; Rosenthal, R.; Lee, I.-F.M.; Krug, S.M.; Schulzke, J.D. The Ginger Component 6-Shogaol Prevents TNF-α-Induced Barrier Loss via Inhibition of PI3K/Akt and NF-ΚB Signaling. Mol. Nutr. Food Res. 2016, 60, 2576–2586. [Google Scholar] [CrossRef]
- Huh, E.; Choi, J.G.; Noh, D.; Yoo, H.-S.; Ryu, J.; Kim, N.-J.; Kim, H.; Oh, M.S. Ginger and 6-Shogaol Protect Intestinal Tight Junction and Enteric Dopaminergic Neurons against 1-Methyl-4-Phenyl 1,2,3,6-Tetrahydropyridine in Mice. Nutr. Neurosci. 2020, 23, 455–464. [Google Scholar] [CrossRef]
- Toschi, A.; Tugnoli, B.; Rossi, B.; Piva, A.; Grilli, E. Thymol Modulates the Endocannabinoid System and Gut Chemosensing of Weaning Pigs. BMC Vet. Res. 2020, 16, 289. [Google Scholar] [CrossRef] [PubMed]
- Bitzer, Z.T.; Glisan, S.L.; Dorenkott, M.R.; Goodrich, K.M.; Ye, L.; O’Keefe, S.F.; Lambert, J.D.; Neilson, A.P. Cocoa Procyanidins with Different Degrees of Polymerization Possess Distinct Activities in Models of Colonic Inflammation. J. Nutr. Biochem. 2015, 26, 827–831. [Google Scholar] [CrossRef]
- Wong, X.; Carrasco-Pozo, C.; Escobar, E.; Navarrete, P.; Blachier, F.; Andriamihaja, M.; Lan, A.; Tomé, D.; Cires, M.J.; Pastene, E.; et al. Deleterious Effect of P-Cresol on Human Colonic Epithelial Cells Prevented by Proanthocyanidin-Containing Polyphenol Extracts from Fruits and Proanthocyanidin Bacterial Metabolites. J. Agric. Food Chem. 2016, 64, 3574–3583. [Google Scholar] [CrossRef]
- Yong, Y.; Fang, B.; Huang, Y.; Li, J.; Yu, T.; Wu, L.; Hu, C.; Liu, X.; Yu, Z.; Ma, X.; et al. Tea Tree Oil Terpinen-4-Ol Protects Gut Barrier Integrity by Upregulation of Tight Junction Proteins via the ERK1/2-Signaling Pathway. Front. Nutr. 2022, 8, 805612. [Google Scholar] [CrossRef]
- Kanda, Y.; Yamasaki, Y.; Sasaki-Yamaguchi, Y.; Ida-Koga, N.; Kamisuki, S.; Sugawara, F.; Nagumo, Y.; Usui, T. TRPA1-Dependent Reversible Opening of Tight Junction by Natural Compounds with an α,β-Unsaturated Moiety and Capsaicin. Sci. Rep. 2018, 8, 2251. [Google Scholar] [CrossRef] [PubMed]
- Tsukura, Y.; Mori, M.; Hirotani, Y.; Ikeda, K.; Amano, F.; Kato, R.; Ijiri, Y.; Tanaka, K. Effects of Capsaicin on Cellular Damage and Monolayer Permeability in Human Intestinal Caco-2 Cells. Biol. Pharm. Bull. 2007, 30, 1982–1986. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Isoda, H. Effects of Capsaicin on Energy Metabolism by Human Intestinal Epithelial Cell Line Caco-2. Cells Cult. 2010, 4, 739–742. [Google Scholar] [CrossRef]
- Liu, S.J.; Wang, J.; He, T.F.; Liu, H.S.; Piao, X.S. Effects of Natural Capsicum Extract on Growth Performance, Nutrient Utilization, Antioxidant Status, Immune Function, and Meat Quality in Broilers. Poult. Sci. 2021, 100, 101301. [Google Scholar] [CrossRef]
- Zsiborás, C.; Mátics, R.; Hegyi, P.; Balaskó, M.; Pétervári, E.; Szabó, I.; Sarlós, P.; Mikó, A.; Tenk, J.; Rostás, I.; et al. Capsaicin and Capsiate Could Be Appropriate Agents for Treatment of Obesity: A Meta-Analysis of Human Studies. Crit. Rev. Food Sci. Nutr. 2018, 58, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, S.; Anwer, S.; Szászi, K. Claudin-2: Roles beyond Permeability Functions. Int. J. Mol. Sci. 2019, 20, 5655. [Google Scholar] [CrossRef]
- Chen, B.; Qiu, J.; Wang, Y.; Huang, W.; Zhao, H.; Zhu, X.; Peng, K. Condensed Tannins Increased Intestinal Permeability of Chinese Seabass (Lateolabrax maculatus) Based on Microbiome-Metabolomics Analysis. Aquaculture 2022, 560, 738615. [Google Scholar] [CrossRef]
- Tretola, M.; Bee, G.; Silacci, P. Gallic Acid Affects Intestinal-Epithelial-Cell Integrity and Selected Amino-Acid Uptake in Porcine in Vitro and Ex Vivo Permeability Models. Br. J. Nutr. 2021, 126, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Frutos, P.; Hervás, G.; Giráldez, F.; Mantecón, A. Review. Tannins and Ruminant Nutrition. Span. J. Agric. Res. 2004, 2, 191. [Google Scholar] [CrossRef] [Green Version]

























| Gene | Primer Sequence (F and R) 5′→3′ | Product Length (bp) | Accession N. | Reference |
|---|---|---|---|---|
| ZO-1 | F: CGGGACTGTTGGTATTGGCTAGA | 184 | NM_001301025.3 | [18] |
| R: GGCCAGGGCCATAGTAAAGTTTG | ||||
| OCCL | F: TCCTATAAATCCACGCCGGTTC | 105 | NM_001205254.2 | [18] |
| R: CTCAAAGTTACCACCGCTGCTG | ||||
| CLDN-2 | F: ATTGTGACAGCAGTTGGCTT | 86 | NM_001171092.1 | [19] |
| R: CTATAGATGTCACACTGGGTGATG | ||||
| CLDN-3 | F: ACATCATCACGTCGCAGAACATC | 103 | NM_001306.4 | [18] |
| R: AGTGCCAGCAGCGAGTCGTA | ||||
| CLDN-15 | F: TCCTATAAATCCACGCCGGTTC | 155 | NM_001185080.2 | [20] |
| R: CTCAAAGTTACCACCGCTGCTG | ||||
| JAM-A | F: CAGAGGTGATTCATGGCTCTGTG | 96 | NM_001382727 | [18] |
| R: TTCCAGGCTGGCAATAACTGAC | ||||
| RPLP0 | F: GCAATGTTGCCAGTGTCTG | 142 | NM_001002.3 | [21] |
| R: GCCTTGACCTTTTCAGCAA | ||||
| GAPDH | F: TGCACCACCAACTGCTTAGC | 87 | NM_02046 | [22] |
| R: GGCATGGACTGTGGTCATGAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toschi, A.; Piva, A.; Grilli, E. Phenol-Rich Botanicals Modulate Oxidative Stress and Epithelial Integrity in Intestinal Epithelial Cells. Animals 2022, 12, 2188. https://doi.org/10.3390/ani12172188
Toschi A, Piva A, Grilli E. Phenol-Rich Botanicals Modulate Oxidative Stress and Epithelial Integrity in Intestinal Epithelial Cells. Animals. 2022; 12(17):2188. https://doi.org/10.3390/ani12172188
Chicago/Turabian StyleToschi, Andrea, Andrea Piva, and Ester Grilli. 2022. "Phenol-Rich Botanicals Modulate Oxidative Stress and Epithelial Integrity in Intestinal Epithelial Cells" Animals 12, no. 17: 2188. https://doi.org/10.3390/ani12172188
APA StyleToschi, A., Piva, A., & Grilli, E. (2022). Phenol-Rich Botanicals Modulate Oxidative Stress and Epithelial Integrity in Intestinal Epithelial Cells. Animals, 12(17), 2188. https://doi.org/10.3390/ani12172188






