Conventional Gel Electrophoresis-Resolvable Insertion/Deletion Markers for Individual Identification and Analysis of Population Genetics in Red-Crowned Cranes in Eastern Hokkaido, Japan
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and DNA Extraction
2.2. Search for InDels
2.3. Selection of Primer Sets to Detect InDels
2.4. PCR Reactions to Detect InDels
2.5. Haplotype Determination and Sexing
2.6. Statistical Analyses
3. Results
3.1. Search for InDel Markers
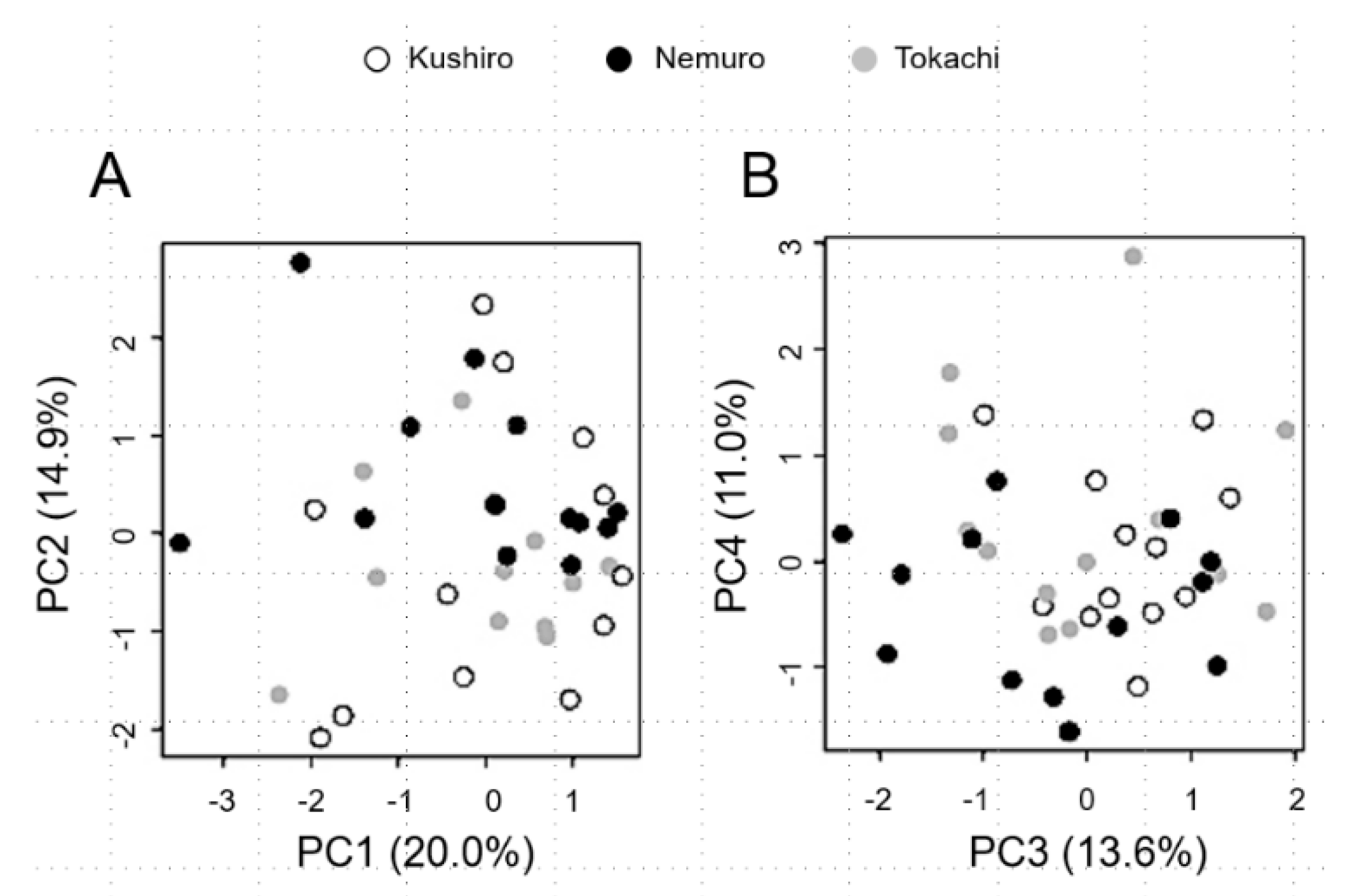
3.2. Basic Population Genetics
3.3. Individual Identification
3.4. Kin Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Momose, Y.S.; Lee, K.; Momose, K.; Qian, F. The International winter censuses of the Red-crowned Crane from 2018/2019 to 2020/2021. In Newslatter of Crane Working Group of Eurasia # 16; Ilyashenko, E., Ilyashenko, V., Eds.; Crane Working Group of Eurasia: Moscow, Russia, 2022; pp. 77–78. [Google Scholar]
- Momose, Y.; Momose, K. Species review: Red-crowned Crane. In Crane Conservation Strategy; Mirande, C.M., Harris, J.T., Eds.; International Crane Foundation: Baraboo, WI, USA, 2019; pp. 245–260. [Google Scholar]
- Masatomi, H.; Masatomi, Y. Ecology of the Red-crowned Crane and Conservation Activities in Japan. In Biodiversity Conservation Using Umbrella Species: Blakiston’s Fish Owl and the Red-crowned Crane; Nakamura, F., Ed.; Ecological Research Monographs; Springer: Singapore, 2018; Chapter 6; pp. 83–105. [Google Scholar]
- Kaneda, H.; Hayashi, J.; Takahama, S.; Taya, C.; Lindahl, K.F.; Yonekawa, H. Elimination of paternal mitochondrial DNA in intraspecific crosses during early mouse embryogenesis. Proc. Natl. Acad. Sci. USA 1995, 92, 4542–4546. [Google Scholar] [CrossRef]
- Taberlet, P. The use of mitochondrial DNA control region sequencing in conservation genetics. In Molecular Genetic Approaches in Conservation; Smith, T.B., Wayne, R.K., Eds.; Oxford University Press: New York, NY, USA, 1996; pp. 125–142. [Google Scholar]
- Glenn, T.C.; Stephan, W.; Braun, M.J. Effects of a population bottleneck on Whooping crane mitochondrial DNA variation. Conserv. Biol. 1999, 13, 1097–1107. [Google Scholar] [CrossRef]
- Petersen, J.L.; Bischof, R.; Krapu, G.L.; Szalanski, A.L. Genetic variation in the midcontinental population of Sandhill crane Grus Canadensis. Biochem. Genet. 2003, 41, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ponomarev, A.; Tatarinova, T.; Bubyakina, V.; Smagulova, F.; Kashentseva, T.; Morozov, I. Variation of mitochondrial DNA D-loop sequences in the endangered Siberian crane Grus leucogeranus Pallas. Conserv. Genet. 2004, 5, 847–851. [Google Scholar] [CrossRef]
- Hasegawa, O.; Takada, S.; Yoshida, M.C.; Abe, S. Variation of mitochondrial control region sequences in three crane species, the Red-crowned crane Grus japonensis, the Common crane G. grus and the Hooded crane G. Monacha. Zoolog. Sci. 1999, 16, 685–692. [Google Scholar] [CrossRef]
- Akiyama, T.; Momose, K.; Onuma, M.; Matsumoto, F.; Masuda, R. Low Genetic Variation of Red-Crowned Cranes on Hokkaido Island, Japan, Over the Hundred Years. Zoolog. Sci. 2017, 34, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, E.; Hasebe, M.; Hwang, J.-H.; Kim, E.-Y.; Lee, L.; Momose, K.; Teraoka, H. Origin of a pair of red-crowned cranes (Grus japonensis) found in Sarobetsu Wetland, northwestern Hokkaido, Japan: A possible crossbreeding between the island and the mainland population. J. Vet. Med. Sci. 2022, 84, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Masatomi, H.; Masatomi, Y. Promoting the coexistence of humans and Tancho in Japan. Jpn. J. Conserv. Ecol. 2009, 14, 223–242. [Google Scholar] [CrossRef]
- Masatomi, Y.; Surmach, S. Distribution of the Red-crowned Crane. In Biodiversity Conservation Using Umbrella Species: Blakiston’s Fish Owl and the Red-Crowned Crane; Nakamura, F., Ed.; Ecological Research Monographs; Springer: Singapore, 2018; Chapter 5; pp. 73–82. [Google Scholar]
- Whitney, K. Bird banding and the environmental humanities: Institutions, intersubjectivities, and the phenomenological method of Margaret Morse Nice. Environ. Humanit. 2021, 13, 113–135. [Google Scholar] [CrossRef]
- Vallant, S.; Niederstätter, H.; Berger, B.; Lentner, R.; Parson, W. Increased DNA typing success for feces and feathers of capercaillie (Tetrao urogallus) and black grouse (Tetrao tetrix). Ecol. Evol. 2018, 8, 3941–3951. [Google Scholar] [CrossRef]
- Manel, S.; Schwartz, M.K.; Luikart, G.; Taberlet, P. Landscape genetics: Combining landscape ecology and population genetics. Trends Ecol. Evol. 2003, 18, 189–197. [Google Scholar] [CrossRef]
- Hasegawa, O.Y.; Ishibashi, Y.; Abe, S. Isolation and characterization of microsatellite loci in the red-crowned crane Grus japonensis. Mol. Ecol. 2000, 9, 1677–1678. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, T.; Hasegawa, O.; Azuma, N.; Masatomi, H.; Sato, F.; Matsumoto, F.; Masatomi, Y.; Izumi, H.; Abe, S. Genetic structure of the endangered red-crowned cranes in Hokkaido, Japan and conservation implications. Conserv. Genet. 2015, 16, 1395–1401. [Google Scholar] [CrossRef]
- Zou, H.; Dong, H.; Kong, W.; Jianhua, M.A.; Liu, J. Characterization of 18 polymorphic microsatellite loci in the red-crowned crane (Grus japonensis), an endangered bird. Anim. Sci. J. 2010, 81, 519–522. [Google Scholar] [CrossRef]
- Uffelmann, E.; Huang, Q.Q.; Munung, N.S.; de Vries, J.; Okada, Y.; Martin, A.R.; Martin, H.C.; Lappalainen, T.; Posthuma, D. Genome-wide association studies. Nat. Rev. Methods Primers 2021, 1, 59. [Google Scholar] [CrossRef]
- Akiyama, T.; Kohyama, T.I.; Nishida, C.; Onuma, M.; Momose, K.; Masuda, R. Genetic variation of major histocompatibility complex genes in the endangered red-crowned crane. Immunogenetics 2017, 69, 451–462. [Google Scholar] [CrossRef]
- Montgomery, S.B.; Goode, D.L.; Kvikstad, E.; Albers, C.A.; Zhang, Z.D.; Mu, X.J.; Ananda, G.; Howie, B.; Karczewski, K.J.; Smith, K.S.; et al. The origin, evolution, and functional impact of short insertion-deletion variants identified in 179 human genomes. Genome. Res. 2013, 23, 749–761. [Google Scholar] [CrossRef]
- Shedlock, A.M.; Okada, N. SINE insertions: Powerful tools for molecular systematics. Bioessays 2000, 22, 148–160. [Google Scholar] [CrossRef]
- Adedze, Y.M.N.; Lu, X.; Xia, Y.; Sun, Q.; Nchongboh, C.G.; Alam, M.A.; Liu, M.; Yang, X.; Zhang, W.; Deng, Z.; et al. Agarose-resolvable InDel markers based on whole genome re-sequencing in cucumber. Sci. Rep. 2021, 11, 3872. [Google Scholar] [CrossRef]
- Jin, R.; Wei, C.; Fang, Y.; Jin, X.; Wang, H.; Lan, Q.; Guo, Y.; Chen, C.; Zhang, X.; Zhu, B. A Novel Panel of 43 Insertion/Deletion Loci for Human Identifications of Forensic Degraded DNA Samples: Development and Validation. Front. Genet. 2021, 12, 610540. [Google Scholar] [CrossRef]
- Bailes, S.; Devers, J.; Kirby, J.; Rhoads, D. An inexpensive, simple protocol for DNA isolation from blood for high-throughput genotyping by polymerase chain reaction or restriction endonuclease digestion. Poult. Sci. 2007, 86, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y.; Shiomi, A.; Shiraishi, J.; Makita, K.; Asakawa, M.; Kitazawa, T.; Hiraga, T.; Momose, Y.; Momose, K.; Masatomi, H.; et al. Large-scale survey of mitochondrial D-loop of the red-crowned crane Grus japonensis in Hokkaido, Japan by convenient genotyping method. J. Vet. Med. Sci. 2013, 75, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.; Koita, N.; Kondo, N.I.; Ito, H.C.; Nakajima, M.; Momose, K.; Iima, H.; Yoshino, T.; Amano, T.; Kitazawa, T.; et al. Metabarcoding of feces and intestinal contents to determine carnivorous diets in red-crowned cranes in eastern Hokkaido, Japan. J. Vet. Med. Sci. 2022, 84, 358–367. [Google Scholar] [CrossRef]
- Kalinowski, S.T.; Taper, M.L.; Marshall, T.C. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 2007, 16, 1099–1106. [Google Scholar] [CrossRef]
- Hardy, O.J.; Vekemans, X. SPAGeDi: A versatile computer program to analyse spatial genetic structure at the individual or population levels. Mol. Ecol. Notes 2002, 2, 618–620. [Google Scholar] [CrossRef]
- Weir, B.S.; Cockerham, C.C. Estimating F-Statistics for the Analysis of Population Structure. Evolution 1984, 38, 1358–1370. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Meir, E. A simple genotyping method to detect small CRISPR-Cas9 induced indels by agarose gel electrophoresis. Sci. Rep. 2019, 9, 4437. [Google Scholar] [CrossRef]
- Yan, Y.; Yi, G.; Sun, C.; Qu, L.; Yang, N. Genome-Wide Characterization of Insertion and Deletion Variation in Chicken Using Next Generation Sequencing. PLoS ONE 2014, 9, e104652. [Google Scholar]
- Boschiero, C.; Gheyas, A.A.; Ralph, H.K.; Eory, L.; Paton, B.; Kuo, R.; Fulton, J.; Preisinger, R.; Kaiser, P.; Burt, D.W. Detection and characterization of small insertion and deletion genetic variants in modern layer chicken genomes. BMC Genom. 2015, 16, 562. [Google Scholar] [CrossRef][Green Version]
- Sun, C.; Liu, H.-Y.; Xu, P.; Lu, C.-H. Genetic diversity of wild wintering red-crowned crane (Grus japonensis) by microsatellite markers and mitochondrial Cyt B gene sequence in the Yancheng reserve. Anim. Biotechnol. 2020, 8, 1–6. [Google Scholar] [CrossRef]
- Pacífico, E.C.; Sánchez-Montes, G.; Miyaki, C.Y.; Tella, J.L. Isolation and characterization of 15 new microsatellite markers for the globally endangered Lear’s macaw Anodorhynchus leari. Mol. Biol. Rep. 2020, 47, 8279–8285. [Google Scholar] [CrossRef] [PubMed]
- Willows-Munro, S.; Kleinhans, C. Testing microsatellite loci for individual identification of captive African grey parrots (Psittacus erithacus): A molecular tool for parentage analysis that will aid in monitoring legal trade. Conserv. Genet. Resour. 2020, 12, 489–495. [Google Scholar] [CrossRef]
- Omote, K.; Nishida, C.; Takenaka, T.; Saito, K.; Shimura, R.; Fujimoto, S.; Sato, T.; Masuda, R. Recent fragmentation of the endangered Blakiston’s fish owl (Bubo blakistoni) population on Hokkaido Island, northern Japan, revealed by mitochondrial DNA and microsatellite analyses. Zool. Lett. 2015, 1, 16. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhan, X.; Dixon, A.; Batbayar, N.; Bragin, E.; Ayas, Z.; Deutschova, L.; Chavko, J.; Domashevsky, S.; Dorosencu, A.; Bagyura, J.; et al. Exonic versus intronic SNPs: Contrasting roles in revealing the population genetic differentiation of a widespread bird species. Heredity 2015, 114, 1–9. [Google Scholar] [CrossRef]
- Masuda, R. Status and Perspective of the Population Based on Genetic Diversity: Introduction. In Biodiversity Conservation Using Umbrella Species: Blakiston’s Fish Owl and the Red-Crowned Crane; Nakamura, F., Ed.; Ecological Research Mono-graphs; Springer: Singapore, 2018; Chapter 8; pp. 131–133. [Google Scholar]
- Kohyama, I.T.; Omote, K.; Nishida, C.; Takenaka, T.; Saito, K.; Fujimoto, S.; Masuda, R. Spatial and temporal variation at major histocompatibility complex class IIB genes in the endangered Blakiston’s fish owl. Zool. Lett. 2015, 1, 13. [Google Scholar] [CrossRef]
- Jang, J.E.; Kim, N.H.; Lim, S.; Kim, K.Y.; Lee, H.J.; Park, Y.C. Genetic integrity and individual identification-based population size estimate of the endangered long-tailed goral, Naemorhedus caudatus from Seoraksan National Park in South Korea, based on a non-invasive genetic approach. Anim. Cells Syst. 2020, 24, 171–179. [Google Scholar] [CrossRef]
- Blåhed, I.-M.; Königsson, H.; Ericsson, G.; Spong, G. Discovery of SNPs for individual identification by reduced representation sequencing of moose (Alces alces). PLoS ONE 2018, 13, e0197364. [Google Scholar] [CrossRef]
- Väli, U.; Brandström, M.; Johansson, M.; Ellegren, E. Insertion-deletion polymorphisms (indels) as genetic markers in natural populations. BMC Genet. 2008, 9, 8. [Google Scholar] [CrossRef]
- Maw, A.A.; Shimogiri, T.; Riztyan; Kawabe, K.; Kawamoto, Y.; Okamoto, S. Genetic diversity of myanmar and indonesia native chickens together with two jungle fowl species by using 102 indels polymorphisms. Asian Australas. J. Anim. Sci. 2012, 25, 927–934. [Google Scholar] [CrossRef]
- Hu, W.; Zhou, T.; Wang, P.; Wang, B.; Song, J.; Han, Z.; Chen, L.; Liu, K.; Xing, Y. Development of Whole-Genome Agarose-Resolvable LInDel Markers in Rice. Rice 2020, 13, 1. [Google Scholar] [CrossRef]

| InDel | Chromosome (Position) | InDel Type | Indel Size (bp) | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | PCR Product (bp) |
|---|---|---|---|---|---|---|
| Id-01 | Grja_16152 (2703..2734) | Deletion | 32 | AATTCAGTGGGTTTGCTCCCGCGGAA | TCAGTTCGTGTCGTACGTTGTCCCA | 200 |
| Id-02 | Grja_24077 (3699..3730) | Deletion | 32 | AGCAGCTCTGGAGTCACCAAAGCA | TCAAAATGGTTCCATGTGCCCTGTGT | 205 |
| Id-03 | Grja_10155 (1061..1090) | Insertion | 30 | GCAGGATAGCAGCCTAATTGTATCAA | AGGTAGGGCAACTTTAAAGTACTTTGA | 160 |
| Id-04 | Grja_25718 (20476..20505) | Deletion | 30 | AAAGGAGGGAATTTGCAGAGCCCA | TGTGTTTTCTGAGACTTTCCACTCT | 234 |
| Id-05 | Grja_8974 (39698..39727) | Deletion | 30 | TGGTATGTCTGCCACTCAGAAAGGGA | AGAAACAGGTAAAGGCTCACACCAGA | 207 |
| Id-06 | Grja_3909 (47326..47361) | Deletion | 36 | TGACTACCTGTGCACCCAGACAGCA | ATGGTGTGGTGGCATGCCAGCAAATA | 147 |
| Id-07 | Grja_36880 (1992..2021) | Deletion | 30 | ATGTCTCTCTTGTGTCCCCTTGGGA | ACAGCCCAGAGATGGTGGCCACAA | 175 |
| Id-08 | Grja_29257 (18954..18984) | Deletion | 31 | TAGCAAAGCTCCAGAACAGGACTCCT | AGAGTTTCAGTACAGAAAGACCTGCT | 236 |
| Id-9 | Grja_13764 (9525..9554) | Deletion | 30 | TATGGTAACGGAACAGGGAGGGGGT | TGTCCCTTGTTTCAGTTCCAGCAGCT | 251 |
| Id-10 | Grja_2883 (30876..30905) | Deletion | 30 | ACATGTTGGCCTTTAAAGGCTGAGCA | AGGAGCACCGACTAGCATTCAGCAT | 183 |
| Id-11 | Grja_3871 (53311..53340) | Deletion | 30 | ACCTCACTACTAGCTCCTTATTCA | TTAGATAAGCCTGCCTCTGTAACTA | 180 |
| InDel Marker | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Area | Crane | Sex | Haplotype | Id-01 | Id-02 | Id-03 | Id-04 | Id-05 | Id-06 | Id-07 | Id-08 | Id-9 | Id-10 | Id-11 |
| Kushiro (13) | 70 | Female | Gj2 | W Homo | D Homo | WI Hetero | W Homo | W Homo | WD Hetero | D Homo | W Homo | W Homo | W Homo | W Homo |
| 89 | Female | Gj1 | W Homo | W Homo | WI Hetero | W Homo | WD Hetero | WD Hetero | WD Hetero | W Homo | W Homo | WD Hetero | W Homo | |
| 123 | Male | Gj1 | W Homo | W Homo | W Homo | W Homo | WD Hetero | W Homo | W Homo | W Homo | W Homo | W Homo | W Homo | |
| 150 | Male | Gj2 | W Homo | WD Hetero | I Homo | W Homo | WD Hetero | W Homo | WD Hetero | WD Hetero | W Homo | WD Hetero | W Homo | |
| 194 | Female | Gj1 | W Homo | W Homo | W Homo | W Homo | WD Hetero | W Homo | D Homo | W Homo | W Homo | W Homo | W Homo | |
| 211 | Male | Gj2 | W Homo | W Homo | W Homo | W Homo | W Homo | WD Hetero | W Homo | WD Hetero | W Homo | W Homo | W Homo | |
| 247 | Male | Gj2 | W Homo | D Homo | W Homo | W Homo | W Homo | W Homo | WD Hetero | WD Hetero | W Homo | WD Hetero | W Homo | |
| 251 | Female | Gj2 | W Homo | W Homo | W Homo | W Homo | W Homo | W Homo | WD Hetero | W Homo | W Homo | W Homo | W Homo | |
| 271 | Male | Gj2 | W Homo | W Homo | W Homo | W Homo | W Homo | W Homo | W Homo | WD Hetero | WD Hetero | W Homo | W Homo | |
| 315 | Female | Gj2 | WD Hetero | WD Hetero | I Homo | WD Hetero | WD Hetero | W Homo | D Homo | W Homo | WD Hetero | W Homo | WD Hetero | |
| 344 | Male | Gj2 | W Homo | W Homo | WI Hetero | W Homo | W Homo | WD Hetero | W Homo | WD Hetero | WD Hetero | WD Hetero | W Homo | |
| 361 | Male | Gj2 | W Homo | D Homo | W Homo | W Homo | W Homo | WD Hetero | WD Hetero | W Homo | W Homo | W Homo | W Homo | |
| 362 | Male | Gj1 | W Homo | W Homo | WI Hetero | W Homo | W Homo | D Homo | W Homo | WD Hetero | W Homo | WD Hetero | W Homo | |
| Nemuro (13) | 54 | Male | Gj2 | W Homo | W Homo | WI Hetero | W Homo | W Homo | WD Hetero | WD Hetero | WD Hetero | W Homo | WD Hetero | W Homo |
| 72 | Female | Gj2 | W Homo | W Homo | WI Hetero | W Homo | WD Hetero | W Homo | WD Hetero | W Homo | W Homo | WD Hetero | W Homo | |
| 127 | Male | Gj2 | W Homo | W Homo | WI Hetero | W Homo | WD Hetero | WD Hetero | WD Hetero | W Homo | W Homo | WD Hetero | W Homo | |
| 137 | Male | Gj2 | W Homo | W Homo | WI Hetero | W Homo | W Homo | WD Hetero | WD Hetero | WD Hetero | W Homo | D Homo | W Homo | |
| 139 | Female | Gj2 | W Homo | W Homo | W Homo | W Homo | W Homo | WD Hetero | W Homo | W Homo | W Homo | W Homo | W Homo | |
| 223 | Male | Gj2 | WD Hetero | WD Hetero | W Homo | WD Hetero | W Homo | WD Hetero | WD Hetero | WD Hetero | W Homo | D Homo | W Homo | |
| 280 | Female | Gj2 | W Homo | WD Hetero | W Homo | WD Hetero | W Homo | W Homo | W Homo | W Homo | W Homo | WD Hetero | W Homo | |
| 287 | Female | Gj2 | WD Hetero | W Homo | W Homo | W Homo | WD Hetero | W Homo | WD Hetero | W Homo | W Homo | D Homo | W Homo | |
| 319 | Male | Gj2 | W Homo | WD Hetero | W Homo | WD Hetero | WD Hetero | W Homo | W Homo | W Homo | W Homo | D Homo | W Homo | |
| 330 | Female | Gj2 | W Homo | D Homo | I Homo | W Homo | W Homo | WD Hetero | D Homo | D Homo | W Homo | W Homo | W Homo | |
| 331 | Female | Gj2 | W Homo | WD Hetero | I Homo | W Homo | W Homo | WD Hetero | W Homo | W Homo | W Homo | W Homo | W Homo | |
| 396 | Female | Gj2 | W Homo | WD Hetero | I Homo | W Homo | WD Hetero | WD Hetero | W Homo | D Homo | W Homo | D Homo | W Homo | |
| 397 | Female | Gj2 | W Homo | W Homo | W Homo | W Homo | W Homo | D Homo | WD Hetero | W Homo | W Homo | W Homo | W Homo | |
| Tokachi (13) | 63 | Female | Gj2 | W Homo | WD Hetero | W Homo | WD Hetero | W Homo | W Homo | WD Hetero | W Homo | W Homo | WD Hetero | W Homo |
| 71 | Female | Gj2 | W Homo | D Homo | I Homo | W Homo | WD Hetero | W Homo | WD Hetero | W Homo | W Homo | W Homo | W Homo | |
| 78 | Male | Gj2 | W Homo | W Homo | WI Hetero | W Homo | W Homo | W Homo | WD Hetero | W Homo | W Homo | W Homo | D Homo | |
| 109 | Female | Gj2 | W Homo | WD Hetero | W Homo | W Homo | WD Hetero | W Homo | WD Hetero | W Homo | WD Hetero | WD Hetero | W Homo | |
| 131 | Female | Gj2 | WD Hetero | WD Hetero | I Homo | W Homo | W Homo | W Homo | W Homo | W Homo | W Homo | W Homo | W Homo | |
| 187 | Male | Gj2 | W Homo | W Homo | WI Hetero | W Homo | W Homo | W Homo | W Homo | W Homo | W Homo | W Homo | W Homo | |
| 201 | Male | Gj2 | WD Hetero | D Homo | W Homo | W Homo | W Homo | WD Hetero | W Homo | W Homo | WD Hetero | WD Hetero | W Homo | |
| 207 | Male | Gj2 | W Homo | WD Hetero | I Homo | W Homo | WD Hetero | WD Hetero | W Homo | W Homo | W Homo | WD Heter | W Homo | |
| 213 | Female | Gj2 | W Homo | W Homo | W Homo | W Homo | W Homo | W Homo | WD Hetero | W Homo | W Homo | WD Heter | W Homo | |
| 254 | Female | Gj2 | WD Hetero | WD Hetero | W Homo | W Homo | WD Hetero | W Homo | W Homo | D Homo | WD Hetero | W Homo | WD Hetero | |
| 256 | Female | Gj2 | W Homo | W Homo | WI Hetero | W Homo | W Homo | W Homo | W Homo | D Homo | W Homo | W Homo | WD Hetero | |
| 371 | Male | Gj1 | WD Hetero | WD Hetero | W Homo | W Homo | WD Hetero | W Homo | W Homo | W Homo | WD Hetero | WD Heter | W Homo | |
| 373 | Female | Gj1 | WD Hetero | W Homo | W Homo | W Homo | W Homo | WD Hetero | WD Hetero | W Homo | WD Hetero | WD Heter | W Homo | |
| N | Ho | He | Fis | P | |
|---|---|---|---|---|---|
| Tokachi | 13 | 0.301 | 0.322 | 0.069 | 0.423 |
| Kushiro | 13 | 0.266 | 0.296 | 0.108 | 0.255 |
| Nemuro | 13 | 0.294 | 0.320 | 0.085 | 0.415 |
| Total | 39 | 0.287 | 0.316 | 0.095 | 0.075 |
| Pop1 | Pop2 | FST | P |
|---|---|---|---|
| Tokachi | Kushiro | −0.006 | 0.566 |
| Tokachi | Nemuro | 0.033 | 0.050 |
| Kushiro | Nemuro | 0.009 | 0.289 |
| InDel Marker | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Banding No. | Relationship | Sex | Haplotype | Id-01 | Id-02 | Id-03 | Id-04 | Id-05 | Id-06 | Id-07 | Id-08 | Id-9 | Id-10 | Id-11 |
| 207 | Father | Male | Gj2 | W Homo | WD Hetero | I Homo | W Homo | WD Hetero | WD Hetero | W Homo | W Homo | W Homo | WD Hetero | W Homo |
| 352 | Sibling of 207 | Male | Gj2 | W Homo | WD Hetero | I Homo | W Homo | WD Hetero | WD Hetero | W Homo | W Homo | W Homo | W Homo | W Homo |
| 387 | Ditto | Female | Gj2 | W Homo | W Homo | I Homo | W Homo | WD Hetero | W Homo | W Homo | W Homo | W Homo | WD Hetero | WD Hetero |
| 388 | Ditto | Female | Gj2 | W Homo | WD Hetero | I Homo | W Homo | W Homo | WD Hetero | W Homo | W Homo | W Homo | W Homo | W Homo |
| 213 | Mother | Female | Gj2 | W Homo | W Homo | W Homo | W Homo | W Homo | W Homo | WD Hetero | W Homo | W Homo | WD Hetero | W Homo |
| 374 | Sibling of 213 | Female | Gj2 | W Homo | W Homo | W Homo | W Homo | WD Hetero | W Homo | W Homo | W Homo | W Homo | WD Hetero | W Homo |
| T73/R568 | Father of 116-390 | Male | Gj2 | W Homo | WD Hetero | WI Hetero | W Homo | WD Hetero | W Homo | WD Hetero | WD Hetero | W Homo | W Homo | W Homo |
| 116 | Mother of 358, 359 | Female | Gj2 | WD Hetero | W Homo | W Homo | W Homo | D Homo | W Homo | W Homo | D Homo | W Homo | WD Hetero | WD Hetero |
| 204 | Sibling of T73 | Female | Gj2 | WD Hetero | WD Hetero | I Homo | W Homo | W Homo | W Homo | WD Hetero | W Homo | W Homo | W Homo | WD Hetero |
| 261 | Ditto | Female | Gj2 | WD Hetero | W Homo | W Homo | W Homo | WD Hetero | W Homo | WD Hetero | D Homo | W Homo | W Homo | W Homo |
| 312 | Ditto | Female | Gj2 | WD Hetero | W Homo | W Homo | W Homo | WD Hetero | W Homo | WD Hetero | D Homo | W Homo | WD Hetero | WD Hetero |
| 313 | Ditto | Female | Gj2 | WD Hetero | WD Hetero | W Homo | W Homo | WD Hetero | W Homo | WD Hetero | W Homo | W Homo | W Homo | W Homo |
| 353 | Ditto | Male | Gj2 | WD Hetero | WD Hetero | I Homo | W Homo | WD Hetero | W Homo | WD Hetero | WD Hetero | W Homo | W Homo | W Homo |
| 390 | Ditto | Female | Gj2 | WD Hetero | WD Hetero | W Homo | W Homo | D Homo | W Homo | W Homo | D Homo | W Homo | WD Hetero | W Homo |
| 253 | Grandsibling of T73 | Male | Gj2 | WD Hetero | W Homo | WI Hetero | W Homo | WD Hetero | W Homo | D Homo | WD Hetero | W Homo | W Hetero | W Homo |
| 384 | Ditto | Female | Gj2 | WD Hetero | W Homo | W Homo | W Homo | WD Hetero | W Homo | D Homo | W Homo | W Homo | W Hetero | W Homo |
| 358 | Ditto | Male | Gj2 | W Homo | W Homo | W Homo | W Homo | WD Hetero | W Homo | W Homo | WD Hetero | W Homo | WD Hetero | WD Hetero |
| 359 | Ditto | Female | Gj2 | W Homo | W Homo | W Homo | W Homo | WD Hetero | W Homo | W Homo | W Homo | W Homo | WD Hetero | WD Hetero |
| 123 | Father | Male | Gj1 | W Homo | W Homo | W Homo | W Homo | WD Hetero | W Homo | W Homo | W Homo | W Homo | W Homo | W Homo |
| 131 | Mother | Female | Gj2 | WD Hetero | WD Hetero | I Homo | W Homo | W Homo | W Homo | W Homo | W Homo | W Homo | W Homo | W Homo |
| 244 | Sibling of 123 and 131 | Male | Gj2 | W Homo | W Homo | WI Hetero | W Homo | WD Hetero | W Homo | W Homo | W Homo | W Homo | W Homo | W Homo |
| 245 | Twins of 244 | Female | Gj2 | W Homo | WD Hetero | W Homo | W Homo | WD Hetero | W Homo | W Homo | W Homo | W Homo | W Homo | W Homo |
| 211 | Father | Male | Gj2 | W Homo | W Homo | W Homo | W Homo | W Homo | WD Hetero | W Homo | WD Hetero | W Homo | W Homo | W Homo |
| 362 | Sibling of 211 | Male | Gj1 | W Homo | W Homo | WI Hetero | W Homo | W Homo | D Homo | W Homo | WD Hetero | W Homo | WD Hetero | W Homo |
| 315 | Twins of 316 | Female | Gj2 | WD Hetero | WD Hetero | I Homo | WD Hetero | WD Hetero | W Homo | D Homo | W Homo | WD Hetero | W Homo | WD Hetero |
| 316 | Twins of 315 | Male | Gj2 | W Homo | W Homo | WI Hetero | WD Hetero | W Homo | W Homo | WD Hetero | WD Hetero | W Homo | W Homo | WD Hetero |
| 201 | Father | Male | Gj2 | WD Hetero | D Homo | W Homo | W Homo | W Homo | WD Hetero | W Homo | W Homo | WD Hetero | WD Hetero | W Homo |
| 375 | Sibling of 201 | Female | Gj2 | W Homo | WD Hetero | W Homo | W Homo | W Homo | WD Hetero | W Homo | W Homo | W Homo | WD Hetero | W Homo |
| 63 | Mother | Female | Gj2 | W Homo | WD Hetero | W Homo | WD Hetero | W Homo | W Homo | WD Hetero | W Homo | W Homo | WD Hetero | W Homo |
| 169 | Sibling of 63 | Male | Gj2 | W Homo | WD Hetero | W Homo | W Homo | W Homo | WD Hetero | WD Hetero | W Homo | WD Hetero | WD Hetero | W Homo |
| 109 | Mother | Female | Gj2 | W Homo | WD Hetero | W Homo | W Homo | WD Hetero | W Homo | WD Hetero | W Homo | WD Hetero | WD Hetero | W Homo |
| 392 | Sibling of 109 | Female | Gj2 | W Homo | WD Hetero | I Homo | W Homo | D Homo | WD Hetero | WD Hetero | W Homo | W Homo | W Homo | W Homo |
| T74/R566 | Father | Male | - | WD Hetero | WD Hetero | W Homo | W Homo | W Homo | W Homo | W Homo | W Homo | WD Hetero | WD Hetero | W Homo |
| 124 | Sibling of T74 | Female | Gj1 | W Homo | D Homo | W Homo | W Homo | WD Hetero | W Homo | W Homo | W Homo | W Homo | WD Hetero | W Homo |
| 125 | Ditto | Male | Gj1 | WD Hetero | WD Hetero | W Homo | W Homo | WD Hetero | W Homo | W Homo | W Homo | WD Hetero | W Homo | W Homo |
| 251 | Mother | Female | Gj2 | W Homo | W Homo | W Homo | W Homo | W Homo | W Homo | WD Hetero | W Homo | W Homo | W Homo | W Homo |
| 415 | Sibling of 251 | Male | Gj2 | W Homo | W Homo | WI Hetero | W Homo | WD Hetero | WD Hetero | WD Hetero | W Homo | WD Hetero | W Homo | W Homo |
| 271 | Father | Male | Gj2 | W Homo | W Homo | W Homo | W Homo | W Homo | W Homo | W Homo | WD Hetero | WD Hetero | W Homo | W Homo |
| 420 | Sibling of 271 | Female | Gj1 | W Homo | W Homo | W Homo | W Homo | W Homo | W Homo | W Homo | W Homo | WD Hetero | W Homo | W Homo |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawasaki, E.; Wenjing, D.; Sawada, A.; Nakajima, M.; Momose, K.; Yoshino, T.; Amano, T.; Endoh, D.; Nakajima, N.; Teraoka, H. Conventional Gel Electrophoresis-Resolvable Insertion/Deletion Markers for Individual Identification and Analysis of Population Genetics in Red-Crowned Cranes in Eastern Hokkaido, Japan. Animals 2022, 12, 2293. https://doi.org/10.3390/ani12172293
Kawasaki E, Wenjing D, Sawada A, Nakajima M, Momose K, Yoshino T, Amano T, Endoh D, Nakajima N, Teraoka H. Conventional Gel Electrophoresis-Resolvable Insertion/Deletion Markers for Individual Identification and Analysis of Population Genetics in Red-Crowned Cranes in Eastern Hokkaido, Japan. Animals. 2022; 12(17):2293. https://doi.org/10.3390/ani12172293
Chicago/Turabian StyleKawasaki, Erika, Dong Wenjing, Akira Sawada, Momoko Nakajima, Kunikazu Momose, Tomoo Yoshino, Tomoko Amano, Daiji Endoh, Nobuyoshi Nakajima, and Hiroki Teraoka. 2022. "Conventional Gel Electrophoresis-Resolvable Insertion/Deletion Markers for Individual Identification and Analysis of Population Genetics in Red-Crowned Cranes in Eastern Hokkaido, Japan" Animals 12, no. 17: 2293. https://doi.org/10.3390/ani12172293
APA StyleKawasaki, E., Wenjing, D., Sawada, A., Nakajima, M., Momose, K., Yoshino, T., Amano, T., Endoh, D., Nakajima, N., & Teraoka, H. (2022). Conventional Gel Electrophoresis-Resolvable Insertion/Deletion Markers for Individual Identification and Analysis of Population Genetics in Red-Crowned Cranes in Eastern Hokkaido, Japan. Animals, 12(17), 2293. https://doi.org/10.3390/ani12172293






