Transcriptome Analysis of the Adipose Tissue of Luchuan and Duroc Pigs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diet
2.2. Hematoxylin and Eosin (HE) Staining
2.3. Transcriptome Sequencing
2.4. Bioinformatic Analysis
2.5. Real-Time qPCR
2.6. Statistics
3. Results
3.1. Phenotype and Overview of Transcriptomics Data between Duroc and Luchuan Pig Adipose Tissues
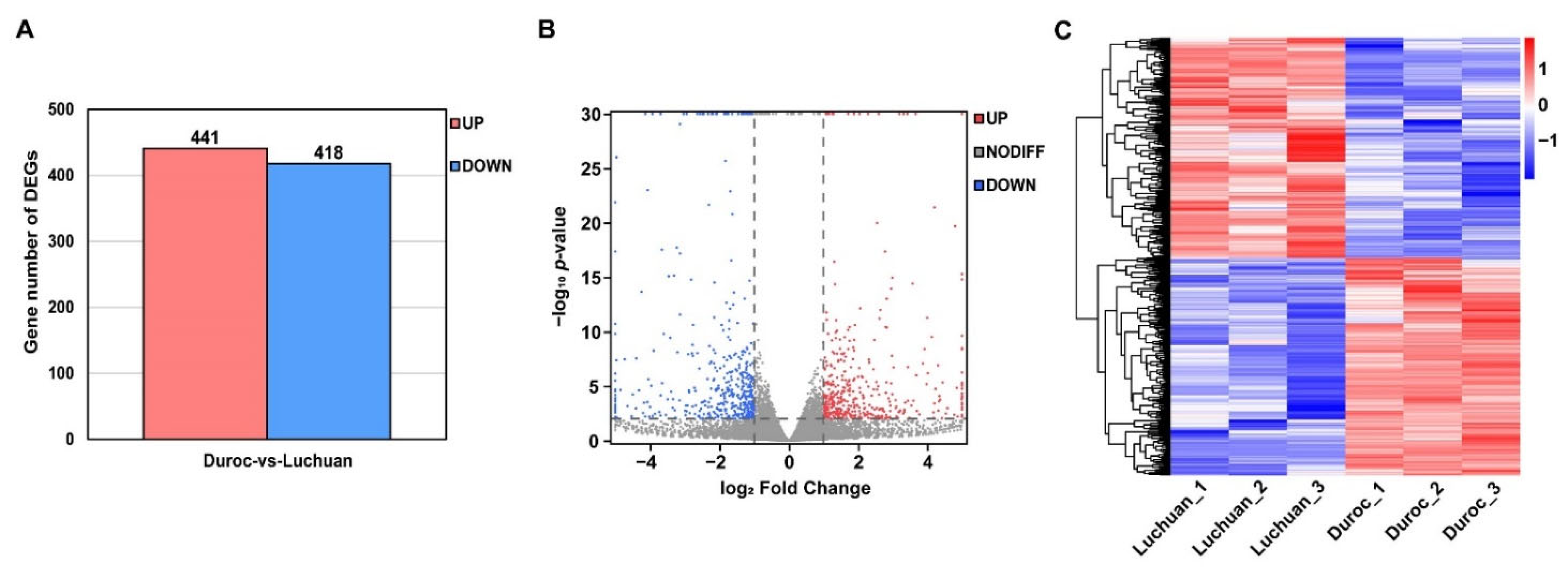
3.2. Differentially Expressed Genes (DEGs) Identification
3.3. GO and KEGG Functional Enrichment Analysis of Duroc and Luchuan Pig Adipose Tissues
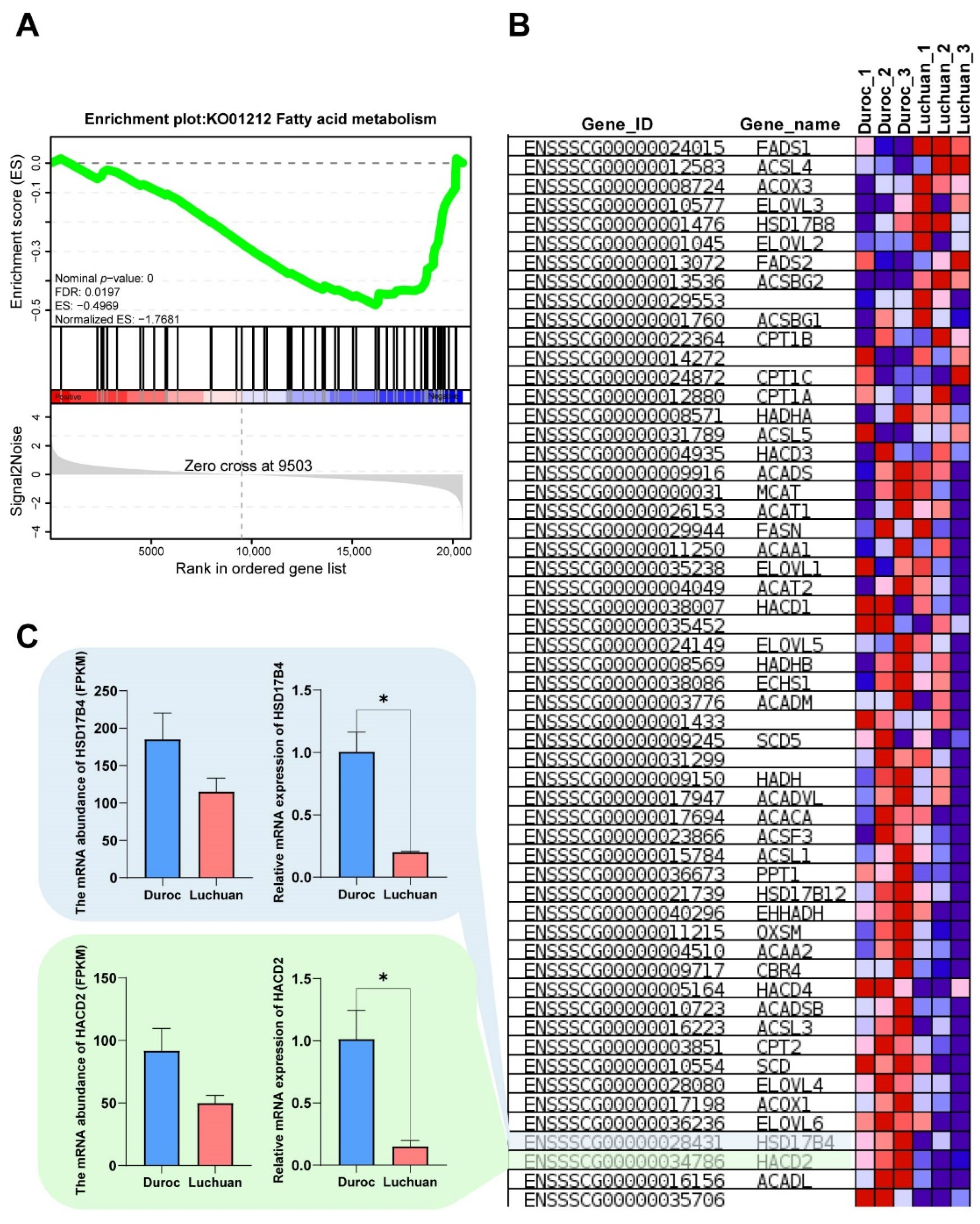
3.4. Gene Set Enrichment Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fernandez, X.; Monin, G.; Talmant, A.; Mourot, J.; Lebret, B. Influence of intramuscular fat content on the quality of pig meat—1. Composition of the lipid fraction and sensory characteristics of m. longissimus lumborum. Meat Sci. 1999, 53, 59–65. [Google Scholar] [CrossRef]
- Tummaruk, P.; Lundeheim, N.; Einarsson, S.; Dalin, A.M. Effect of birth litter size, birth parity number, growth rate, backfat thickness and age at first mating of gilts on their reproductive performance as sows. Anim. Reprod. Sci. 2001, 66, 225–237. [Google Scholar] [CrossRef]
- van Laack, R.L.; Stevens, S.G.; Stalder, K.J. The influence of ultimate pH and intramuscular fat content on pork tenderness and tenderization. J. Anim. Sci. 2001, 79, 392–397. [Google Scholar] [CrossRef]
- Shao, J.; Bai, X.; Pan, T.; Li, Y.; Jia, X.; Wang, J.; Lai, S. Genome-Wide DNA Methylation Changes of Perirenal Adipose Tissue in Rabbits Fed a High-Fat Diet. Animals 2020, 10, 2213. [Google Scholar] [CrossRef] [PubMed]
- Resurreccion, A.V. Sensory aspects of consumer choices for meat and meat products. Meat Sci. 2004, 66, 11–20. [Google Scholar] [CrossRef]
- Suzuki, K.; Irie, M.; Kadowaki, H.; Shibata, T.; Kumagai, M.; Nishida, A. Genetic parameter estimates of meat quality traits in Duroc pigs selected for average daily gain, longissimus muscle area, backfat thickness, and intramuscular fat content. J. Anim. Sci. 2005, 83, 2058–2065. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhao, P.; Zheng, X.; Zhou, L.; Wang, C.; Liu, J.F. Genome-Wide Detection of Selection Signatures in Duroc Revealed Candidate Genes Relating to Growth and Meat Quality. G3 Genes Genomes Genet. 2020, 10, 3765–3773. [Google Scholar] [CrossRef]
- Ran, M.L.; He, J.; Tan, J.Y.; Yang, A.Q.; Li, Z.; Chen, B. The complete sequence of the mitochondrial genome of Luchuan pig (Sus scrofa). Mitochondrial DNA Part A 2016, 27, 1880–1881. [Google Scholar] [CrossRef]
- Miao, W.; Ma, Z.; Tang, Z.; Yu, L.; Liu, S.; Huang, T.; Wang, P.; Wu, T.; Song, Z.; Zhang, H.; et al. Integrative ATAC-seq and RNA-seq Analysis of the Longissimus Muscle of Luchuan and Duroc Pigs. Front. Nutr. 2021, 8, 742672. [Google Scholar] [CrossRef]
- Tarazona, S.; García-Alcalde, F.; Dopazo, J.; Ferrer, A.; Conesa, A. Differential expression in RNA-seq: A matter of depth. Genome Res. 2011, 21, 2213–2223. [Google Scholar] [CrossRef] [Green Version]
- Shi, G.; Chen, L.; Chen, G.; Zou, C.; Li, J.; Li, M.; Fang, C.; Li, C. Identification and Functional Prediction of Long Intergenic Non-coding RNAs Related to Subcutaneous Adipose Development in Pigs. Front. Genet. 2019, 10, 160. [Google Scholar] [CrossRef]
- Davoli, R.; Gaffo, E.; Zappaterra, M.; Bortoluzzi, S.; Zambonelli, P. Identification of differentially expressed small RNAs and prediction of target genes in Italian Large White pigs with divergent backfat deposition. Anim. Genet. 2018, 49, 205–214. [Google Scholar] [CrossRef]
- Xing, K.; Zhao, X.; Liu, Y.; Zhang, F.; Tan, Z.; Qi, X.; Wang, X.; Ni, H.; Guo, Y.; Sheng, X.; et al. Identification of Differentially Expressed MicroRNAs and Their Potential Target Genes in Adipose Tissue from Pigs with Highly Divergent Backfat Thickness. Animals 2020, 10, 624. [Google Scholar] [CrossRef]
- Xing, K.; Liu, H.; Zhang, F.; Liu, Y.; Shi, Y.; Ding, X.; Wang, C. Identification of key genes affecting porcine fat deposition based on co-expression network analysis of weighted genes. J. Anim. Sci. Biotechnol. 2021, 12, 100. [Google Scholar] [CrossRef]
- Zhang, Z.; Liao, Q.; Sun, Y.; Pan, T.; Liu, S.; Miao, W.; Li, Y.; Zhou, L.; Xu, G. Lipidomic and Transcriptomic Analysis of the Longissimus Muscle of Luchuan and Duroc Pigs. Front. Nutr. 2021, 8, 667622. [Google Scholar] [CrossRef]
- Sirén, J.; Välimäki, N.; Mäkinen, V. Indexing Graphs for Path Queries with Applications in Genome Research. IEEE/ACM Trans. Comput. Biol. Bioinform. 2014, 11, 375–388. [Google Scholar] [CrossRef]
- Mortazavi, A.; Williams, B.A.; Mccue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods. 2008, 5, 621–628. [Google Scholar] [CrossRef]
- Wang, L.; Feng, Z.; Wang, X.; Wang, X.; Zhang, X. DEGseq: An R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 2010, 26, 136–138. [Google Scholar] [CrossRef]
- Huang, T.; Yu, J.; Ma, Z.; Fu, Q.; Liu, S.; Luo, Z.; Liu, K.; Yu, L.; Miao, W.; Yu, D.; et al. Translatomics Probes into the Role of Lycopene on Improving Hepatic Steatosis Induced by High-Fat Diet. Front. Nutr. 2021, 8, 727785. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Whittington, F.M.; Hughes, S.I. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef]
- Cameron, N.D.; Enser, M.; Nute, G.R.; Whittington, F.M.; Penman, J.C.; Fisken, A.C.; Perry, M.A.; Wood, J.D. Genotype with nutrition interaction on fatty acid composition of intramuscular fat and the relationship with flavour of pig meat. Meat Sci. 2000, 55, 187–195. [Google Scholar] [CrossRef]
- Tapiero, H.; Ba, G.N.; Couvreur, P.; Tew, K.D. Polyunsaturated fatty acids (PUFA) and eicosanoids in human health and pathologies. Biomed. Pharmacother. 2002, 56, 215–222. [Google Scholar] [CrossRef]
- Xi, B.; Luo, J.; Gao, Y.Q.; Yang, X.L.; Guo, T.F.; Li, W.H.; Du, T.Q. Transcriptome-metabolome analysis of fatty acid of Bamei pork and Gansu Black pork in China. Bioprocess Biosyst. Eng. 2021, 44, 995–1002. [Google Scholar] [CrossRef]
- Yu, K.; Shu, G.; Yuan, F.; Zhu, X.; Gao, P.; Wang, S.; Wang, L.; Xi, Q.; Zhang, S.; Zhang, Y.; et al. Fatty acid and transcriptome profiling of longissimus dorsi muscles between pig breeds differing in meat quality. Int. J. Biol. Sci. 2013, 9, 108–118. [Google Scholar] [CrossRef]
- Ge, L.; Gordon, J.S.; Hsuan, C.; Stenn, K.; Prouty, S.M. Identification of the delta-6 desaturase of human sebaceous glands: Expression and enzyme activity. J. Invest Dermatol. 2003, 120, 707–714. [Google Scholar] [CrossRef]
- Yamashita, S.; Hirashima, A.; Lin, I.; Bae, J.; Nakahara, K.; Murata, M.; Yamada, S.; Kumazoe, M.; Yoshitomi, R.; Kadomatsu, M.; et al. Saturated fatty acid attenuates anti-obesity effect of green tea. Sci Rep. 2018, 8, 10023. [Google Scholar] [CrossRef]
- Qin, Y.M.; Haapalainen, A.M.; Kilpeläinen, S.H.; Marttila, M.S.; Koski, M.K.; Glumoff, T.; Novikov, D.K.; Hiltunen, J.K. Human peroxisomal multifunctional enzyme type 2. Site-directed mutagenesis studies show the importance of two protic residues for 2-enoyl-CoA hydratase 2 activity. J. Biol. Chem. 2000, 275, 4965–4972. [Google Scholar] [CrossRef]
- Swinnen, J.V.; Ulrix, W.; Heyns, W.; Verhoeven, G. Coordinate regulation of lipogenic gene expression by androgens: Evidence for a cascade mechanism involving sterol regulatory element binding proteins. Proc. Natl. Acad. Sci. USA 1997, 94, 12975–12980. [Google Scholar] [CrossRef]
- Ikeda, M.; Kanao, Y.; Yamanaka, M.; Sakuraba, H.; Mizutani, Y.; Igarashi, Y.; Kihara, A. Characterization of four mammalian 3-hydroxyacyl-CoA dehydratases involved in very long-chain fatty acid synthesis. FEBS Lett. 2008, 582, 2435–2440. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Nutrition Level | Content (%) |
|---|---|
| Energy (kj/kg) | 11.98 |
| Crude protein (%) | 16 |
| Crude fiber (%) | 6 |
| Crude ash (%) | 7 |
| Calcium (%) | 0.6–1.2 |
| Total phosphorus (%) | 0.4–1.0 |
| NaCI (%) | 0.2–0.8 |
| Lysine (%) | 0.8 |
| Gene Name | Primer Sequence (5′ to 3′) |
|---|---|
| TBP_F | GAACTGGCGGAAGTGACGTT |
| TBP_R | GCACAGCAAGAAAGAGTGATGC |
| HSD17B4_F | AGGCAGTGGCCAACTATGATTC |
| HSD17B4_R | AGGAAGAGTTTTCCCCCGATG |
| HACD2_F | ACTGGAGCCTTGTTGGAGATTT |
| HACD2_R | ACGCTATGTGTTACTGCCCA |
| Sample | Raw Reads | Clean Reads | Clean Reads Rate (%) | Q30 | Mapped Reads | Mapping Rate |
|---|---|---|---|---|---|---|
| Duroc_1 | 47,456,140 | 45,889,484 | 96.7 | 95.91% | 117,943,789 | 94.28% |
| Duroc_2 | 49,962,578 | 47,937,230 | 95.95 | 95.91% | 118,682,360 | 94.68% |
| Duroc_3 | 46,846,584 | 45,082,868 | 96.23 | 95.98% | 118,441,470 | 93.54% |
| Luchuan_1 | 45,890,832 | 43,859,842 | 95.57 | 95.90% | 115,923,370 | 91.04% |
| Luchuan_2 | 48,171,536 | 46,262,262 | 96.04 | 95.88% | 114,285,253 | 89.87% |
| Luchuan_3 | 47,453,720 | 45,651,590 | 96.2 | 95.88% | 116,073,783 | 92.24% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, H.; Huang, T.; Yu, L.; Wang, P.; Su, S.; Wu, T.; Bai, Y.; Teng, Y.; Wei, Y.; Zhou, L.; et al. Transcriptome Analysis of the Adipose Tissue of Luchuan and Duroc Pigs. Animals 2022, 12, 2258. https://doi.org/10.3390/ani12172258
Pan H, Huang T, Yu L, Wang P, Su S, Wu T, Bai Y, Teng Y, Wei Y, Zhou L, et al. Transcriptome Analysis of the Adipose Tissue of Luchuan and Duroc Pigs. Animals. 2022; 12(17):2258. https://doi.org/10.3390/ani12172258
Chicago/Turabian StylePan, Hongyuan, Tengda Huang, Lin Yu, Peng Wang, Songtao Su, Tian Wu, Yin Bai, Yonghao Teng, Yutian Wei, Lei Zhou, and et al. 2022. "Transcriptome Analysis of the Adipose Tissue of Luchuan and Duroc Pigs" Animals 12, no. 17: 2258. https://doi.org/10.3390/ani12172258





