Adaptive Party Choice of Low-Ranking Males in Fission–Fusion Dynamics of Chimpanzees in Kalinzu Forest Reserve, Uganda
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Subjects
2.2. Data Collection
2.2.1. Definition of the Presence of Females with Maximum Swelling
2.2.2. Fruit Abundance
2.3. Statistical Analyses
2.3.1. Male Attendance at Parties
2.3.2. Frequency of Receiving Aggression
3. Results
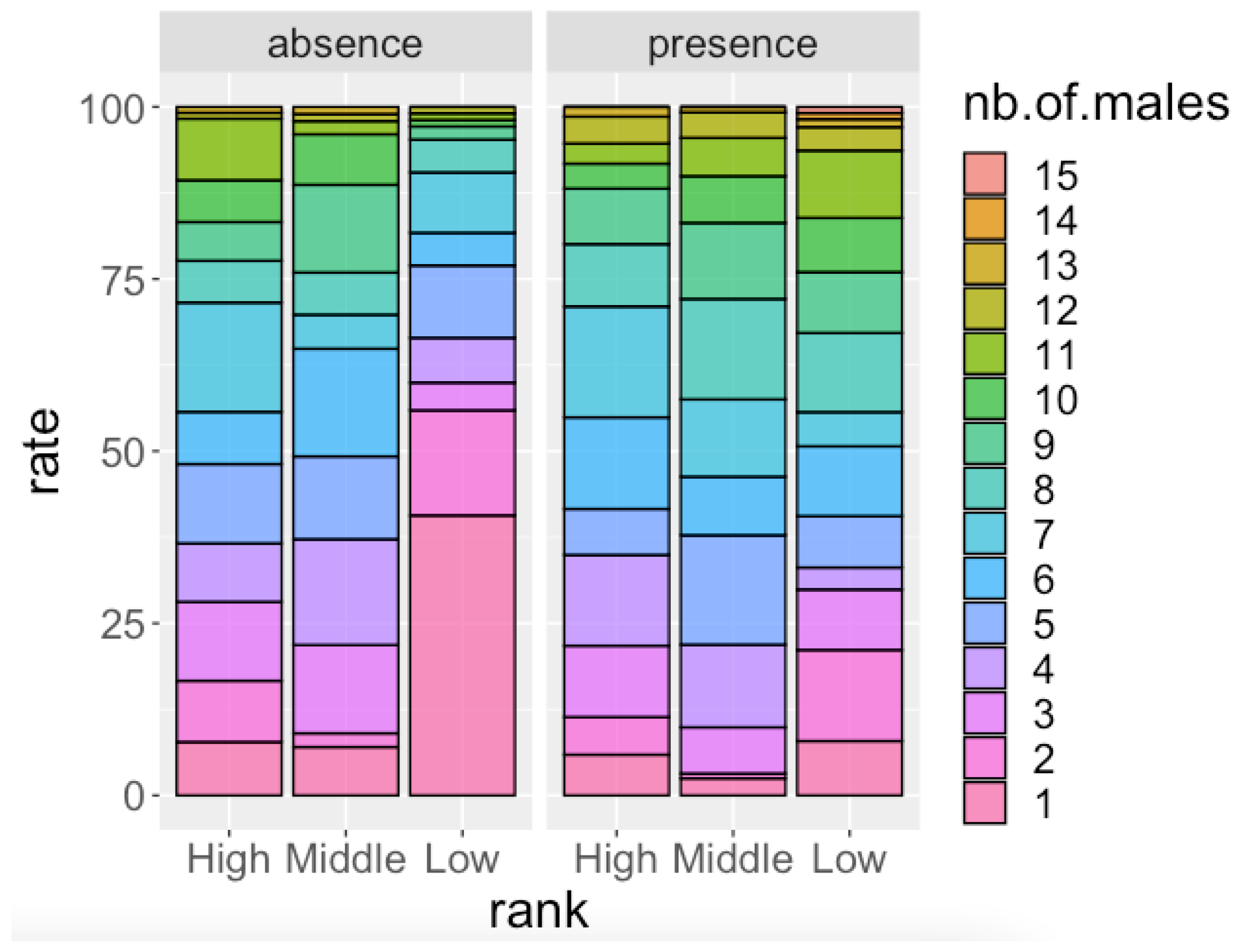
3.1. Male Attendance at Parties
3.2. Frequency of Receiving Aggression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parra, G.J.; Corkeron, P.J.; Arnold, P. Grouping and Fission-Fusion Dynamics in Australian Snubfin and Indo-Pacific Humpback Dolphins. Anim. Behav. 2011, 82, 1423–1433. [Google Scholar] [CrossRef]
- Carter, K.D.; Seddon, J.M.; Frère, C.H.; Carter, J.K.; Goldizen, A.W. Fission-Fusion Dynamics in Wild Giraffes May Be Driven by Kinship, Spatial Overlap and Individual Social Preferences. Anim. Behav. 2013, 85, 385–394. [Google Scholar] [CrossRef]
- Wittemyer, G.; Douglas-Hamilton, I.; Getz, W.M. The Socioecology of Elephants: Analysis of the Processes Creating Multitiered Social Structures. Anim. Behav. 2005, 69, 1357–1371. [Google Scholar] [CrossRef]
- Smith, J.E.; Kolowski, J.M.; Graham, K.E.; Dawes, S.E.; Holekamp, K.E. Social and Ecological Determinants of Fission-Fusion Dynamics in the Spotted Hyaena. Anim. Behav. 2008, 76, 619–636. [Google Scholar] [CrossRef]
- Aguilar-Melo, A.R.; Calmé, S.; Smith-Aguilar, S.E.; Ramos-Fernandez, G. Fission-Fusion Dynamics as a Temporally and Spatially Flexible Behavioral Strategy in Spider Monkeys. Behav. Ecol. Sociobiol. 2018, 72, 150. [Google Scholar] [CrossRef]
- Lehmann, J.; Korstjens, A.H.; Dunbar, R.I.M. Fission-Fusion Social Systems as a Strategy for Coping with Ecological Constraints: A Primate Case. Evol. Ecol. 2007, 21, 613–634. [Google Scholar] [CrossRef]
- Aureli, F.; Schaffner, C.M.; Boesch, C.; Bearder, S.K.; Call, J.; Chapman, C.A.; Connor, R.; Di Fiore, A.; Dunbar, R.I.M.; Peter Henzi, S.; et al. Fission-Fusion Dynamics New Research Frameworks. Curr. Anthropol. 2008, 49, 627–654. [Google Scholar] [CrossRef]
- Van Schaik, C.P.; Van Hooff, J. On the Ultimate Causes of Primate Social Systems. Behaviour 1983, 85, 91–117. [Google Scholar] [CrossRef]
- Holekamp, K.E.; Smith, J.E.; Strelioff, C.C.; Van Horn, R.C.; Watts, H.E. Society, Demography and Genetic Structure in the Spotted Hyena. Mol. Ecol. 2012, 21, 613–632. [Google Scholar] [CrossRef]
- Kummer, H. Primate Societies: Group Techniques of Ecological Adaptation; Wiley; Aldine-Atherton: Chicago, IL, USA, 1971. [Google Scholar]
- Nishida, T. The Social Group of Wild Chimpanzees in the Mahali Mountains. Primates 1968, 9, 167–224. [Google Scholar] [CrossRef] [Green Version]
- Boesch, C.; Boesch-Achermann, H. The Chimpanzees of the Tai Forest; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Matsumoto-Oda, A.; Hosaka, K.; Huffman, M.A.; Kawanaka, K. Factors Affecting Party Size in Chimpanzees of the Mahale Mountains. Int. J. Primatol. 1998, 19, 999–1011. [Google Scholar] [CrossRef]
- Lehmann, J.; Boesch, C. To Fission or to Fusion: Effect of Community Size on Wild Chimpanzee (Pan Troglodytes versus) Social Organisation. Behav. Ecol. Sociobiol. 2004, 56, 207–216. [Google Scholar] [CrossRef]
- Boesch, C. The Effect of Leopard Predation on Grouping Patterns in Forest Chimpanzees. Behaviour 1991, 117, 220–241. [Google Scholar] [CrossRef]
- Sakura, O. Factors Affecting Party Size and Composition of Chimpanzees (Pan Troglodytes Verus) Bossou, Guinea. Int. J. Primatol. 1994, 15, 167–183. [Google Scholar] [CrossRef]
- Hashimoto, C.; Furuichi, T.; Tashiro, Y. What Factors Affect the Size of Chimpanzee Parties in the Kalinzu Forest, Uganda? Examination of Fruit Abundance and Number of Estrous Females. Int. J. Primatol. 2001, 22, 947–959. [Google Scholar] [CrossRef]
- Doran, D.M. Influence of Seasonality on Activity Patterns, Feeding Behavior, Ranging, and Grouping Patterns in Tai Chimpanzees. Int. J. Primatol. 1997, 18, 183–206. [Google Scholar] [CrossRef]
- Basabose, A.K. Fruit Availability and Chimpanzee Party Size at Kahuzi Montane Forest, Democratic Republic of Congo. Primates 2004, 45, 211–219. [Google Scholar] [CrossRef]
- Newton-Fisher, N.E.; Reynolds, V.; Plumptre, A.J. Food Supply and Chimpanzee (Pan troglodytes schweinfurthii) Party Size in the Budongo Forest Reserve, Uganda. Int. J. Primatol. 2000, 21, 613–628. [Google Scholar] [CrossRef]
- Hashimoto, C.; Suzuki, S.; Takenoshita, Y.; Yamagiwa, J.; Basabose, A.K.; Furuichi, T. How Fruit Abundance Affects the Chimpanzee Party Size: A Comparison between Four Study Sites. Primates 2003, 44, 77–81. [Google Scholar] [CrossRef]
- Williams, J.M.; Lonsdorf, E.V.; Wilson, M.L.; Schumacher-Stankey, J.; Goodall, J.; Pusey, A.E. Causes of Death in the Kasekela Chimpanzees of Gombe National Park, Tanzania. Am. J. Primatol. 2008, 70, 766–777. [Google Scholar] [CrossRef]
- Watts, D.P. Intracommunity Coalitionary Killing of an Adult Male Chimpanzee at Ngogo, Kibale National Park, Uganda. Int. J. Primatol. 2004, 25, 507–521. [Google Scholar] [CrossRef]
- Otali, E.; Gilchrist, J.S. Why Chimpanzee (Pan troglodytes schweinfurthii) Mothers Are Less Gregarious than Nonmothers and Males: The Infant Safety Hypothesis. Behav. Ecol. Sociobiol. 2006, 59, 561–570. [Google Scholar] [CrossRef]
- Lowe, A.E.; Hobaiter, C.; Newton-Fisher, N.E. Countering Infanticide: Chimpanzee Mothers Are Sensitive to the Relative Risks Posed by Males on Differing Rank Trajectories. Am. J. Phys. Anthropol. 2018, 168, 3–9. [Google Scholar] [CrossRef]
- Nishie, H.; Nakamura, M. A Newborn Infant Chimpanzee Snatched and Cannibalized Immediately after Birth: Implications for “Maternity Leave” in Wild Chimpanzee. Am. J. Phys. Anthropol. 2018, 165, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, C. Population Census of the Chimpanzees in the Kalinzu Forest, Uganda: Comparison Between Methods with Nest Counts. Primates 1995, 36, 477–488. [Google Scholar] [CrossRef]
- Hashimoto, C.; Furuichi, T. Comparison of Behavioral Sequence of Copulation between Chimpanzees and Bonobos. Primates 2006, 47, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Goodall, J. The Chimpanzees of Gombe: Patterns of Behavior; Harvard University Press: Cambridge, UK, 1986. [Google Scholar]
- David, H.A. Ranking from Unbalanced Paired-Comparison Data. Biometrika 1987, 74, 432–436. [Google Scholar] [CrossRef]
- Altmann, J. Observational Study of Behavior: Sampling Methods. Behaviour 1974, 49, 227–266. [Google Scholar] [CrossRef]
- Tokuyama, N.; Furuichi, T. Leadership of Old Females in Collective Departures in Wild Bonobos (Pan Paniscus) at Wamba. Behav. Ecol. Sociobiol. 2017, 71, 55. [Google Scholar] [CrossRef]
- Mulavwa, M.; Furuichi, T.; Yangozene, K. Seasonal Changes in Fruit Production and Party Size of Bonobos at Wamba. In Developments in Primatology: Progress and Prospects; Springer: Boston, MA, USA, 2008. [Google Scholar] [CrossRef]
- Tutin, C.E.G.; McGinnis, P.R. Chimpanzee Reproduction in the Wild. In Reproductive Biology of the Great Apes: Comparative and Biomedical Perspectives; Graham, C.E., Ed.; Academic Press: New York, NY, USA, 1981. [Google Scholar]
- Hasegawa, T.; Hiraiwa-Hasegawa, M. Sperm Competition and Mating Behavior. In The Chimpanzees of the Mahale Mountains; Nishida, T., Ed.; University of Tokyo Press: Tokyo, Japan, 1990; pp. 115–132. [Google Scholar]
- Deschner, T.; Heistermann, M.; Hodges, K.; Boesch, C. Timing and Probability of Ovulation in Relation to Sex Skin Swelling in Wild West African Chimpanzees, Pan Troglodytes Verus. Anim. Behav. 2003, 66, 551–560. [Google Scholar] [CrossRef]
- Furuichi, T.; Hashimoto, C.; Tashiro, Y. Extended Application of a Marked-Nest Census Method to Examine Seasonal Changes in Habitat Use by Chimpanzees. Int. J. Primatol. 2001, 22, 913–928. [Google Scholar] [CrossRef]
- Hancock, T. Generalized Linear Mixed Models Using Template Model Builder. Available online: https://cran.r-project.org/web/packages/glmmTMB/glmmTMB.pdf (accessed on 15 August 2022).
- Barton, K. Multi-Model Inference (1.46.0). Available online: https://cran.r-project.org/web/packages/MuMIn/index.html (accessed on 1 July 2022).
- Itoh, N.; Nishida, T. Chimpanzee Grouping Patterns and Food Availability in Mahale Mountains National Park, Tanzania. Primates 2007, 48, 87–96. [Google Scholar] [CrossRef]
- Matthews, J.K.; Ridley, A.; Kaplin, B.A.; Grueter, C.C. Ecological and Reproductive Drivers of Fission-Fusion Dynamics in Chimpanzees (Pan troglodytes schweinfurthii) Inhabiting a Montane Forest. Behav. Ecol. Sociobiol. 2021, 75, 23. [Google Scholar] [CrossRef]
- Sugardjito, J.; te Boekhorst, I.J.A.; van Hooff, J.A.R.A.M. Ecological Constraints on the Grouping of Wild Orang-Utans (Pongo Pygmaeus) in the Gunung Leuser National Park, Sumatra, Indonesia. Int. J. Primatol. 1987, 8, 17–41. [Google Scholar] [CrossRef]
- Muller, M.N.; Wrangham, R.W. Dominance, Cortisol and Stress in Wild Chimpanzees (Pan troglodytes schweinfurthii). Behav. Ecol. Sociobiol. 2004, 55, 332–340. [Google Scholar] [CrossRef]
- Watts, D.P. Coalitionary Mate Guarding by Male Chimpanzees at Ngogo, Kibale National Park, Uganda. Behav. Ecol. Sociobiol. 1998, 44, 43–55. [Google Scholar] [CrossRef]
- Newton-Fisher, N.E. Association by Male Chimpanzees: A Social Tactic? Behaviour 1999, 136, 705–730. [Google Scholar] [CrossRef]
- Gilby, I.C.; Wrangham, R.W. Association Patterns among Wild Chimpanzees (Pan troglodytes schweinfurthii) Reflect Sex Differences in Cooperation. Behav. Ecol. Sociobiol. 2008, 62, 1831–1842. [Google Scholar] [CrossRef]
- Chapman, C.A.; Chapman, L.J.; Wrangham, R.W. Ecological Constraints on Group Size: An Analysis of Spider Monkey and Chimpanzee Subgroups. Behav. Ecol. Sociobiol. 1995, 36, 59–70. [Google Scholar] [CrossRef]
- Symington, M. Fission-Fusion Social Organization in Ateles and Pan. Int. J. Primatol. 1990, 11, 47–61. [Google Scholar] [CrossRef]
- Asensio, N.; Korstjens, A.H.; Schaffner, C.M.; Aureli, F. Intragroup Aggression, Fission-Fusion Dynamics and Feeding Competition in Spider Monkeys. Behaviour 2008, 145, 983–1001. [Google Scholar] [CrossRef]
- Valero, A.; Schaffner, C.M.; Vick, L.G.; Aureli, F.; Ramos-Fernandez, G. Intragroup Lethal Aggression in Wild Spider Monkeys. Am. J. Primatol. 2006, 68, 732–737. [Google Scholar] [CrossRef]

| Name (Abbreviation) | Dominance Rank | Birth Year | Age Class | OHU |
|---|---|---|---|---|
| Goku (GK) | Alpha | 1993 * | Prime | 90 |
| Ponta (PO) | High | 1995 * | Prime | 53 |
| Ichiro (IC) | High | 1980s * | Old | 76 |
| Buru (BR) | High | 1970s * | Old | 65 |
| Prince (PR) | Middle | 1997 * | Prime | 59 |
| Taiki (TK) | Middle | 1999 | Young | 77 |
| Deo (DO) | Middle | 1970s * | Old | 62 |
| Pietan (PT) | Low | 2001 | Young | 65 |
| Black (BL) | Low | 1998 * | Young | 72 |
| Jo (JO) | Low | 2000 * | Young | 58 |
| Variable Statistics | ||||||
|---|---|---|---|---|---|---|
| Predictor Variables | Estimate | SE | z Value | p Value | Random Effects | Variance |
| Intercept | −0.61791 | 0.27047 | −2.285 | 0.022 * | Subjects | 0.096 |
| Rank: Low vs. High | −1.63861 | 0.46026 | −3.560 | <0.001 *** | ||
| Rank: Low vs. Middle | −2.13921 | 0.60170 | −3.555 | <0.001 *** | ||
| MS: absence vs. presence | −1.84479 | 0.44766 | −4.121 | <0.001 *** | ||
| FAI | 0.14836 | 0.08311 | 1.785 | 0.074. | ||
| Variable Statistics | ||||||
|---|---|---|---|---|---|---|
| Predictor Variables | Estimate | SE | z Value | p Value | Random Effects | Variance |
| Intercept | 1.8417 | 0.0587 | 31.394 | <0.001 *** | Subjects | 0.006 |
| Rank: Low vs. High | 0.1348 | 0.0660 | 2.043 | 0.041 * | ||
| Rank: Low vs. Middle | 0.1444 | 0.0693 | 2.082 | 0.037 * | ||
| FAI | −0.0488 | 0.0080 | −6.091 | <0.001 *** | ||
| MS: absence vs. presence | 0.2723 | 0.0673 | 4.048 | <0.001 *** | ||
| Low vs. High: MS | −0.1928 | 0.0845 | −2.282 | 0.022 * | ||
| Variable Statistics | ||||||
|---|---|---|---|---|---|---|
| Predictor Variables | Estimate | SE | z Value | p Value | Random Effects | Variance |
| Intercept | 1.7930 | 0.0688 | 26.068 | <0.001 *** | Subjects | 0.002 |
| Rank: Low vs. High | 0.1367 | 0.0660 | 2.071 | 0.038 * | ||
| Rank: Low vs. Middle | 0.1493 | 0.0694 | 2.150 | 0.031 * | ||
| FAI | −0.0349 | 0.0129 | −2.717 | 0.007 ** | ||
| Variable Statistics | ||||||
|---|---|---|---|---|---|---|
| Predictor Variables | Estimate | SE | z Value | p Value | Random Effects | Variance |
| Intercept | 2.1468 | 0.0636 | 33.730 | <0.001 *** | Subjects | 0.004 |
| Rank: Low vs. High | −0.0671 | 0.0675 | −0.993 | 0.321 | ||
| Rank: Low vs. Middle | 0.0267 | 0.0719 | 0.371 | 0.711 | ||
| FAI | −0.0578 | 0.0104 | −5.553 | <0.001 *** | ||
| Variable Statistics | ||||||
|---|---|---|---|---|---|---|
| Predictor Variables | Estimate | SE | z Value | p Value | Random Effects | Variance |
| Intercept | −4.5668 | 1.0009 | −4.563 | <0.001 *** | Subjects | 0.005 |
| Number of males | 0.20789 | 0.0860 | 2.420 | 0.016 * | ||
| Rank: Low vs. High | −1.60612 | 0.7848 | −2.047 | 0.041 * | ||
| Rank: Low vs. Middle | −0.52884 | 0.5923 | −0.893 | 0.372 | ||
| MS: absence vs. presence | 0.68867 | 0.5837 | 1.180 | 0.238 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shibata, S.; Furuichi, T.; Hashimoto, C. Adaptive Party Choice of Low-Ranking Males in Fission–Fusion Dynamics of Chimpanzees in Kalinzu Forest Reserve, Uganda. Animals 2022, 12, 2240. https://doi.org/10.3390/ani12172240
Shibata S, Furuichi T, Hashimoto C. Adaptive Party Choice of Low-Ranking Males in Fission–Fusion Dynamics of Chimpanzees in Kalinzu Forest Reserve, Uganda. Animals. 2022; 12(17):2240. https://doi.org/10.3390/ani12172240
Chicago/Turabian StyleShibata, Shohei, Takeshi Furuichi, and Chie Hashimoto. 2022. "Adaptive Party Choice of Low-Ranking Males in Fission–Fusion Dynamics of Chimpanzees in Kalinzu Forest Reserve, Uganda" Animals 12, no. 17: 2240. https://doi.org/10.3390/ani12172240
APA StyleShibata, S., Furuichi, T., & Hashimoto, C. (2022). Adaptive Party Choice of Low-Ranking Males in Fission–Fusion Dynamics of Chimpanzees in Kalinzu Forest Reserve, Uganda. Animals, 12(17), 2240. https://doi.org/10.3390/ani12172240





