Effect of the Inclusion of Bacillus spp. in Growing–Finishing Pigs’ Diets: A Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search for Studies
2.2. Inclusion and Exclusion Criteria
2.3. Data Mining
2.4. Statistical Analysis
2.4.1. Effect size and Forest Plots
2.4.2. Heterogeneity
2.4.3. Meta-Regression
2.4.4. Publication Bias
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fuller, R. Probiotics in man and animals. J. Appl. Bacteriol. 1989, 66, 365–378. [Google Scholar] [PubMed]
- Cromwell, G.L. Why and how antibiotics are used in swine production. Anim. Biotechnol. 2006, 13, 7–27. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. Animal Production and Health Division (NSA). 2019. Available online: http://www.fao.org/ag/againfo/themes/en/pigs/home.html (accessed on 1 May 2022).
- Al-Dobaib, S.N.; Mousa, H.M. Benefits and risks of growth promoters in animal production. J. Food Agric. Environ. 2009, 7, 202–208. [Google Scholar]
- Li, J. Current status and prospects for in-feed antibiotics in the different stages of pork production—A review. Asian-Australas. J. Anim. Sci. 2017, 30, 1667–1673. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Antimicrobial Resistance Surveillance in Europe. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). 2011. Available online: http://www.ecdc.europa.eu/en/publications/Publications/antimicrobial-resistance-surveillance-europe-2011.pdf (accessed on 24 August 2021).
- Teillant, A.; Laxminarayan, R. Economics of Antibiotic Use in U.S. Swine and Poultry Production. Choices 2015, 30, 1–11. [Google Scholar]
- Maron, D.F.; Smith, T.J.; Nachman, K.E. Restrictions on antimicrobial use in food animal production: An international regulatory and economic survey. Glob. Health 2013, 9, 48. [Google Scholar] [CrossRef]
- Van Lankveld, M.R. Antibiotic-Free. The European Experience A. Biomin 2014, 30. Available online: https://nuevo-group.com/wp-content/uploads/2019/12/Issue-8-Swine.pdf (accessed on 30 May 2022).
- Diana, A.; Manzanilla, E.G.; Calderón Díaz, J.A.; Leonard, F.C.; Boyle, L.A. Do weaner pigs need in-feed antibiotics to ensure good health and welfare? PLoS ONE 2017, 12, e0193505. [Google Scholar] [CrossRef]
- Yu, K.; Mu, C.; Yang, Y.; Su, Y.; Zhu, W. Segment-specific responses of intestinal epithelium transcriptome to in-feed antibiotics in pigs. Physiol. Genom. 2017, 49, 582–591. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Reviews. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Weichelsebaum, E. Probiotics and health: A review of the evidence. Nutr. Bull. 2009, 34, 340–373. [Google Scholar] [CrossRef]
- VanBelle, M. Current status and future perspectives in E.U. for antibiotics, probiotics, enzymes and organic acids in nutrition. In Gut Environment of Pigs; Piva, A., Bach Knudsen, K.E., Lindberg, J.E., Eds.; Nottingham University Press: Nottingham, UK, 2001; pp. 231–256. [Google Scholar]
- Yang, F.; Hou, C.; Zeng, X.; Qiao, S. The Use of Lactic Acid Bacteria as a Probiotic in Swine Diets. Pathogens 2015, 4, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Kim, I.H. The apparent total tract digestibility, apparent ileal digestibility and fecal noxious gas content of growing pigs fed probiotics in diets. Wayamba J. Anim. Sci. 2011, 3, 121–123. [Google Scholar]
- Liao, S.F.; Nyachoti, M. Using probiotics to improve swine gut health and nutrient utilization. Anim. Nutr. 2017, 3, 331–343. [Google Scholar] [CrossRef]
- Abdelqader, A.; Irshaid, R.; Al-Fataftah, A.R. Effects of dietary probiotic inclusion on performance, eggshell quality, cecal microflora composition, and tibia traits of laying hens in the late phase of production. Trop. Anim. Health Prod. 2013, 45, 1017–1024. [Google Scholar] [CrossRef]
- Cutting, S.M. Bacillus probiotics. Food Microbiol. 2011, 28, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, C.; Georgoulakis, I.E.; Tzivara, A.; Kyriakis, C.S.; Govaris, A.; Kyriakis, S.C. Field Evaluation of the Effect of a Probiotic-containing Bacillus licheniformis and Bacillus subtilis Spores on the Health Status, Performance, and Carcass Quality of Grower and Finisher Pigs. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2004, 51, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Giang, H.H.; Viet, T.Q.; Ogle, B.; Lindberg, J.E. Effects of Supplementation of Probiotics on the Performance, Nutrient Digestibility and Faecal Microflora in Growing-finishing Pigs. Asian-Australas. J. Anim. Sci. 2011, 24, 655–661. [Google Scholar] [CrossRef]
- Liu, X.; Cao, G.; Wang, Q.; Yao, X.; Fang, B. The effect of Bacillus coagulans-fermented and nonfermented Ginkgo biloba on the immunity status of broiler chickens. J. Anim. Sci. 2015, 93, 3384–3394. [Google Scholar] [CrossRef]
- Mandel, D.R.; Eichas, K.; Holmes, J. Bacillus coagulans: A viable adjunct therapy for relieving symptoms of rheumatoid arthritis according to a randomized, controlled trial. BMC Complement. Altern. Med. 2010, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sutton, A.J.; Higgins, J.P. Recent developments in meta-analysis. Stat. Med. 2008, 27, 625–650. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, G.M.; Dila, K.A.S.; Mohamed, M.Y.F.; Tam, D.N.H.; Kien, N.D.; Ahmed, A.M.; Huy, N.T. A step by step guide for conducting a systematic review and meta-analysis with simulation data. Trop. Med. Health 2019, 47, 46. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, B.; Li, T.; Kim, I.H. Effects of supplementing growing-finishing pig diets with Bacillus spp. probiotic on growth performance and meat-carcass grade qualitytraits. Rev. Bras. Zootec. 2016, 45, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Upadhaya, S.D.; Kim, S.C.; Valientes, R.A.; Kim, I.H. The Effect of Bacillus-based Feed Additive on Growth Performance, Nutrient Digestibility, Fecal Gas Emission, and Pen Cleanup Characteristics of Growing-finishing Pigs. Asian-Australas. J. Anim. Sci. 2015, 28, 999–1005. [Google Scholar] [CrossRef]
- Nitikanchana, S.; Tokach, M.D.; DeRouchey, J.M.; Goodband, R.D.; Nelssen, J.L.; Dritz, S.S. The Effect of Bacillus Probiotic on Growth Performance and Fecal Consistency of Growing-Finishing Pigs. Kans. Agric. Exp. Stan. Res. Rep. 2011, 10, 1968–2014. Available online: http://hdl.handle.net/2097/13496 (accessed on 22 May 2021). [CrossRef]
- Patarapreecha, P.; Jaikan, W.; Juangsaman, A.; Khajarern, J. Effects of Dietary Bacillus subtilis Supplementation as Probiotics on Growth Performance and Nutrients Digestibility in Fattening Pigs. Pak. J. Nutr. 2018, 17, 634–640. [Google Scholar] [CrossRef]
- Silva, N.V.P.; Dadalt, J.C.; Budiño, F.E.L.; Gamairo, A.H.; Trindade Neto, M.A. Apparent total tract digestibility, performance, and methane emissions in pigs maintained under different sanitary conditions and supplemented with antibiotic or Bacillus subtilis. Can. J. Anim. Sci. 2017, 97, 553–561. [Google Scholar] [CrossRef]
- Fu, R.; Chen, D.; Tian, G.; Zheng, P.; Mao, X.; Yu, J.; He, J.; Huang, Z.; Luo, Y.; Yu, B. Effect of dietary supplementation of Bacillus coagulans or yeast hydrolysates on growth performance, antioxidant activity, cytokines and intestinal microflora of growing-finishing pigs. Anim. Nutr. 2019, 5, 366–372. [Google Scholar] [CrossRef]
- Rybarczyk, A.; Bogusławska-Wa, S.E.; Dłubała, A. Effect of BioPlus YC Probiotic Supplementation on Gut Microbiota, Production Performance, Carcass and Meat Quality of Pigs. Animals 2021, 11, 1581. [Google Scholar] [CrossRef]
- Van der Peet-Schwering, C.M.C.; Verheijen, R.; Jørgensen, L.; Raff, L. Effects of a mixture of Bacillus amyloliquefaciens and Bacillus subtilis on the performance of growing-finishing pigs. Anim. Feed. Sci. Technol. 2020, 261, 114409. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. Introduction to Meta-Analysis; John Wiley & Sons Ltd.: Chichester, UK, 2009. [Google Scholar] [CrossRef]
- Lean, I.J.; Rabiee, A.R.; Duffield, T.F.; Dohoo, I.R. Use of meta-analysis in animal health and reproduction: Methods and applications. J. Dairy Sci. 2009, 92, 3545–3565. [Google Scholar] [CrossRef]
- Huedo-Medina, T.B.; Sanchez-Meca, J.; Marín-Martínez, F.; Botella, J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol. Methods 2006, 11, 193–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J. Am. Stat. Assoc. 2000, 95, 89–98. [Google Scholar] [CrossRef]
- Ravindran, V. Feed enzymes: The science, practice, and meta- bolic realities. J. Appl. Poult. Res. 2013, 22, 628–636. [Google Scholar] [CrossRef]
- Zimmermann, J.A.; Fusari, M.L.; Rossler, E.; Blajman, J.; Romero Scharpen, A.; Olivero, C.R.; Berisvil, A.P.; Signorini, M.L.; Zbrun, M.V.; Frizzo, L.S.; et al. Effects of probiotics in swines growth performance: A meta-analysis of randomised controlled trials. Anim. Feed Sci. Tech. 2016, 219, 280–293. [Google Scholar] [CrossRef]
- Robison, O.W.; Christian, L.L.; Goodwin, R.; Johnson, R.K.; Mabry, J.W. Effects of genetic type and protein levels on growth of swine. J. Anim. Sci. 2000, 77, 1–9. [Google Scholar] [CrossRef]
- Lee, S.H.; Ingale, S.L.; Kim, J.S.; Kim, K.H.; Lokhande, A.; Kim, E.K.; Kwon, I.K.; Kim, Y.H.; Chae, B.J. Effects of dietary supplementation with Bacillus subtilis LS 1–2 fermentation biomass on growth performance, nutrient digestibility, cecal microbiota and intestinal morphology of weanling pig. Anim. Feed Sci. Technol. 2014, 188, 102–110. [Google Scholar] [CrossRef]
- Zhao, P.Y.; Kim, I.H. Effect of direct-fed microbial on growth performance, nutrient digestibility, fecal noxious gas emission, fecal microbial flora and diarrhea score in weanling pigs. Anim. Feed Sci. Tech. 2015, 200, 86–92. [Google Scholar] [CrossRef]
- Meng, Q.W.; Yan, L.; Zhou, T.X.; Wang, J.P.; Lee, J.H.; Kim, I.H. Influence of probiotics in different energy and nutrient density diets on growth performance, nutrient digestibility, meat quality, and blood characteristics in growing-finishing pigs. J. Anim. Sci. 2010, 88, 3320–3326. [Google Scholar] [CrossRef] [PubMed]
- NRC—National Research Council. Nutrient Requirements of Swine, 11th ed.; National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Wang, X.; Tsai, T.; Wei, X.; Zuo, B.; Davis, E.; Rehberger, T.; Hernandez, S.; Jochems, E.J.M.; Maxwell, C.V.; Zhao, J. Effect of Lactylate and Bacillus subtilis on Growth Performance, Peripheral Blood Cell Profile, and Gut Microbiota of Nursery Pigs. Microorganisms 2021, 9, 803. [Google Scholar] [CrossRef]
- Deng, B.; Wu, J.; Li, X.; Zhang, C.; Men, X.; Xu, Z. Effects of Bacillus subtilis on growth performance, serum parameters, digestive enzyme, intestinal morphology, and colonic microbiota in piglets. AMB Express 2020, 10, 212. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Jinno, C.; Kim, K.; Wu, Z.; Tan, B.; Li, X.; Whelan, R.; Liu, Y. Dietary Bacillus spp. enhanced growth and disease resistance of weaned pigs by modulating intestinal microbiota and systemic immunity. J. Animal. Sci. Biotechnol. 2020, 11, 101. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, E.; Conway, P. Probiotics for pigs. In Probiotics: The Scientific Basis; Fuller, R., Ed.; Chapman & Hall: London, UK, 1992; pp. 260–316. [Google Scholar]
- Davis, M.E.; Parrat, T.; Brown, D.C.; de Rodas, B.Z.; Johnson, Z.B.; Maxwell, C.V.; Rehberger, T. Effect of a Bacillus-based direct-fed microbial feed supplement on growth performance and pen cleaning characteristics of growing-finishing pigs. J. Anim. Sci. 2008, 86, 1459–1467. [Google Scholar] [CrossRef]
- Li, H.H.; Jiang, X.R.; Qiao, J.Y. Effect of dietary Bacillus subtilis on growth performance and serum biochemical and immune indexes in weaned piglets. J. Appl. Anim. Res. 2021, 49, 83–88. [Google Scholar] [CrossRef]
- Kornegay, E.T.; Risley, C.R. Nutrient digestibilities of a corn-soybean meal diet as influenced by Bacillus products fed to finishing swine. J. Anim. Sci. 1996, 74, 799–805. [Google Scholar] [CrossRef]
- Turner, J.L.T.; Dritz, S.S.; Minton, J.E. Review: Alternatives to conventional antimicrobials in swine diets. Prof. Anim. Sci. 2001, 17, 217–226. [Google Scholar] [CrossRef]
- Djouzi, Z.; Andrieux, C.; Degivry, M.C.; Bouley, C.; Szylit, O. The association of yogur t starters with Lactobacillus casei DN 114.001 in fermented milk alters the composition and metabolism of intestinal microflora in germ free rats and in human flora associated rats. J. Nutr. 1997, 127, 2260–2266. [Google Scholar] [CrossRef]
- Balasubramanian, B.; Lee, S.I.; Kim, I.H. Inclusion of dietary multi-species probiotic on growth performance, nutrient digestibility, meat quality traits, faecal microbiota and diarrhoea score in growing–finishing pigs. Ital. J. Anim. Sci. 2018, 17, 100–106. [Google Scholar] [CrossRef]
- Hong, H.A.; Duc, L.H.; Cutting, S.M. The use of bacterial spore formers as probiotics. FEMS Microbiol. Rev. 2005, 29, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, E.; Jarnagin, A.; Schmidt, B. Commercial Production of Extracellular Enzymes. In Bacillus subtilis and Other Gram-Positive Bacteria: Biochemistry, Physiology, and Molecular Genetics; Sonenshein, A.L., Hoch, J.A., Losick, R., Eds.; American Society Microbiology: Washington, DC, USA, 1993; pp. 917–937. [Google Scholar]
- Ghani, M.; Ansari, A.; Aman, A.; Zohra, R.R.; Siddiqui, N.N.; Qader, S.A. Isolation and characterization of different strains of Bacillus licheniformis for the production of commercially significant enzymes. Pak. J. Pharm. Sci. 2013, 26, 691–697. [Google Scholar] [PubMed]
- Hmidet, N.; Ali, N.E.; Haddar, A.; Kanoun, S.; Alya, S.K.; Nasri, M. Alkaline proteases and thermostable αamylase co-produced by Bacillus licheniformis NH1: Characterization and potential application as detergent additive. Biochem. Eng. J. 2009, 47, 71–79. [Google Scholar] [CrossRef]
- Schofield, B.J.; Skarshewski, A.; Lachner, N.; Ouwerkerk, D.; Klieve, A.V.; Dart, P.; Hugenholtz, P. Near complete genome sequence of the animal feed probiotic, Bacillus amyloliquefaciens H57. Stand. Genom. Sci. 2016, 11, 60. [Google Scholar] [CrossRef]
- Maruta, K.; Miyazaki, H.; Tadano, Y.; Masuda, S.; Suzuki, A.; Takahashi, H.; Takahashi, M. Effects of Bacillus subtilis C-3102 intake on fecal flora of sows and on diarrhea and mortality rate of their piglets. Anim. Sci. Technol. 1996, 67, 403–409. [Google Scholar]
- Marubashi, T.; Gracia, M.I.; Vilà, B.; Bontempo, V.; Kritas, S.K.; Piskoríková, M. The efficacy of the probiotic feed additive Calsporin® (Bacillus subtilis C-3102) in weaned piglets: Combined analysis of four different studies. J. Appl. Anim. Nutr. 2012, 1, 1–5. [Google Scholar] [CrossRef]
- Chesson, A. Probiotics and other intestinal mediators. In Principles of Pig Science; Cole, D.J.A., Wiseman, J., Varley, M.A., Eds.; Nottingham University Press: Loughborough, UK, 1993; pp. 197–214. [Google Scholar]


| Reference | 1 NC | Breed | Sex | Based Diet | 2 IBW | Td (days) | 3 N | 4 CP g/kg | 5 ME MJ/kg | 6 Anti | Bacillus spp. | Bacillus spp. (mg/d) | 7 Ca | 8 P | 9 Lys | 10 Met |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Giang et al. [21] | 1 | [Yorkshire × Landrace] | 8 gilts and 12 barrows per treatment | Corn-SBM | 28.70 ± 0.90 | 42 | 20 | 19.4 ± 1.1 | 14.45 | NU | B. subtilis H4 (6 × 1011 CFU/mL) | 6480.0 | 9.1 | 4.0 | 9.0 | 2.9 |
| Balasubramanian et al. [27] | 2 | [(Yorkshire × Landrace) × Duroc] | Three barrows and two gilts per pen | SBM | 23.3 ± 1.40 | 112 | 25 | 185.6 ± 15 | 13.21 | NU | B. coagulans (1 × 109 cfu/g), B. lichenformis (5 × 108 cfu/g), B. subtilis (1 × 109 cfu/g) | 211.7–426.8 | 8.0 | 5.1 | 9.8 | 2.9 |
| Upadhaya et al. [28] | 1 | [(Yorkshire × Landrace) × Duroc] | 2 gilts and 3 barrows per pen | Corn-SBM-Wheat | 23.6 ± 1.41 | 112 | 60 | 182.8 ± 11 | NR | UN | B. Linchenformis and B. subtilis (1.47 × 10 8 CFU/g) | NR | 8.0 | 5.0 | 9.5 | 2.8 |
| Nitikanchana et al. [29] | 4 | PIC 1050 × 337 [(Large White × Landrace) × (Pietrain × Duroc)] | NR | Corn-SBM | 34.01 | 105 | 183 | 180 ± 15 | 14.08 | NU | Sporzyme ® (4.36 × 1012 CFU/lb) | NR | 5.0 | 4.5 | 9.3 | 0.05 |
| Patarapreecha et al. [30] | 4 | [(Yorkshire × Landrace) × Duroc] | Half barrows and half gilts | Corn-SBM | 60 ± 1.2 | 52 | 100 | 180.1 ± 10 | 12.57 | NS | B. subtilis (1.0 × 1012 CFU g) | 248.0–1989.0 | 2.28 | ND | 2.2 | 0.2 |
| Silva et al. [31] | 2 | [Large White × Landrace] | Half barrows and half gilts | Corn-SBM | 26.07 ± 0.07 | 82 | 10 | 171.0 ± 15 | 13.73 | Lincomycin | B. subtilis C-3102 (1.0 × 1010 CFU g) | 66.0–78.9 | 6.5 | 2.9 | 7.5 | 2.4 |
| Fu et al. [32] | 1 | [(Yorkshire × Landrace) × Duroc] | NR | Corn-SBM | 26.87 ± 2.65 | 105 | 12 | 138.8 ± 11 | 14.23 | Enramycin | B coagulans (5.0 × 109 cfu/g) | 48.6 | 4.6 | 3.9 | 6.1 | 1.8 |
| Rybarczyk et al. [33] | 1 | [(Yorkshire × Landrace) × Duroc] | Half barrows and half gilts | Wheat -Triticale | 33.15 ± 2.36 | 77 | 60 | 166.5 ± 15 | 11.70 | NU | B. licheniformis DSM 5749 (1.6 × 109 CFU/g), and B. subtilis DSM 5750— (1.6 × 109 CFU/g) | 1012.0 | 5.8 | 4.5 | 9.8 | 2.8 |
| van der Peet-Schwering et al. [34] | 1 | [(York × Dutch Landrace) × Large White boar] | 6 barrows and 6 gilts per pen | Corn-SBM-Wheat-Barley | 23.2 ± 2.95 | 102 | 288 | 174.5 ± 1 | 14.33 | NU | B. amyloliquefaciens DSM 25,840 and B. subtilis DSM 3232 (1.5 × 109 CFU/g) | 784.0 | 4.7 | ND | ND | ND |
| SMD 2 (95% CI 3) | Heterogeneity | RMD 4 (95% CI) | Publication Bias | |||||
|---|---|---|---|---|---|---|---|---|
| Outcomes 1 | No. of Comparisons | Random Effect | p-Value | Q | p-Value | I 2 | Random Effect | Egger |
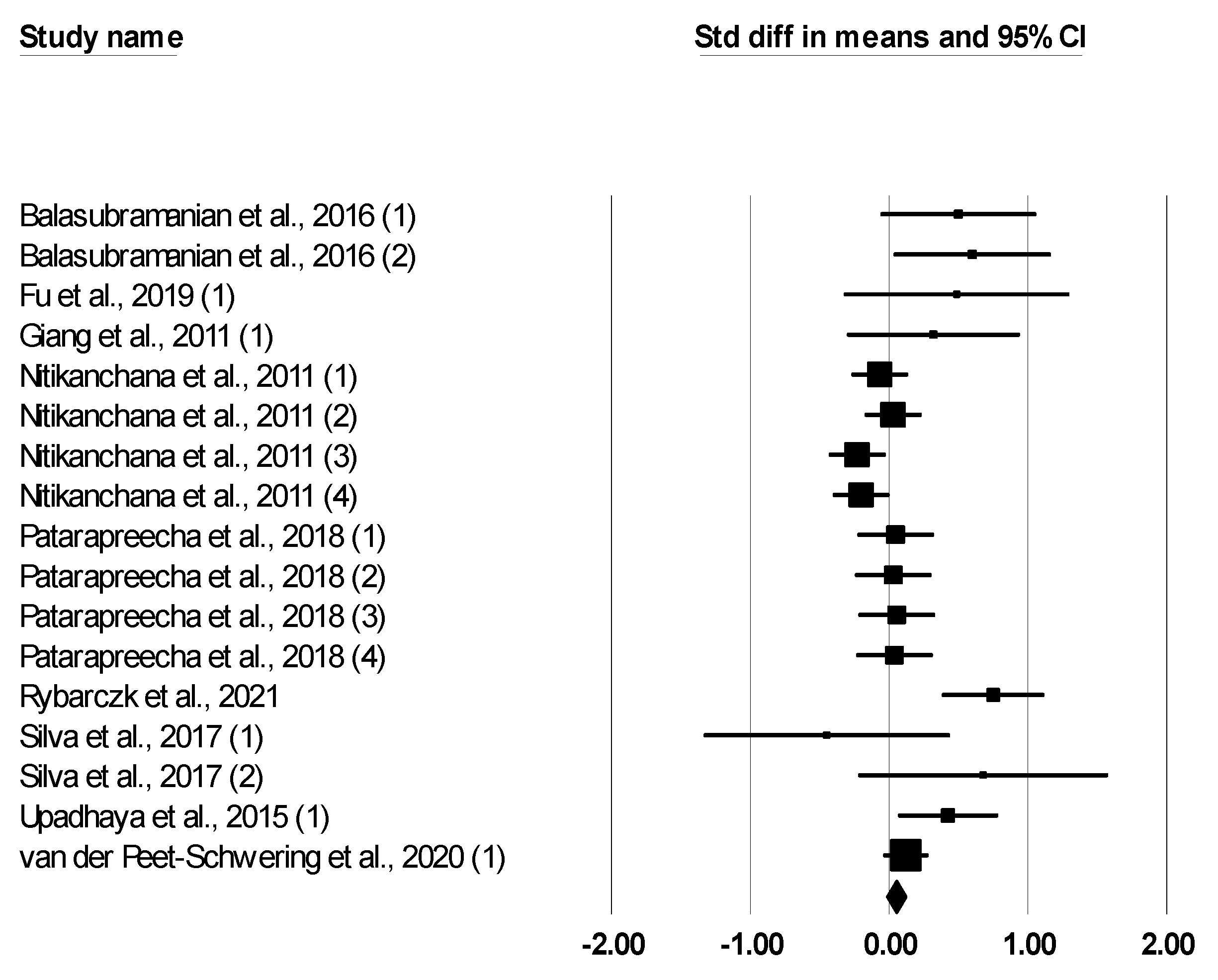
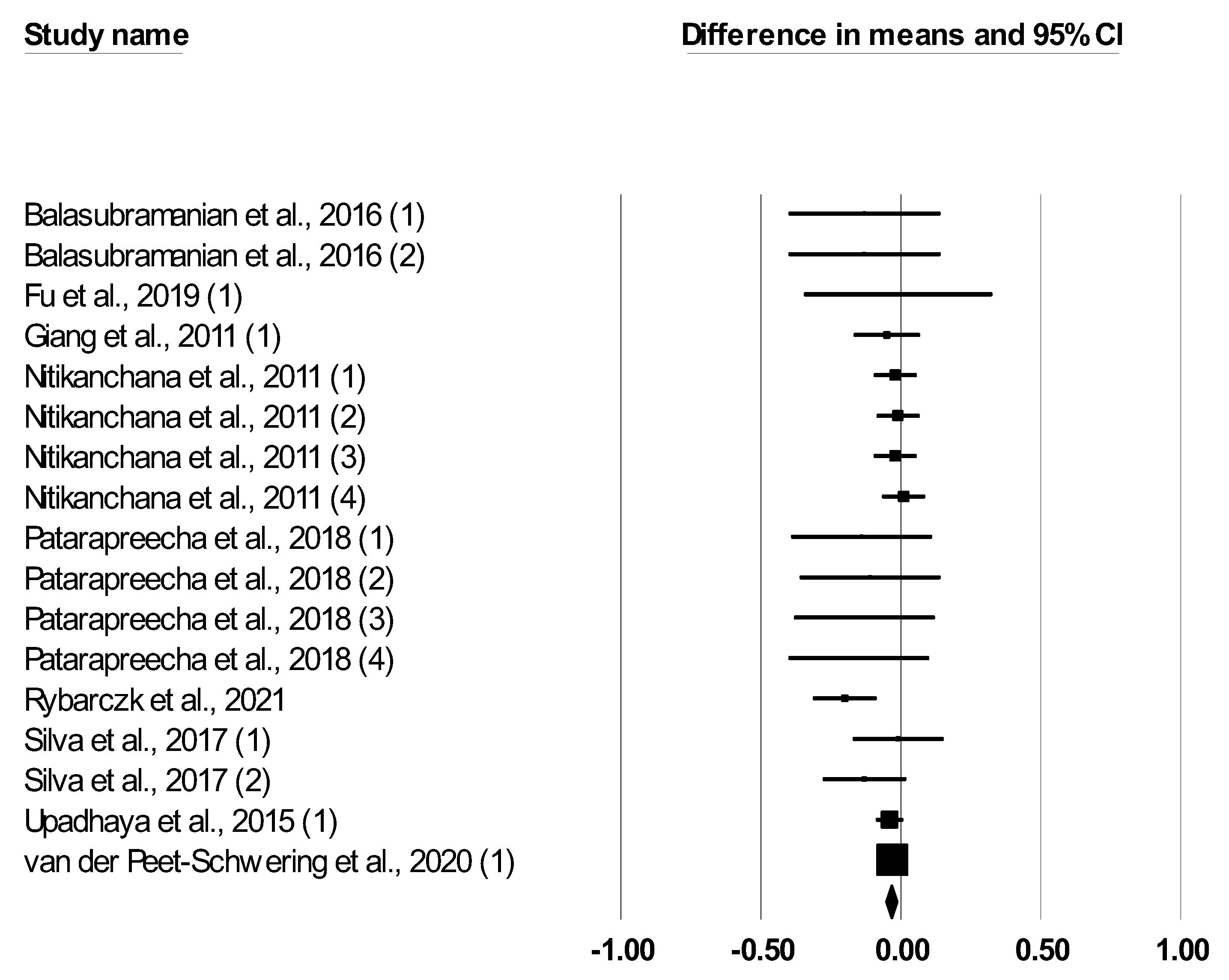
| ADFI, kg/d | 17 | −0.052 (−0.120, 0.017) | 0.138 | 15.123 | 0.516 | 0.00 | −0.012 (−0.029, 0.006) | 0.829 |
| ADG, kg/d | 17 | 0.113 (−0.014, 0.240) | 0.081 | 43.704 | <0.001 | 63.39 | 0.011 (−0.003, 0.025) | 0.033 |
| F:G ratio | 17 | −0.127 (−0.195, 0.058) | <0.001 | 15.048 | 0.521 | 0.00 | −0.037 (−0.056, −0.017) | 0.060 |
| Covariate | Slope | p-Value | Intercept | p-Value |
|---|---|---|---|---|
| Initial body weight | −0.015 | 0.194 | 0.537 | 0.109 |
| Experiment period | −0.005 | 0.459 | 0.714 | 0.380 |
| Number of animals per group | −0.001 | 0.015 | 0.367 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez-Ronquillo, M.; Villegas-Estrada, D.; Robles-Jimenez, L.E.; Garcia Herrera, R.A.; Villegas-Vázquez, V.L.; Vargas-Bello-Pérez, E. Effect of the Inclusion of Bacillus spp. in Growing–Finishing Pigs’ Diets: A Meta-Analysis. Animals 2022, 12, 2269. https://doi.org/10.3390/ani12172269
Gonzalez-Ronquillo M, Villegas-Estrada D, Robles-Jimenez LE, Garcia Herrera RA, Villegas-Vázquez VL, Vargas-Bello-Pérez E. Effect of the Inclusion of Bacillus spp. in Growing–Finishing Pigs’ Diets: A Meta-Analysis. Animals. 2022; 12(17):2269. https://doi.org/10.3390/ani12172269
Chicago/Turabian StyleGonzalez-Ronquillo, Manuel, Daniela Villegas-Estrada, Lizbeth E. Robles-Jimenez, Ricardo A Garcia Herrera, Vanessa L. Villegas-Vázquez, and Einar Vargas-Bello-Pérez. 2022. "Effect of the Inclusion of Bacillus spp. in Growing–Finishing Pigs’ Diets: A Meta-Analysis" Animals 12, no. 17: 2269. https://doi.org/10.3390/ani12172269







