The Trophic Niche of Two Sympatric Species of Salamanders (Plethodontidae and Salamandridae) from Italy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
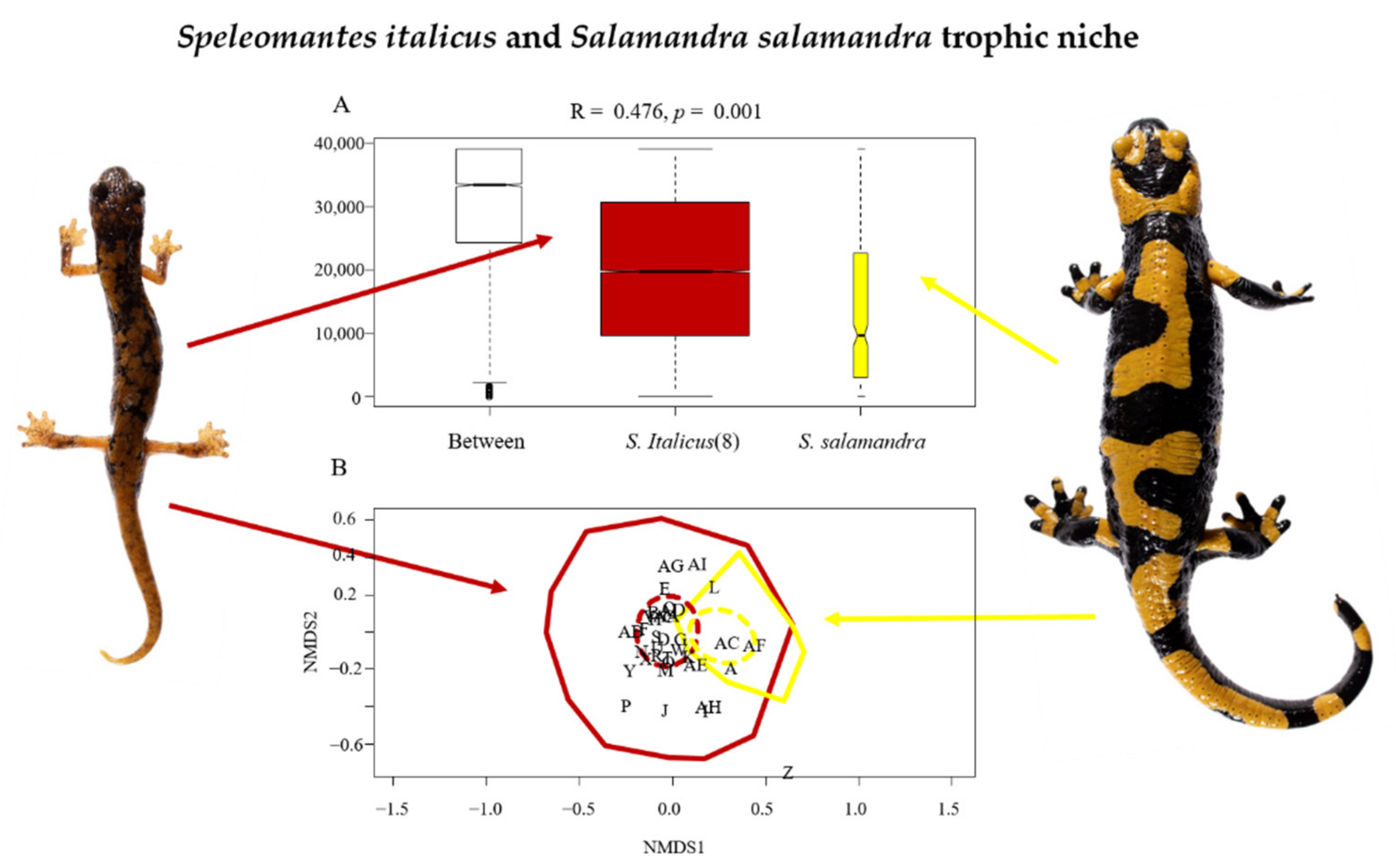
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hutchinson, G.E. Concluding remarks. Cold Spring Harb. Symp. Quant. Biol. 1957, 22, 415–427. [Google Scholar] [CrossRef]
- Campbell, C.J.; Nelson, D.M.; Ogawa, N.O.; Chikaraishi, Y.; Ohkouchi, N. Trophic position and dietary breadth of bats revealed by nitrogen isotopic composition of amino acids. Sci. Rep. 2017, 7, 15932. [Google Scholar] [CrossRef] [PubMed]
- Manenti, R.; Denoël, M.; Ficetola, G.F. Foraging plasticity favours adaptation to new habitats in fire salamanders. Anim. Behav. 2013, 86, 375–382. [Google Scholar] [CrossRef]
- Lunghi, E.; Cianferoni, F.; Ceccolini, F.; Veith, M.; Manenti, R.; Mancinelli, G.; Corti, C.; Ficetola, G.F. What shapes the trophic niche of European plethodontid salamanders? PLoS ONE 2018, 13, e0205672. [Google Scholar] [CrossRef]
- Fraenkel, G.; Blewett, M. The basic food requirements of several insects. J. Exp. Biol. 1943, 20, 28–34. [Google Scholar] [CrossRef]
- Stuart, S.N.; Chanson, J.S.; Cox, N.A.; Young, B.E.; Rodrigues, A.S.L.; Fischman, D.L.; Waller, R.W. Status and trends of amphibian declines and extinctions worldwide. Science 2004, 306, 1783–1786. [Google Scholar] [CrossRef]
- Persson, L. Optimal foraging: The difficulty of exploiting different feeding strategies simultaneously. Oecologia 1985, 67, 338–341. [Google Scholar] [CrossRef]
- Araújo, M.S.; Bolnick, D.L.; Layman, C.A. The ecological causes of individual specialisation. Ecol. Lett. 2011, 14, 948–958. [Google Scholar] [CrossRef]
- Bolnick, D.I.; Ingram, T.; Stutz, W.E.; Snowberg, L.K.; Lee Lau, O.; Paull, J.S. Ecological release from interspecific competition leads to decoupled changes in population and individual niche width. Proc. R. Soc. B 2010, 277, 1789–1797. [Google Scholar] [CrossRef]
- Costa-Pereira, R.; Araújo, M.S.; Souza, F.L.; Ingram, T. Competition and resource breadth shape niche variation and overlap in multiple trophic dimensions. Proc. R. Soc. B 2019, 286, 20190369. [Google Scholar] [CrossRef] [Green Version]
- Costa-Pereira, R.; Rudolf, V.H.W.; Souza, F.L.; Araújo, M.S. Drivers of individual niche variation in coexisting species. J. Anim. Ecol. 2018, 87, 1452–1464. [Google Scholar] [CrossRef] [PubMed]
- Vignoli, L.; Caldera, F.; Bologna, M.A. Trophic niche of cave populations of Speleomantes italicus. J. Nat. Hist. 2006, 40, 1841–1850. [Google Scholar] [CrossRef]
- Marques, A.J.D.; Mata, V.A.; Velo-Antón, G. COI metabarcoding provides insights into the highly diverse diet of a generalist salamander, Salamandra salamandra (Caudata: Salamandridae). Diversity 2022, 14, 89. [Google Scholar] [CrossRef]
- Costa, A.; Baroni, D.; Romano, A.; Salvidio, S. Individual diet variation in Salamandra salamandra larvae in a Mediterranean stream (Amphibia: Caudata). Salamandra 2017, 53, 148–152. [Google Scholar]
- Lanza, B.; Pastorelli, C.; Laghi, P.; Cimmaruta, R. A review of systematics, taxonomy, genetics, biogeography and natural history of the genus Speleomantes Dubois, 1984 (Amphibia Caudata Plethodontidae). Atti Mus Civ Stor Nat Trieste 2006, 52, 5–135. [Google Scholar]
- Ficetola, G.F.; Lunghi, E.; Manenti, R. Microhabitat analyses support relationships between niche breadth and range size when spatial autocorrelation is strong. Ecography 2020, 43, 724–734. [Google Scholar] [CrossRef]
- Spotila, J.R. Role of temperature and water in the ecology of lungless salamanders. Ecol. Monogr. 1972, 42, 95–125. [Google Scholar] [CrossRef]
- Ficetola, G.F.; Lunghi, E.; Canedoli, C.; Padoa-Schioppa, E.; Pennati, R.; Manenti, R. Differences between microhabitat and broad-scale patterns of niche evolution in terrestrial salamanders. Sci. Rep. 2018, 8, 10575. [Google Scholar] [CrossRef]
- Lunghi, E.; Corti, C.; Manenti, R.; Barzaghi, B.; Buschettu, S.; Canedoli, C.; Cogoni, R.; De Falco, G.; Fais, F.; Manca, A.; et al. Comparative reproductive biology of European cave salamanders (genus Hydromantes): Nesting selection and multiple annual breeding. Salamandra 2018, 54, 101–108. [Google Scholar]
- Lunghi, E.; Manenti, R.; Manca, S.; Mulargia, M.; Pennati, R.; Ficetola, G.F. Nesting of cave salamanders (Hydromantes flavus and H. italicus) in natural environments. Salamandra 2014, 50, 105–109. [Google Scholar]
- Bruni, G. Tail-straddling walk and spermatophore transfer in Hydromantes italicus: First observations for the genus and insights about courtship behavior in plethodontid salamanders. Herpetol. Rev. 2020, 51, 673–680. [Google Scholar]
- Oneto, F.; Ottonello, D.; Pastorino, M.V.; Salvidio, S. Posthatching parental care in salamanders revealed by infrared video surveillance. J. Herpetol. 2010, 44, 649–653. [Google Scholar] [CrossRef]
- Lunghi, E. Doubling the lifespan of European plethodontid salamanders. Ecology 2022, 103, e03581. [Google Scholar] [CrossRef] [PubMed]
- Rondinini, C.; Battistoni, A.; Peronace, V.; Teofili, C. Lista Rossa IUCN dei Vertebrati Italiani; Comitato Italiano IUCN e Ministero dell’Ambiente e della Tutela del Territorio e del Mare: Roma, Italy, 2013; p. 54.
- Lunghi, E.; Manenti, R.; Ficetola, G.F. Seasonal variation in microhabitat of salamanders: Environmental variation or shift of habitat selection? PeerJ 2015, 3, e1122. [Google Scholar] [CrossRef]
- Lunghi, E.; Cianferoni, F.; Ceccolini, F.; Mulargia, M.; Cogoni, R.; Barzaghi, B.; Cornago, L.; Avitabile, D.; Veith, M.; Manenti, R.; et al. Field-recorded data on the diet of six species of European Hydromantes cave salamanders. Sci. Data 2018, 5, 180083. [Google Scholar] [CrossRef] [Green Version]
- Salvidio, S.; Pasmans, F.; Bogaerts, S.; Martel, A.; van de Loo, M.; Romano, A. Consistency in trophic strategies between populations of the Sardinian endemic salamander Speleomantes imperialis. Anim. Biol. 2017, 67, 1–16. [Google Scholar] [CrossRef]
- Salvidio, S. Diet and food utilization in the European plethodontid Speleomantes ambrosii. Vie et Milieu 1992, 42, 35–39. [Google Scholar]
- Salvidio, S.; Romano, A.; Oneto, F.; Ottonello, D.; Michelon, R. Different season, different strategies: Feeding ecology of two syntopic forest-dwelling salamanders. Acta Oecol. 2012, 43, 42–50. [Google Scholar]
- Lunghi, E.; Ficetola, G.F.; Zhao, Y.; Manenti, R. Are the neglected Tipuloidea crane flies (Diptera) an important component for subterranean environments? Diversity 2020, 12, 333. [Google Scholar] [CrossRef]
- Manenti, R.; Lunghi, E.; Ficetola, G.F. Distribution of spiders in cave twilight zone depends on microclimatic features and trophic supply. Invertebr. Biol. 2015, 134, 242–251. [Google Scholar] [CrossRef]
- Speybroeck, J.; Beukema, W.; Bok, B.; Van Der Voort, J.; Velikov, I. Field Guide to the Amphibians & Reptiles of Britain and Europe; Bloomsbury: London, UK, 2016. [Google Scholar]
- Martel, A.; Spitzen-van der Sluijs, A.; Blooi, M.; Bert, W.; Ducatelle, R.; Fisher, M.C.; Woeltjes, A.; Bosman, W.; Chiers, K.; Bossuyt, F.; et al. Batrachochytrium salamandrivorans sp. nov. causes lethal chytridiomycosis in amphibians. Proc. Natl. Acad. Sci. USA 2012, 110, 15325–15329. [Google Scholar] [CrossRef]
- Caldonazzi, M.; Nistri, A.; Tripepi, S. Salamandra salamandra. In Fauna d’Italia. Vol. XLII. Amphibia; Lanza, B., Andreone, F., Bologna, M.A., Corti, C., Razzetti, E., Eds.; Edizioni Calderini de Il Sole 24 ORE Editoria Specializzata S.r.l.: Bologna, Italy, 2007; pp. 221–227. [Google Scholar]
- Lunghi, E.; Guillaume, O.; Blaimont, P.; Manenti, R. The first ecological study on the oldest allochthonous population of European cave salamanders (Hydromantes sp.). Amphib.-Reptil. 2018, 39, 113–119. [Google Scholar] [CrossRef]
- Manenti, R.; Lunghi, E.; Ficetola, G.F. Cave exploitation by an usual epigean species: A review on the current knowledge on fire salamander breeding in cave. Biogeographia 2017, 32, 31–46. [Google Scholar] [CrossRef]
- Bressi, N.; Dolce, S.; Stoch, F. Observations on the feeding of amphibians: IV. Larval diet of the fire salamander, Salamandra salamandra salamandra (Linnaeus, 1758), near Trieste (North-eastern Italy) (Amphibia, Caudata, Salamandridae). Atti Mus Civ Stor Nat Trieste 1996, 47, 275–283. [Google Scholar]
- Melotto, A.; Ficetola, G.F.; Manenti, R. Safe as a cave? Intraspecific aggressiveness rises in predator-devoid and resource-depleted environments. Behav. Ecol. Sociobiol. 2019, 73, 68. [Google Scholar] [CrossRef]
- Balogová, M.; Miková, E.; Orendáš, P.; Uhrin, M. Trophic spectrum of adult Salamandra salamandra in the Carpathians with the first note on food intake by the species during winter. Herpeto. Notes 2015, 8, 371–377. [Google Scholar]
- Maier, A.-R.-M.; Ferenți, S.; Dumbravă, A.-R.; Cadar, A.-M. A late autumn feeding: Diet of Salamandra salamandra (Linnaeus, 1758) (Amphibia: Salamandridae) in October and November in the Iron Gates natural park, Romania. Acta Zoologica Bulgarica 2022, 74, 195–201. [Google Scholar]
- Cloyed, C.S.; Eason, P.K. Niche partitioning and the role of intraspecific niche variation in structuring a guild of generalist anurans. R. Soc. Open Sci. 2017, 4, 170060. [Google Scholar] [CrossRef]
- Novak, T.; Tkavc, T.; Kuntner, M.; Arnett, A.E.; Lipovšek Delakorda, S.; Perc, M.; Janžekovič, F. Niche partitioning in orbweaving spiders Meta menardi and Metellina merianae (Tetragnathidae). Acta Oecol. 2010, 36, 522–529. [Google Scholar] [CrossRef]
- Lunghi, E.; Corti, C.; Manenti, R.; Ficetola, G.F. Consider species specialism when publishing datasets. Nat. Ecol. Evol. 2019, 3, 319. [Google Scholar] [CrossRef]
- Lunghi, E.; Corti, C.; Biaggini, M.; Merilli, S.; Manenti, R.; Zhao, Y.; Ficetola, G.F.; Cianferoni, F. Capture-mark-recapture data on the strictly protected Speleomantes italicus. Ecology 2022, 103, e3641. [Google Scholar] [CrossRef]
- Lunghi, E.; Bacci, F.; Zhao, Y. How can we record reliable information on animal colouration in the wild? Diversity 2021, 13, 356. [Google Scholar] [CrossRef]
- Lunghi, E.; Giachello, S.; Manenti, R.; Zhao, Y.; Corti, C.; Ficetola, G.F.; Bradley, J.G. The post hoc measurement as a safe and reliable method to age and size plethodontid salamanders. Ecol. Evol. 2020, 10, 11111–11116. [Google Scholar] [CrossRef]
- Lunghi, E.; Biaggini, M.; Corti, C. Reliability of the post-hoc measurement on Salamandra salamandra. Il Nat. Sicil. 2021; in press. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Lanza, B.; Nistri, A.; Vanni, S. Classe Amphibia Gray, 1825 (Morfologia e Biologia). In Fauna d’Italia. Vol. XLII. Amphibia; Lanza, B., Andreone, F., Bologna, M.A., Corti, C., Razzetti, E., Eds.; Edizioni Calderini de Il Sole 24 ORE Editoria Specializzata S.r.l.: Bologna, Italy, 2007; p. 2. [Google Scholar]
- Lunghi, E.; Romeo, D.; Mulargia, M.; Cogoni, R.; Manenti, R.; Corti, C.; Ficetola, G.F.; Veith, M. On the stability of the dorsal pattern of European cave salamanders (genus Hydromantes). Herpetozoa 2019, 32, 249–253. [Google Scholar] [CrossRef]
- Speybroeck, J.; Steenhoudt, K. A pattern-based tool for long-term, large-sample capture-markrecapture studies of fire salamanders Salamandra species (Amphibia: Urodela: Salamandridae). Acta Herpetol. 2017, 12, 55–63. [Google Scholar]
- Lunghi, E.; Bruni, G. Long-term reliability of Visual Implant Elastomers in the Italian cave salamander (Hydromantes italicus). Salamandra 2018, 54, 283–286. [Google Scholar]
- Ferenti, S.; David, A.; Nagy, D. Feeding-behaviour responses to anthropogenic factors on Salamandra salamandra (Amphibia, Caudata). Biharean Biol. 2010, 4, 139–143. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria.
- Kohl, M. MKmisc: Miscellaneous Functions from M. Kohl. R Package Version 0.993. 2016. Available online: http://www.stamats.de (accessed on 13 August 2022).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-7. Available online: https://github.com/vegandevs/vegan (accessed on 13 August 2022).
- Anderson, M.J.; Walsh, D.C.I. PERMANOVA, ANOSIM, and the Mantel test in the face of heterogeneous dispersions: What null hypothesis are you testing? Ecol. Monogr. 2013, 83, 557–574. [Google Scholar] [CrossRef]
- Anderson, M.J. Distance-based tests for homogeneity of multivariate dispersions. Biometrics 2006, 62, 245–253. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, B.; Christensen, H.B. lmerTest: Tests in Linear Mixed Effects Models, R package version 2.0-2.9; 2016. Available online: www.r-project.org (accessed on 13 August 2022).
- Douglas, B.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Bolnick, D.I.; Svanbäck, R.; Fordyce, J.A.; Yang, L.H.; Davis, J.M.; Hulsey, C.D.; Forister, M.L. The ecology of individuals: Incidence and implications of individual specialization. Am. Nat. 2003, 161, 1–28. [Google Scholar] [CrossRef]
- Bolnick, D.I.; Yang, L.H.; Fordyce, J.A.; Davis, J.M.; Svanbäck, R. Measuring individual-level resource specialization. Ecology 2002, 83, 2936–2941. [Google Scholar] [CrossRef]
- Zaccarelli, N.; Bolnick, D.I.; Mancinelli, G. RInSp: An R package for the analysis of individual specialization in resource use. Methods Ecol. Evol. 2013, 4, 1018–1023. [Google Scholar] [CrossRef]
- Lunghi, E.; Manenti, R.; Cianferoni, F.; Ceccolini, F.; Veith, M.; Corti, C.; Ficetola, G.F.; Mancinelli, G. Interspecific and inter-population variation in individual diet specialization: Do environmental factors have a role? Ecology 2020, 101, e03088. [Google Scholar] [CrossRef]
- Bolnick, D.I.; Svanbäck, R.; Araújo, M.S.; Persson, L. Comparative support for the niche variation hypothesis that more generalized populations also are more heterogeneous. Proc. Natl. Acad. Sci. USA 2007, 104, 10075–10079. [Google Scholar] [CrossRef]
- Lunghi, E.; Giachello, S.; Zhao, Y.; Corti, C.; Ficetola, G.F.; Manenti, R. Photographic database of the European cave salamanders, genus Hydromantes. Sci. Data 2020, 7, 171. [Google Scholar] [CrossRef]
- Lunghi, E.; Cianferoni, F.; Giachello, S.; Zhao, Y.; Manenti, R.; Corti, C.; Ficetola, G.F. Updating salamander datasets with phenotypic and stomach content information for two mainland Speleomantes. Sci. Data 2021, 8, 150. [Google Scholar] [CrossRef]
- Lunghi, E.; Cianferoni, F.; Merilli, S.; Zhao, Y.; Manenti, R.; Ficetola, G.F.; Corti, C. Ecological observations on hybrid populations of European plethodontid salamanders, genus Speleomantes. Diversity 2021, 13, 285. [Google Scholar] [CrossRef]
- Deban, S.M.; Marks, S.B. Metamorphosis and evolution of feeding behaviour in salamanders of the family Plethodontidae. Zool. J. Linn. Soc. 2002, 134, 375–400. [Google Scholar] [CrossRef]
- O’Donnell, M.K.; Deban, S.M. Cling performance and surface area of attachment in plethodontid salamanders. J. Exp. Biol. 2020, 233, jeb211706. [Google Scholar] [CrossRef]
- Roth, G. Experimental analysis of the prey catching behavior of Hydromantes italicus Dunn (Amphibia, Plethodontidae). J. Comp. Physiol. A 1976, 109, 47–58. [Google Scholar] [CrossRef]
- Roth, G. Responses in the optic tectum of the salamander Hydromantes italicus to moving prey stimuli. Exp. Brain Res. 1982, 45, 386–392. [Google Scholar] [CrossRef]
- Lunghi, E.; Manenti, R.; Mulargia, M.; Veith, M.; Corti, C.; Ficetola, G.F. Environmental suitability models predict population density, performance and body condition for microendemic salamanders. Sci. Rep. 2018, 8, 7527. [Google Scholar] [CrossRef]
- Culver, D.C.; Pipan, T. The Biology of Caves and Other Subterranean Habitats, 2nd ed.; Oxford University Press: New York, NY, USA, 2019; p. 336. [Google Scholar]
- Lunghi, E.; Valle, B.; Guerrieri, A.; Bonin, A.; Cianferoni, F.; Manenti, R.; Ficetola, G.F. Complex patterns of environmental DNA transfers from surface to subterranean soils revealed by analyses of cave insects and springtails. Sci. Total Environ. 2022, 826, 154022. [Google Scholar] [CrossRef]
- Luthardt, G.; Roth, G. The relationship between stimulus orientation and stimulus movement pattern in the prey catching behavior of Salamandra salamandra. Copeia 1979, 1979, 442–447. [Google Scholar] [CrossRef]
- Bas Lopez, S.; Gutian Rivera, J.; Castro Lorenzo, A.D.; Sanchez Canals, J.L. Datos sobre l’alimentacion de la salamandra (Salamandra salamandra L.) in Galicia. Bol. De La Estac. Cent. De Ecol. 1979, 8, 73–78. [Google Scholar]
- Wang, Y.; Smith, H.K.; Goossens, E.; Hertzog, L.; Bletz, M.C.; Bonte, D.; Verheyen, K.; Lens, L.; Vences, M.; Pasmans, F.; et al. Diet diversity and environment determine the intestinal microbiome and bacterial pathogen load of fire salamanders. Sci. Rep. 2021, 11, 20493. [Google Scholar] [CrossRef]
- Feder, M.E. Integrating the ecology and physiology of plethodontid salamanders. Herpetologica 1983, 39, 291–310. [Google Scholar]
- Feder, M.E. Oxygen consumption and activity in salamanders: Effect of body size and lunglessness. J. Exp. Zool. 1977, 202, 403–414. [Google Scholar] [CrossRef]
- Lunghi, E.; Cianferoni, F.; Ceccolini, F.; Zhao, Y.; Manenti, R.; Corti, C.; Ficetola, G.F.; Mancinelli, G. Same diet, different strategies: Variability of individual feeding habits across three populations of Ambrosi’s cave salamander (Hydromantes ambrosii). Diversity 2020, 12, 180. [Google Scholar] [CrossRef]
- Salvidio, S.; Pastorino, M.V. Spatial segregation in the European plethodontid Speleomantes strinatii in relation to age and sex. Amphib.-Reptil. 2002, 23, 505–510. [Google Scholar]
- Ficetola, G.F.; Pennati, R.; Manenti, R. Spatial segregation among age classes in cave salamanders: Habitat selection or social interactions? Popul. Ecol. 2013, 55, 217–226. [Google Scholar] [CrossRef]
| Prey Code | Prey Order | Number Recognized in Speleomantes italicus and Relative Importance (%) | Number Recognized in Salamandra salamandra and Relative Importance (%) |
|---|---|---|---|
| A | Pulmonata | 54 (1.86) | 59 (35.12) |
| B | Sarcoptiformes | 78 (2.69) | 0 |
| C | Mesostigmata | 15 (0.52) | 0 |
| S | Trombidiformes | 7 (0.24) | 0 |
| E | Araneae | 359 (12.38) | 9 (5.36) |
| F | Pseudoscorpiones | 125 (4.31) | 0 |
| G | Opiliones | 30 (1.03) | 8 (4.76) |
| H | Lithobiomorpha | 22 (0.76) | 2 (1.19) |
| I | Geophilomorpha | 14 (0.48) | 0 |
| J | Scolopendromorpha | 5 (0.17) | 0 |
| K | Julida | 16 (0.55) | 7 (4.17) |
| L | Polydesmida | 92 (3.17) | 13 (7.74) |
| M | Isopoda | 81 (2.79) | 2 (1.19) |
| N | Symphypleona | 11 (0.38) | 0 |
| O | Poduromorpha | 35 (1.21) | 0 |
| P | Entomobryomorpha | 288 (9.93) | 0 |
| Q | Blattodea | 4 (0.14) | 0 |
| R | Hemiptera | 186 (6.41) | 0 |
| S | Hymenoptera | 22 (0.76) | 0 |
| T | Hymenoptera-Formicidae | 121 (4.17) | 1 (0.6) |
| U | Coleoptera | 275 (9.48) | 2 (1.19) |
| V | Coleoptera-Staphylinidae | 82 (2.83) | 0 |
| W | Coleoptera-larvae | 35 (1.21) | 4 (2.38) |
| X | Trichoptera-larvae | 3 (0.10) | 0 |
| Y | Plecoptera | 179 (6.17) | 3 (1.79) |
| Z | Lepidoptera | 1 (0.03) | 1 (0.6) |
| AA | Lepidoptera-larvae | 24 (0.83) | 0 |
| AB | Diptera | 582 (20.07) | 10 (5.95) |
| AC | Diptera-larvae | 109 (3.76) | 23 (13.69) |
| AD | Archaeognatha | 10 (0.34) | 0 |
| AE | Speleomantes-skin | 5 (0.17) | 0 |
| AF | Haplotaxida | 22 (0.76) | 24 (14.29) |
| AG | Siphonaptera | 3 (0.10) | 0 |
| AH | Dermaptera | 4 (0.14) | 0 |
| AI | Ixodida | 1 (0.03) | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lunghi, E.; Corti, C.; Biaggini, M.; Zhao, Y.; Cianferoni, F. The Trophic Niche of Two Sympatric Species of Salamanders (Plethodontidae and Salamandridae) from Italy. Animals 2022, 12, 2221. https://doi.org/10.3390/ani12172221
Lunghi E, Corti C, Biaggini M, Zhao Y, Cianferoni F. The Trophic Niche of Two Sympatric Species of Salamanders (Plethodontidae and Salamandridae) from Italy. Animals. 2022; 12(17):2221. https://doi.org/10.3390/ani12172221
Chicago/Turabian StyleLunghi, Enrico, Claudia Corti, Marta Biaggini, Yahui Zhao, and Fabio Cianferoni. 2022. "The Trophic Niche of Two Sympatric Species of Salamanders (Plethodontidae and Salamandridae) from Italy" Animals 12, no. 17: 2221. https://doi.org/10.3390/ani12172221






