Dietary Epidermal Growth Factor Supplementation Alleviates Intestinal Injury in Piglets with Intrauterine Growth Retardation via Reducing Oxidative Stress and Enhancing Intestinal Glucose Transport and Barrier Function
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Sample Collection
2.3. Serum Biochemical Profile Detection
2.4. Intestinal Histomorphology
2.5. Jejunal Mucosal Antioxidant and Immune Indices Detection
2.6. AKP and Na+/K+-ATPase Activity in Jejunum Mucosa
2.7. Real-Time PCR
2.8. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Serum Biochemical Profiles
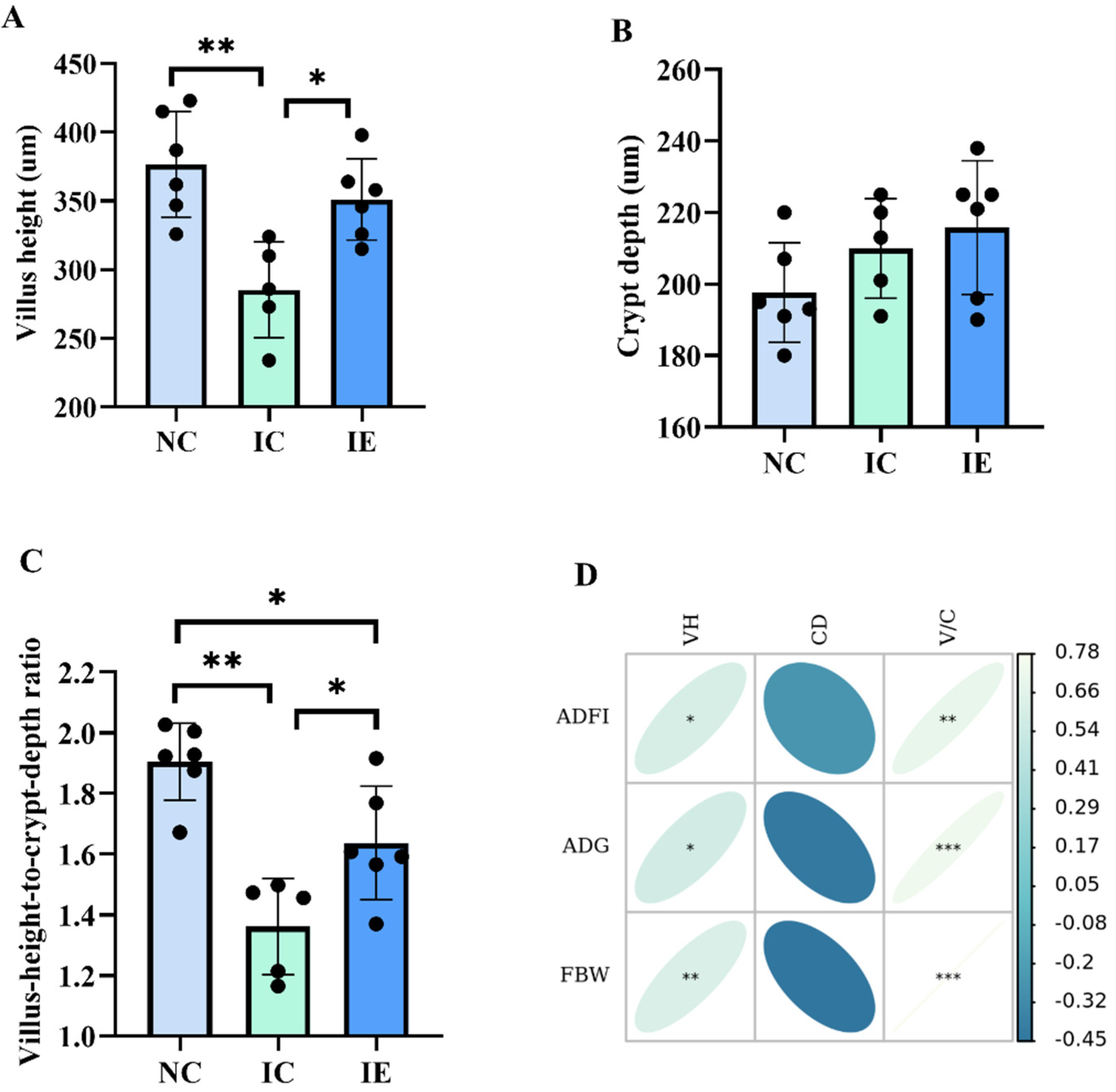
3.3. Intestinal Morphology and Its Correlations Analysis with Growth Performance
3.4. Glucose Absorption Capacity and Its Correlation Analysis with Growth Performance and Intestinal Morphology
3.5. Antioxidant Activities and Its Correlation Analysis with Growth Performance and Intestinal Morphology
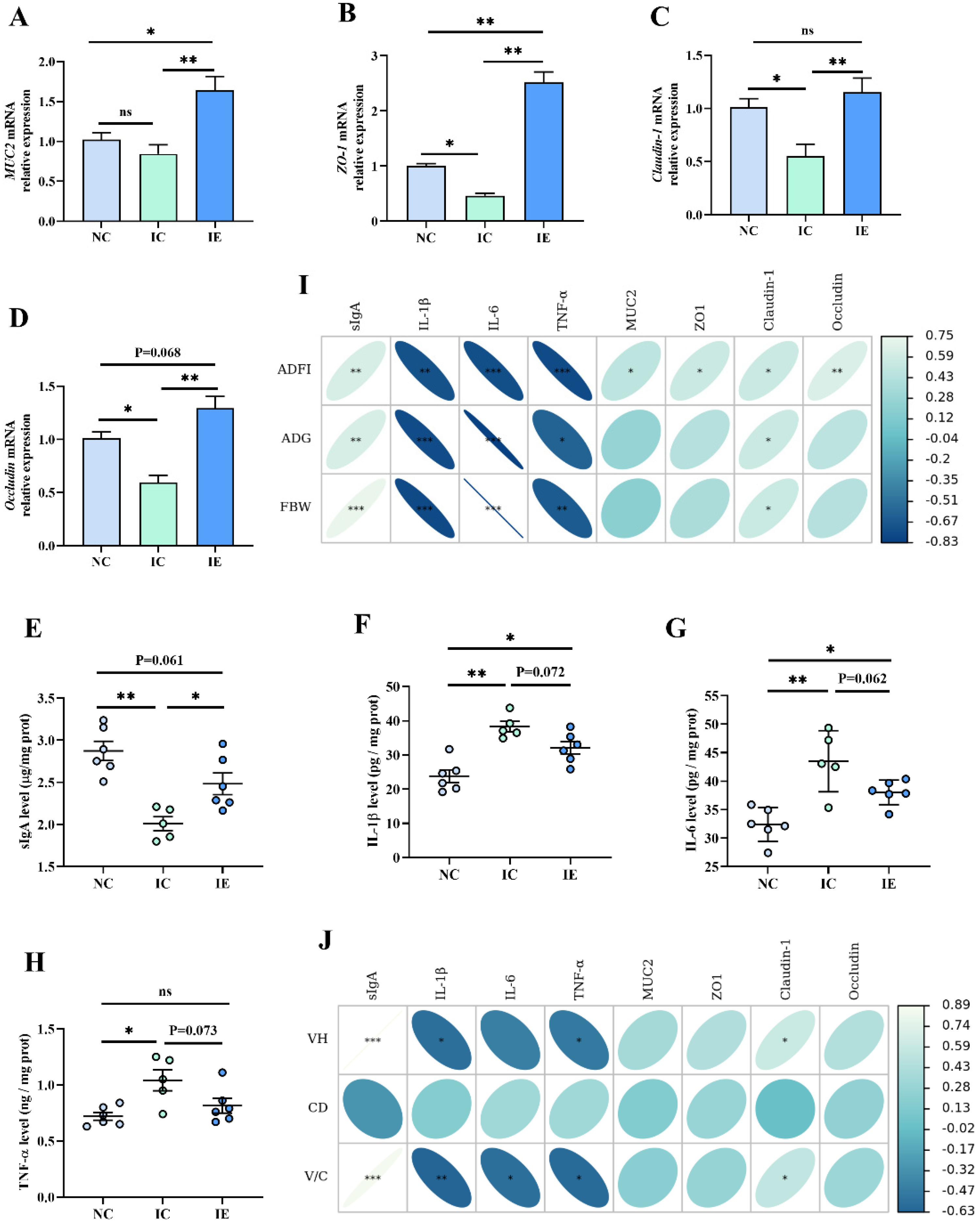
3.6. Intestinal Barrier Function and Its Correlation Analysis with Growth Performance and Intestinal Morphology
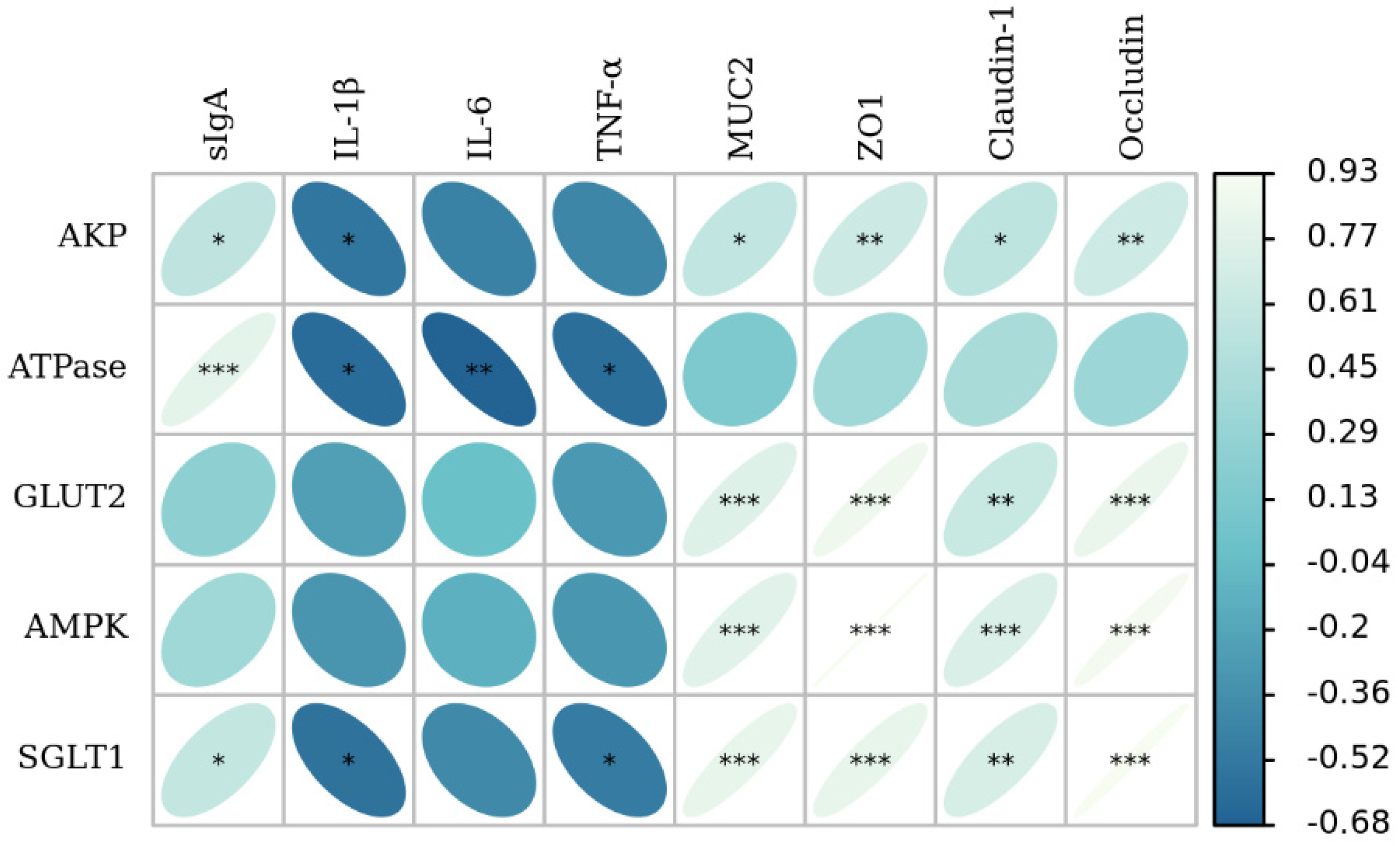
3.7. The Correlation Analysis of Intestinal Barrier Function with Glucose Absorption Capacity
4. Discussion
4.1. Effects of EGF on the Growth Performance of IUGR Piglets
4.2. Effects of EGF on the Serum Biochemical Indices of Piglets with IUGR
4.3. Effects of EGF on the Intestinal Morphology of IUGR Piglets
4.4. Effects of EGF on the Glucose Absorption Capacity of Piglets with IUGR
4.5. Effects of EGF on the Antioxidant Activities of Piglets with IUGR
4.6. Effects of EGF on the Intestinal Barrier Function of Piglets with IUGR
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, G.; Bazer, F.W.; Wallace, J.M.; Spencer, T.E. Board-invited review: Intrauterine growth retardation: Implications for the animal sciences. J. Anim. Sci. 2006, 84, 2316–2337. [Google Scholar] [CrossRef]
- Wang, J.; Chen, L.; Li, D.; Yin, Y.; Wang, X.; Li, P.; Dangott, L.J.; Hu, W.; Wu, G. Intrauterine growth restriction affects the proteomes of the small intestine, liver, and skeletal muscle in newborn pigs. J. Nutr. 2008, 138, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; You, J.; Zhang, W.; Zhu, Q.; Blachier, F.; Yin, Y.; Kong, X. Intrauterine growth restriction alters growth performance, plasma hormones, and small intestinal microbial communities in growing-finishing pigs. J. Anim. Sci. Biotechnol. 2020, 11, 86. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Xiong, K. Intrauterine Growth Retardation Affects Intestinal Health of Suckling Piglets via Altering Intestinal Antioxidant Capacity, Glucose Uptake, Tight Junction, and Immune Responses. Oxid. Med. Cell. Longev. 2022, 2022, 2644205. [Google Scholar] [CrossRef] [PubMed]
- Fung, C.M.; White, J.R.; Brown, A.S.; Gong, H.; Weitkamp, J.H.; Frey, M.R.; McElroy, S.J. Intrauterine growth restriction alters mouse intestinal architecture during development. PLoS ONE 2016, 11, e0146542. [Google Scholar] [CrossRef] [PubMed]
- Matheson, S.M.; Walling, G.A.; Edwards, S.A. Genetic selection against intrauterine growth retardation in piglets: A problem at the piglet level with a solution at the sow level. Genet. Sel. Evol. 2018, 50, 46. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, T.; Huang, S.; Wang, W.; Dai, Z.; Feng, C.; Wu, G.; Wang, J. Maternal L-glutamine supplementation during late gestation alleviates intrauterine growth restriction-induced intestinal dysfunction in piglets. Amino Acids 2018, 50, 1289–1299. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Y.; Li, Y.; Wang, T. Protective Effect of Polydatin on Jejunal Mucosal Integrity, Redox Status, Inflammatory Response, and Mitochondrial Function in Intrauterine Growth-Retarded Weanling Piglets. Oxid. Med. Cell. Longev. 2020, 2020, 7178123. [Google Scholar] [CrossRef]
- Che, L.; Zhou, Q.; Liu, Y.; Hu, L.; Peng, X.; Wu, C.; Zhang, R.; Tang, J.; Wu, F.; Fang, Z.; et al. Flaxseed oil supplementation improves intestinal function and immunity, associated with altered intestinal microbiome and fatty acid profile in pigs with intrauterine growth retardation. Food Funct. 2019, 10, 8149–8160. [Google Scholar] [CrossRef]
- Tang, X.; Liu, H.; Yang, S.; Li, Z.; Zhong, J.; Fang, R. Epidermal Growth Factor and Intestinal Barrier Function. Mediators Inflamm. 2016, 2016, 1927348. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Liu, X.; Zhong, J.; Fang, R. Potential Application of Lonicera japonica Extracts in Animal Production: From the Perspective of Intestinal Health. Front. Microbiol. 2021, 12, 719877. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, J.; Zabielski, R.; Skrzypek, T.; Matyba, P.; Wierzbicka, M.; Adamski, A.; Grzesiuk, E.; Sady, M.; Gajewski, Z.; Ferenc, K. Differences in Intestinal Barrier Development between Intrauterine Growth Restricted and Normal Birth Weight Piglets. Animals 2021, 11, 990. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, H.; Chen, Y.; Jia, P.; Ji, S.; Zhang, Y.; Wang, T. Resveratrol and its derivative pterostilbene ameliorate intestine injury in intrauterine growth-retarded weanling piglets by modulating redox status and gut microbiota. J. Anim. Sci. Biotechnol. 2021, 12, 70. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Xiong, K.; Wassie, T.; Wu, X. Curcumin and Intestinal Oxidative Stress of Pigs with Intrauterine Growth Retardation: A Review. Front. Nutr. 2022, 9, 847673. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, F.; Yang, H.; Li, J.; Li, Y.; Ding, X.; Xiong, X.; Ji, F.; Zhou, H.; Yin, Y. Epidermal growth factor improves intestinal morphology by stimulating proliferation and differentiation of enterocytes and mTOR signaling pathway in weaning piglets. Sci. China Life Sci. 2020, 63, 259–268. [Google Scholar] [CrossRef]
- Tang, X.; Liu, B.; Wang, X.; Yu, Q.; Fang, R. Epidermal growth factor, through alleviating oxidative stress, protect IPEC-J2 cells from lipopolysaccharides-induced apoptosis. Int. J. Mol. Sci. 2018, 19, 848. [Google Scholar] [CrossRef]
- Xue, J.; Xie, L.; Liu, B.; Zhou, L.; Hu, Y.; Ajuwon, K.M.; Fang, R. Dietary Supplementation of EGF Ameliorates the Negatively Effects of LPS on Early-Weaning Piglets: From Views of Growth Performance, Nutrient Digestibility, Microelement Absorption and Possible Mechanisms. Animals 2021, 11, 1598. [Google Scholar] [CrossRef]
- Tang, X.; Xiong, K. Effects of epidermal growth factor on glutamine and glucose absorption by IPEC-J2 cells challenged by lipopolysaccharide using the Ussing chamber system. Pak. J. Zool. 2021, 53, 417–422. [Google Scholar] [CrossRef]
- Guntaka, S.R.; Samak, G.; Seth, A.; LaRusso, N.F.; Rao, R. Epidermal growth factor protects the apical junctional complexes from hydrogen peroxide in bile duct epithelium. Lab. Investig. 2011, 91, 1396–1409. [Google Scholar] [CrossRef]
- Chen, Y.L.; Peng, H.C.; Hsieh, Y.C.; Yang, S.C. Epidermal growth factor improved alcohol-induced inflammation in rats. Alcohol 2014, 48, 701–706. [Google Scholar] [CrossRef]
- Suzuki, T.; Seth, A.; Rao, R. Role of phospholipase Cγ-induced activation of protein kinase Cϵ (PKCϵ) and PKCβ1 in epidermal growth factor-mediated protection of tight junctions from acetaldehyde in Caco-2 cell monolayers. J. Biol. Chem. 2008, 283, 3574–3583. [Google Scholar] [CrossRef] [PubMed]
- Arda-Pirincci, P.; Bolkent, S. The role of epidermal growth factor in prevention of oxidative injury and apoptosis induced by intestinal ischemia/reperfusion in rats. Acta Histoch. 2014, 116, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Xiong, K. Epidermal growth factor activates EGFR/AMPK signalling to up-regulate the expression of SGLT1 and GLUT2 to promote intestinal glucose absorption in lipopolysaccharide challenged IPEC-J2 cells and piglets. Ital. J. Anim. Sci. 2022, 21, 943–954. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Swine, 11th ed.; National Academy Press: Washington, DC, USA, 2012. [Google Scholar]
- Tang, X.; Liu, X.; Zhang, K. Effects of Microbial Fermented Feed on Serum Biochemical Profile, Carcass Traits, Meat Amino Acid and Fatty Acid Profile, and Gut Microbiome Composition of Finishing Pigs. Front. Vet. Sci. 2021, 8, 744630. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Deng, D.; Chen, S.; Li, C.; Luo, J.; Romeo, A.; Li, T.; Tang, X.; Fang, R. The effects of dietary porous zinc oxide supplementation on growth performance, inflammatory cytokines and tight junction’s gene expression in early-weaned piglets. J. Nutr. Sci. Vitaminol. 2020, 66, 311–318. [Google Scholar] [CrossRef]
- Tang, X.; Su, W.; Fang, R. Effects of calcitonin on porcine intestinal epithelial cells proliferation, phosphorus absorption, and NaPi-IIb expression. Pak. J. Zool. 2019, 51, 2167–2174. [Google Scholar] [CrossRef]
- Wang, L.X.; Zhu, F.; Li, J.Z.; Li, Y.L.; Ding, X.Q.; Yin, J.; Xiong, X.; Yang, H.S. Epidermal growth factor promotes intestinal secretory cell differentiation in weaning piglets via Wnt/β-catenin signalling. Animal 2020, 14, 790–798. [Google Scholar] [CrossRef]
- Wang, X.; Tan, B.; Liao, P.; Cui, Z.; Zhang, S.; Li, X.; Yin, Y.; Xiao, D. Functional bioactive substance improves the growth performance, antioxidant capacity and immune function of growth retardation pigs. Food Agric. Immunol. 2020, 31, 329–340. [Google Scholar] [CrossRef]
- Bai, K.; Jiang, L.; Li, Q.; Zhang, J.; Zhang, L.; Wang, T. Dietary dimethylglycine sodium salt supplementation improves growth performance, redox status, and skeletal muscle function of intrauterine growth-restricted weaned piglets. J. Anim. Sci. 2021, 99, skab186. [Google Scholar] [CrossRef]
- Bedford, A.; Huynh, E.; Fu, M.; Zhu, C.; Wey, D.; de Lange, C.; Li, J. Growth performance of early-weaned pigs is enhanced by feeding epidermal growth factor-expressing Lactococcus lactis fermentation product. J. Biotechnol. 2014, 173, 47–52. [Google Scholar] [CrossRef]
- Carobene, A.; Braga, F.; Roraas, T.; Sandberg, S.; Bartlett, W.A. A systematic review of data on biological variation for alanine aminotransferase, aspartate aminotransferase and γ-glutamyl transferase. Clin. Chem. Lab. Med. 2013, 51, 1997–2007. [Google Scholar] [CrossRef]
- Morrill, J.L.; Morrill, J.M.; Feyerherm, A.M.; Laster, J.F. Plasma proteins and a probiotic as ingredients in milk replacer. J. Dairy Sci. 1995, 78, 902–907. [Google Scholar] [CrossRef]
- Wang, S.; Guo, C.; Zhou, L.; Zhong, Z.; Zhu, W.; Huang, Y.; Zhang, Z.; Gorgels, T.G.M.F.; Berendschot, T.T.J.M. Effects of dietary supplementation with epidermal growth factor-expressing saccharomyces cerevisiae on duodenal development in weaned piglets. Brit. J. Nutr. 2015, 115, 1509–1520. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, H.W.; Cavacini, L. Structure and function of immunoglobulins. J. Allergy Clin. Immunol. Pract. 2010, 125, S41–S52. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Xiong, X.; Liu, H.; Zhou, J.; Liu, Y.; Yin, Y. Effects of dietary lysozyme levels on growth performance, intestinal morphology, immunity response and microbiota community of growing pigs. J. Sci. Food Agric. 2019, 99, 1643e50. [Google Scholar] [CrossRef]
- Chen, C.; Yin, Y.; Tu, Q.; Yang, H. Glucose and amino acid in enterocyte: Absorption, metabolism and maturation. Front. Biosci. (Landmark Ed) 2018, 23, 1721–1739. [Google Scholar]
- Chen, L.; Tuo, B.; Dong, H. Regulation of Intestinal Glucose Absorption by Ion Channels and Transporters. Nutrients 2016, 8, 43. [Google Scholar] [CrossRef]
- Schmitt, C.C.; Aranias, T.; Viel, T.; Chateau, D.; Le Gall, M.; Waligora-Dupriet, A.J.; Melchior, C.; Rouxel, O.; Kapel, N.; Gourcerol, G.; et al. Intestinal invalidation of the glucose transporter GLUT2 delays tissue distribution of glucose and reveals an unexpected role in gut homeostasis. Mol. Metab. 2016, 6, 61–72. [Google Scholar] [CrossRef]
- Koepsell, H. Glucose transporters in the small intestine in health and disease. Pflugers Arch. 2020, 472, 1207–1248. [Google Scholar] [CrossRef]
- Pirinccioglu, A.G.; Gökalp, D.; Pirinccioglu, M.; Kizil, G.; Kizil, M. Malondialdehyde (MDA) and protein carbonyl (PCO) levels as biomarkers of oxidative stress in subjects with familial hypercholesterolemia. Clin. Biochem. 2010, 43, 1220–1224. [Google Scholar] [CrossRef]
- Yan, E.; Zhang, J.; Han, H.; Wu, J.; Gan, Z.; Wei, C.; Zhang, L.; Wang, C.; Wang, T. Curcumin Alleviates IUGR Jejunum Damage by Increasing Antioxidant Capacity through Nrf2/Keap1 Pathway in Growing Pigs. Animals 2019, 10, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, S.; Wang, D.; Zhang, P.; Lin, Y.; Fang, Z.; Che, L.; Wu, D. Oral administration of Lactococcus lactis-expressed recombinant porcine epidermal growth factor (rpEGF) stimulates the development and promotes the health of small intestines in early-weaned piglets. J. Appl. Microbiol. 2015, 119, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.A.; Doelle, S.M.; Halpern, M.D.; Saunders, T.A.; Holubec, H.; Dvorak, K.; Boitano, S.A.; Dvorak, B. Intestinal barrier failure during experimental necrotizing enterocolitis: Protective effect of EGF treatment. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G938–G949. [Google Scholar] [CrossRef]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef] [PubMed]
- Pelaseyed, T.; Bergström, J.H.; Gustafsson, J.K.; Ermund, A.; Birchenough, G.M.; Schütte, A.; van der Post, S.; Svensson, F.; Rodríguez-Piñeiro, A.M.; Nyström, E.E.; et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol. Rev. 2014, 260, 8–20. [Google Scholar] [CrossRef]
- Bedford, A.; Chen, T.; Huynh, E.; Zhu, C.; Medeiros, S.; Wey, D.; de Lange, C.; Li, J. Epidermal growth factor containing culture supernatant enhances intestine development of early-weaned pigs in vivo: Potential mechanisms involved. J. Biotechnol. 2015, 196–197, 9–19. [Google Scholar] [CrossRef]
- Schoultz, I.; Keita, Å.V. The Intestinal Barrier and Current Techniques for the Assessment of Gut Permeability. Cells 2020, 9, 1909. [Google Scholar] [CrossRef]


| Ingredients 1 | Content (%) | Item | Nutrient Levels 3 |
|---|---|---|---|
| Corn | 57.30 | DE/(MJ/Kg) | 14.08 |
| Squeezed soybean meal | 14.20 | Crude protein (%) | 20.14 |
| Expanded soybean | 13.53 | Total Lys (%) | 1.50 |
| Whey powder | 5.03 | Total Thr (%) | 0.75 |
| Fish meal | 4.60 | Total Met (%) | 0.82 |
| Glucose | 2.00 | Met + Cys (%) | 0.73 |
| Limestone | 0.84 | Calcium (%) | 0.86 |
| CaHPO4 | 0.81 | Available Pi (%) | 0.45 |
| L-lysine-HCl (98.0%) | 0.34 | ||
| L-methionine (98.0%) | 0.11 | ||
| NaCl | 0.24 | ||
| Premix 2 | 1.00 | ||
| Total | 100.00 |
| Gene | Primers Sequence | Product Length | Reference |
|---|---|---|---|
| AMPK-α1 | F:5′-GGTGAAAATCGGCCACTACA-3′ R:5′-TTGCCAACCTTCACTTTGCC-3′ | 72 bp | [23] |
| SGLT1 | F:5′-ATATGCCCTTATATTCCCCTT-3′ R:5′-AAATCGTGTTGATAGCGCCAA-3′ | 138 bp | [23] |
| GLUT2 | F:5′-CAGCCTATTCTAGTAGCACTG-3′ R:5′-AAATCGTGTTGATAGCGCCAA-3′ | 151 bp | [23] |
| MUC2 | F: 5′-ACGCCATCCTGGGTGAGCT-3′ R: 5′-ACGCTGCCGTCCGACTTGA-3′ | 121 bp | [28] |
| ZO-1 | F:5′-TGCTGGCACTGACCAACGTA-3′ R:5′-CACTGGGCATAATTCAGACGA-3′ | 129 bp | [4] |
| Claudin-1 | F: 5′-TCCTGCTGGGACTAATAGCCAT-3′ R: 5′-CAATGACAGCCATCCGCATC-3′ | 102 bp | [4] |
| Occludin | F: 5′-CATTGCCATTGTACTAGGGTT-3′ R: 5′-GCTGCTCGTCATAAATACGTT-3′ | 140 bp | [4] |
| β-actin | F: 5′-CATCCTGCGTCTGGACCTGG-3′ R:5′-TAATGTCACGCACGATTTCC-3′ | 116 bp | [16] |
| Items | NC | IC | IE | SEM | p Value |
|---|---|---|---|---|---|
| IBW (kg) | 6.77 a | 5.02 b | 5.04 b | 0.20 | <0.001 |
| FBW (kg) | 9.74 a | 6.95 c | 7.63 b | 0.30 | <0.001 |
| ADG (g/d) | 212.14 a | 137.14 c | 184.88 b | 8.25 | <0.001 |
| ADFI (g/d) | 301.71 a | 216.13 c | 268.47 b | 10.30 | <0.001 |
| F/G (g/g) | 1.42 | 1.60 | 1.46 | 0.05 | 0.297 |
| Items | NC | IC | IE | SEM | p-Value |
|---|---|---|---|---|---|
| ALT(U/L) | 50.28 | 64.16 | 62.30 | 3.48 | 0.214 |
| AST (U/L) | 110.88 c | 163.31 a | 137.78 ab | 7.86 | 0.015 |
| TG (mmol/L) | 0.57 | 0.43 | 0.48 | 0.03 | 0.168 |
| TP (g/L) | 44.19 a | 33.10 b | 41.31 a | 1.83 | 0.032 |
| BUN (mmol/L) | 3.87 | 3.21 | 4.28 | 0.27 | 0.294 |
| GLU (mmol/L) | 4.29 a | 2.30 b | 3.91 a | 0.29 | 0.005 |
| IgA (g/L) | 1.23 | 0.96 | 1.14 | 0.08 | 0.368 |
| IgG (g/L) | 2.83 a | 1.76 b | 2.54 a | 0.16 | 0.011 |
| IgM (g/L) | 0.49 a | 0.34 b | 0.40 ab | 0.02 | 0.025 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.; Xiong, K. Dietary Epidermal Growth Factor Supplementation Alleviates Intestinal Injury in Piglets with Intrauterine Growth Retardation via Reducing Oxidative Stress and Enhancing Intestinal Glucose Transport and Barrier Function. Animals 2022, 12, 2245. https://doi.org/10.3390/ani12172245
Tang X, Xiong K. Dietary Epidermal Growth Factor Supplementation Alleviates Intestinal Injury in Piglets with Intrauterine Growth Retardation via Reducing Oxidative Stress and Enhancing Intestinal Glucose Transport and Barrier Function. Animals. 2022; 12(17):2245. https://doi.org/10.3390/ani12172245
Chicago/Turabian StyleTang, Xiaopeng, and Kangning Xiong. 2022. "Dietary Epidermal Growth Factor Supplementation Alleviates Intestinal Injury in Piglets with Intrauterine Growth Retardation via Reducing Oxidative Stress and Enhancing Intestinal Glucose Transport and Barrier Function" Animals 12, no. 17: 2245. https://doi.org/10.3390/ani12172245
APA StyleTang, X., & Xiong, K. (2022). Dietary Epidermal Growth Factor Supplementation Alleviates Intestinal Injury in Piglets with Intrauterine Growth Retardation via Reducing Oxidative Stress and Enhancing Intestinal Glucose Transport and Barrier Function. Animals, 12(17), 2245. https://doi.org/10.3390/ani12172245





