1. Introduction
Nowadays, donkeys are of high interest, both for clinicians and owners, due to their inner working abilities and as companion animals. Thus, in recent years, people’s awareness of the well-being and care of these animals has continued to increase, and the demand for professional veterinary services has increased. According to reports, there are many differences between donkeys and horses. Therefore, clinical data, treatment and diagnostic protocols from horse to donkey may lead to misdiagnosis and unnecessary or inadequate treatment [
1]. It was found that cardiovascular disease is underreported in donkeys, possibly related to their limited athletic posture and frequent poor performance-related examinations. Reports on treatments for cardiovascular disease are anecdotal in donkeys [
2]. Normal echocardiographic parameters have also been reported in healthy donkeys [
3]. Amory et al. [
4] described normal values of echocardiographic dimensions and functional indexes, as well as quantitative reference values for Doppler flow in healthy donkeys. However, those references must be taken with restraint because marked variations can be seen depending on the breed, body size, age, growth rate and training of the animal.
Pulsed wave (PW) Doppler uses short ultrasound bursts, which are transmitted to a point (designated as “sample volume”) distant from the transducer [
5]. PW Doppler allows the calculation of blood flow velocity, direction and spectral characteristics from a specified point in the heart or blood vessel, but the measurement of the maximum velocity is limited as the pulse repetition frequency is limited [
6]. Consequently, it is used for the evaluation of hemodynamic abnormalities in the heart, myocardial and pericardial disorders, transvalvular gradients, intracardiac pressures and shunts, diastolic and systolic cardiac performance and severity of valvular lesions. In humans, assessment of ventricular function can distinguish patients with normal LV function and LV dysfunction and provide knowledge about the hemodynamic alterations as a side effect of the use of therapeutic agents [
7,
8]. Blood flow velocity patterns are altered in human patients with cardiac dysfunction and valvular regurgitation [
9]. Mitral and aortic valve regurgitation can be assessed by regurgitant volume or volumetric volume [
10,
11].
In humans and horses, the velocity time integral (VTI) is a hemodynamic echocardiographic parameter measured from the Doppler spectrum across the valves through the left ventricular outflow tract (LVOT) [
12]. The area under the flow velocity curve represents the distance of the blood volume passing through the valve [
13]. Doppler echocardiography has been used in the assessment of valvular regurgitation in horses with cardiac murmurs [
14,
15,
16]. Moreover, peak blood flow velocity in the mitral valve has been assessed in healthy warm blood horses using PW Doppler echocardiography [
17]. Furthermore, Blissitt and Bonagura [
18] measured peak velocity and deceleration time of mitral inflow E-wave and peak velocity, peak acceleration, acceleration time and VTI of aortic outflow using Doppler echocardiography in thoroughbred and thoroughbred cross horses.
In horses, the pressure gradient, which was calculated using a simplified Bernoulli equation, is applied to insufficient valves, ventricular and atrial septal defects, and intracardiac pressure determination [
19]. The pressure half time (PHT) is the time interval in milliseconds between the maximal mitral gradient in early diastole and the time point where the gradient is half the maximum initial value [
20]. It is a simple Doppler method used for assessing the MVA, severity of aortic regurgitation and pressure deceleration [
21].
Till now, echocardiographic parameters, including pulsatility index (PI), resistance index (RI) and myocardial performance index (MPI), which are used in human studies to evaluate the cardiac performance, was not recorded in horses with cardiac disorders [
22,
23]. PI and RI are useful in the measurement of blood flow resistance [
24]. The myocardial performance index is an easily measured index used for the assessment of global heart function, combining both systolic and diastolic cardiac performance [
22].
Data regarding reference values of Doppler echocardiographic parameters in healthy donkeys are scarce. Consequently, the current study was conducted to determine the reference values and repeatability for PW Doppler echocardiographic variables of the mitral valve, aortic valve and myocardial performance in normal donkeys.
4. Discussion
PW Doppler is used in combination with the 2D image to assess flow velocities within discrete regions of the heart and great vessels, which are used to evaluate the cardiac performance [
21]. In the current study, E-wave was higher than A-wave for the mitral inflow during filling of the left ventricle in healthy donkeys. The same results were recorded in normal thoroughbred and thoroughbred cross horses [
18], normal dogs [
34] and normal human subjects [
35].
PW Doppler evaluates the mitral velocity, which provides intuition into the dynamics of LV filling and helps to evaluate the diastolic function [
36]. Furthermore, the evaluation of transmitral velocity, together with tricuspid and hepatic vein velocities, is useful when evaluating cardiac tamponade and constrictive pericarditis [
37].
The ventricular filling results from isovolumic relaxation, ventricular compliance, filling pressures from the left atrium to left ventricle, pericardial restraint, ventricular interaction and atrial function, and it may be influenced by afterload and contractility [
7]. Consequently, changes in the ventricular relaxation affect early filling of the left ventricular chamber, while changes in ventricular compliance affect late diastolic filling of the ventricle with a resultant increase of E-wave [
38]. Moreover, E-wave velocity is increased with increased left atrial pressure, decreased left ventricular pressure associating the increased rate of ventricular relaxation, and decreased MVA [
39]. Meanwhile, the early ventricular filling is decreased in cases of decreased atrial pressure, decreased rate of ventricular relaxation, increased ventricular compliance and increased MVA with a subsequent decrease of transmitral E-wave amplitude, which resulted in increased A-wave velocity as late diastole contributes more to total left ventricular filling [
39]. Thus, changes in the velocity occur with alterations in the left atrial and left ventricular diastolic pressures [
40].
The pressure gradient of the E-wave is higher than the A-wave of the mitral inflow during filling of the left ventricle in healthy donkeys. In equine, the pressure gradient is a reflection of normal intra-cardiac pressure and pathological increased pressure [
41]. The pressure gradient quantifies the severity of stenotic lesions and can differentiate the unknown pressure from the known pressure. The pressure gradient will be increased in the presence of conduct-type lesions as tunnel sub-valvular stenosis and in cases of decreased blood viscosity. In contrast, the increased blood viscosity may underestimate the pressure gradient [
42].
In the current study, the velocity of the aortic outflow is 64.9 ± 10.4 during the first third of systole in healthy donkeys. The aortic flow pattern is found in normal thoroughbred and thoroughbred cross horses (0.937 ± 0.094) [
18], in normal adult Turkmen horses (101.948 ± 15.341) [
43], in clinically normal dogs (106.0 ± 21.0) [
34] and in human (92 ± 11) [
44]. This is probably due to differences in alignment with aortic flow in donkeys. However, this may represent a species variation in actual flow velocities.
The flow velocity of aortic flow is affected by heart rate. A faster heart rate will increase peak and mean velocity [
42]. The velocity of blood flow depends on the blood volume moving through the vessels or orifice. When there is a high blood flow [
18], severe valvular insufficiency or stenosis, a coexisting shunt, anemia and/or sepsis [
45], the pressure gradient will be inaccurate.
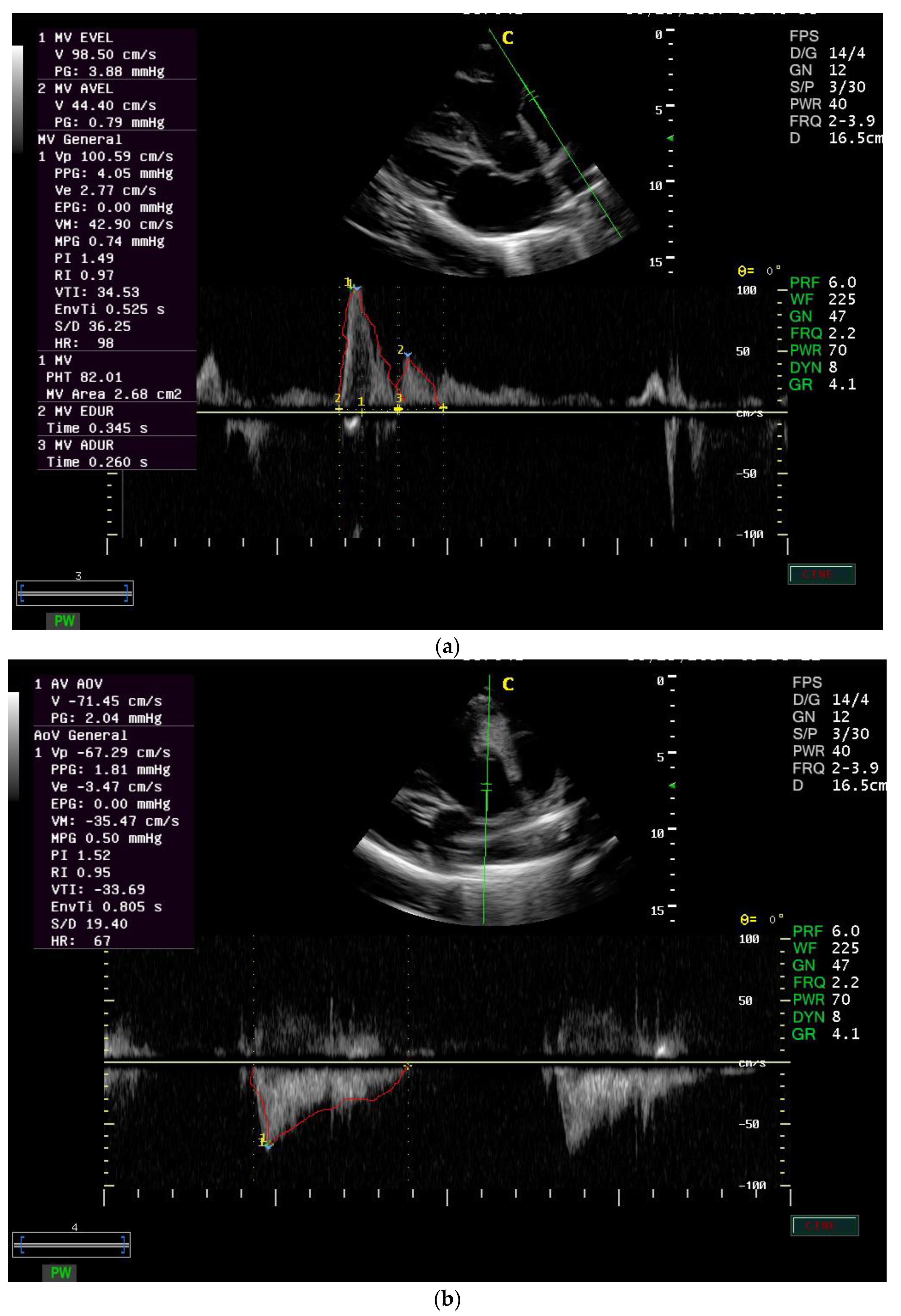
In the current study, the MVA equals 2.4 ± 1.5 cm
2, and the PHT equals 135 ± 93.9 ms in healthy donkeys. However, in humans, the MVA is found to be 4.0–6.0, and the PHT is 40–70 ms [
20].
The Doppler study can be used to calculate pressure half-time (PHT), which is defined as the time required for the pressure gradient across an obstruction to decrease to half of its maximal value. Thus, PHT increases as the severity of stenosis increases.
Overestimation of the MVA occurs when PHT is shortened by concomitant significant aortic insufficiency, decreased ventricular compliance and atrial septal defect [
21]. PHT is useful in the detection of mitral stenosis with coexistent mitral regurgitation [
21].
In patients with aortic regurgitation, the Doppler velocity becomes significantly shorter <250 ms because of the rapid increase in left ventricular diastolic pressure and decrease in aortic pressure. Furthermore, it may be affected by severe diastolic dysfunction with marked elevation of left ventricular diastolic pressure without severe aortic regurgitation [
30].
In the current study, PI was 1.4 ± 0.4, RI was 0.9 ± 0.03 for the mitral valve, the PI was 1.4 ± 0.3 and RI was 0.9 ± 0.02 for the aortic valve in healthy donkeys. Normal values for the PI and RI are 1.36–1.56 and 0.6–0.8, respectively.
PI is equal to the difference between the peak systolic velocity and the minimum diastolic velocity divided by the mean velocity during the cardiac cycle. The value of PI decreases with distance from the heart [
46]. The two indices, pulsatility index and resistance index, measure the resistance of blood flow. Furthermore, they are affected by input pressure waveform pulsatility, impedance and resistance changes [
24].
In the current study, the VTI is 19.1 ± 5.7 cm for mitral inflow and equals 25.02 ± 6.2 for aortic outflow in healthy donkeys. The VTI is found to be 25.369 ± 3.209 cm in normal horses for aortic flow [
18], 0.146 ± 0.029 cm in dog for aortic outflow [
34] and 25.1 ± 3.4 cm in humans for aortic flow [
47]. Left ventricular outflow tract velocity time integral (LVOT VTI) is a measure of cardiac systolic function and cardiac output. Heart failure patients with low cardiac output are known to have poor cardiovascular outcomes. Thus, extremely low LVOT VTI may predict heart failure patients at highest risk for mortality [
12].
In the present study, Myocardial Performance Index (LV)–Tei Index was 1.7 ± 0.7, isovolumic contraction time was 0.3 ± 0.1, isovolumic relaxation time was 0.3 ± 0.1, and ejection time was 0.4 ± 0.1. The MPI was found to be 0.52 ± 0.12 in dog [
48] and in human (0.39 ± 0.05) [
22]. Systolic dysfunction prolongs pre-ejection (ICT) and a shortening of the ejection time (ET). Both systolic and diastolic dysfunction results in abnormality in myocardial relaxation, which prolongs the relaxation period (IRT) [
22].
The Myocardial Performance Index (LV)–Tei Index is independent of heart rate, arterial blood pressure, ventricular geometry, atrioventricular valve regurgitation, loading condition as afterload and preload, and can be used to evaluate the function of both the LV and the RV [
49]. The Myocardial Performance Index (LV)–Tei Index have strong prognostic value in severe cardiac diseases such as cardiac amyloidosis (0.54 ± 0.16), dilated cardiomyopathy (0.59 ± 0.10), ischemic heart diseases (0.85 ± 0.32), pulmonary hypertension (0.93 ± 0.34), congestive heart failure (0.37 ± 0.05), valvular diseases (0.6 ± 0.2), myocardial infarction, congenital heart diseases (0.35 ± 0.03) and cardiotoxicity (0.45 ± 0.06) [
50].