In Vitro Antiparasitic Activities of Monovalent Ionophore Compounds for Human and Canine Leishmaniases
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Cells and Leishmania spp. Promastigote Cultures
2.2. Isolation of Canine Peripheral Blood Mononuclear Cells
2.3. Cytotoxicity and Leishmania Promastigote Viability Assay
2.4. In Vitro Differentiation of Human and Canine Macrophages and Intracellular Amastigote Susceptibility Assays
3. Results
3.1. In Vitro Activity of Ionophores against L. infantum, L. tropica and L. braziliensis Promastigotes
3.2. In Vitro Cytotoxicity of Ionophores against Human Cells and Antiparasitic Activity against Intracellular Amastigotes of L. infantum
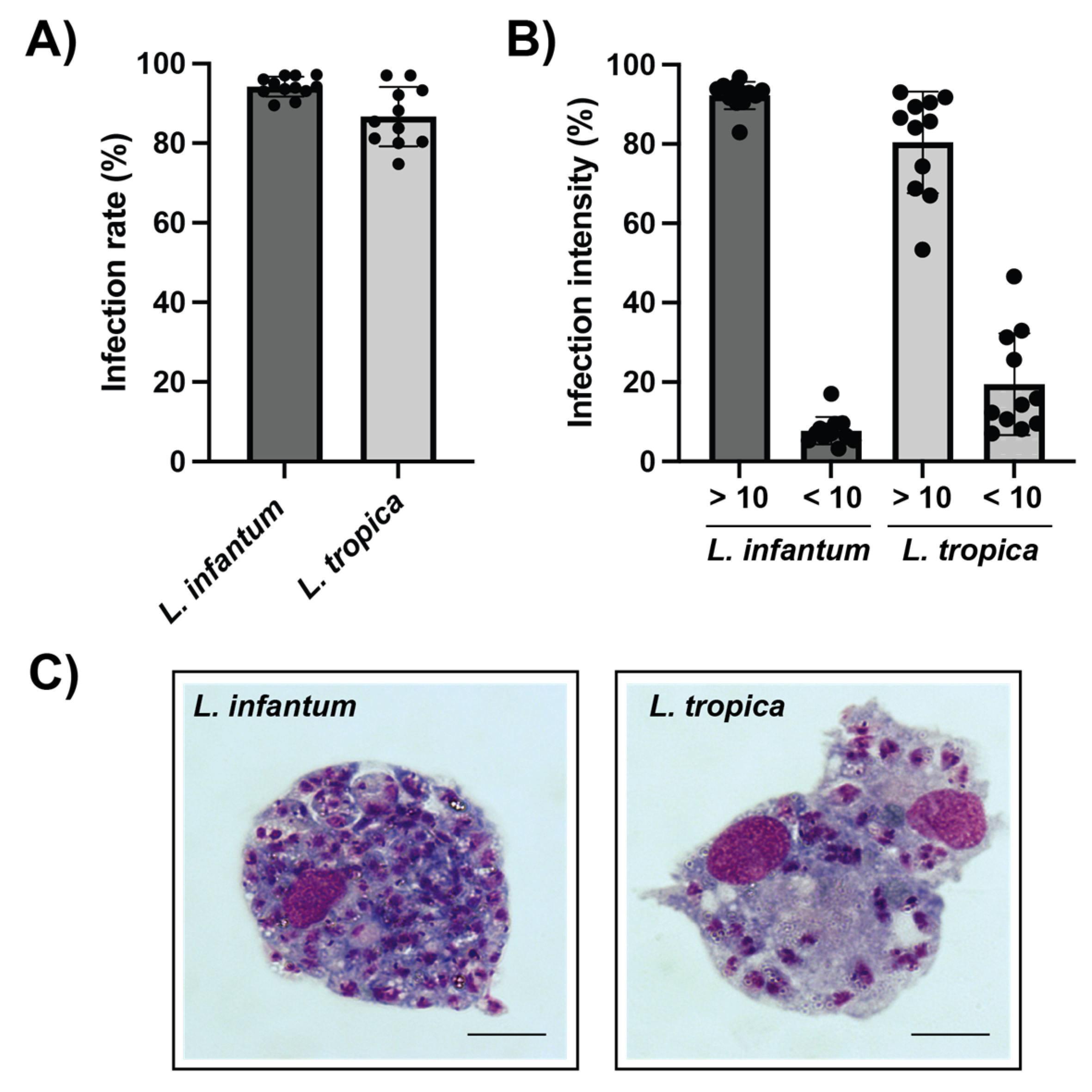
3.3. L. infantum and L. tropica Infection of Canine Macrophages
3.4. Anti-Leishmania In Vitro Activity of Ionophores against Intracellular L. infantum Amastigotes in Canine Macrophages
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Desjeux, P. Leishmaniasis: Current situation and new perspectives. Comp. Immunol. Microbiol. Infect. Dis. 2004, 27, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F.; Miró, G.; Baneth, G.; Bourdeau, P.; Breitschwerdt, E.; Capelli, G.; Cardoso, L.; Day, M.J.; Dobler, G.; Ferrer, L.; et al. Canine Leishmaniasis Control in the Context of One Health. Emerg. Infect. Dis. 2019, 25, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef]
- Ribeiro, R.R.; Michalick, M.S.M.; da Silva, M.E.; dos Santos, C.C.P.; Frézard, F.J.G.; da Silva, S.M. Canine Leishmaniasis: An Overview of the Current Status and Strategies for Control. Biomed. Res. Int. 2018, 2018, 3296893. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Control of the Leishmaniases; Report of a Meeting of the WHO Expert Committee on the Control of Leishmaniases, Geneva, 22–26 March 2010; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- Velez, R.; Gállego, M. Commercially approved vaccines for canine leishmaniosis: A review of available data on their safety and efficacy. Trop. Med. Int. Health 2020, 25, 540–557. [Google Scholar] [CrossRef] [PubMed]
- Ponte-Sucre, A.; Gamarro, F.; Dujardin, J.C.; Barrett, M.P.; López-Vélez, R.; García-Hernández, R.; Pountain, A.W.; Mwenechanya, R.; Papadopoulou, B. Drug resistance and treatment failure in leishmaniasis: A 21st century challenge. PLoS Negl. Trop. Dis. 2017, 11, e0006052. [Google Scholar] [CrossRef]
- Gonçalves, A.A.M.; Leite, J.C.; Resende, L.A.; Mariano, R.M.D.S.; Silveira, P.; Melo-Júnior, O.A.O.; Ribeiro, H.S.; de Oliveira, D.S.; Soares, D.F.; Santos, T.A.P.; et al. An Overview of Immunotherapeutic Approaches Against Canine Visceral Leishmaniasis: What Has Been Tested on Dogs and a New Perspective on Improving Treatment Efficacy. Front. Cell Infect. Microbiol. 2019, 9, 427. [Google Scholar] [CrossRef]
- Klug, D.M.; Gelb, M.H.; Pollastri, M.P. Repurposing strategies for tropical disease drug discovery. Bioorg. Med. Chem. Lett. 2016, 26, 2569–2576. [Google Scholar] [CrossRef]
- Kevin Ii, D.A.; Meujo, D.A.; Hamann, M.T. Polyether ionophores: Broad-spectrum and promising biologically active molecules for the control of drug-resistant bacteria and parasites. Expert Opin. Drug Discov. 2009, 4, 109–146. [Google Scholar] [CrossRef]
- Mitani, M.; Yamanishi, T.; Miyazaki, Y.; Otake, N. Salinomycin effects on mitochondrial ion translocation and respiration. Antimicrob. Agents Chemother. 1976, 9, 655–660. [Google Scholar] [CrossRef]
- Boesch, M.; Sopper, S.; Wolf, D. Ionophore Antibiotics as Cancer Stem Cell-Selective Drugs: Open Questions. Oncologist 2016, 21, 1291–1293. [Google Scholar] [CrossRef] [PubMed]
- Huczyński, A. Polyether ionophores-promising bioactive molecules for cancer therapy. Bioorg. Med. Chem. Lett. 2012, 22, 7002–7010. [Google Scholar] [CrossRef] [PubMed]
- Antoszczak, M.; Steverding, D.; Huczyński, A. Anti-parasitic activity of polyether ionophores. Eur. J. Med. Chem. 2019, 166, 32–47. [Google Scholar] [CrossRef]
- D’Alessandro, S.; Corbett, Y.; Ilboudo, D.P.; Misiano, P.; Dahiya, N.; Abay, S.M.; Habluetzel, A.; Grande, R.; Gismondo, M.R.; Dechering, K.J.; et al. Salinomycin and Other Ionophores as a New Class of Antimalarial Drugs with Transmission-Blocking Activity. Antimicrob. Agents Chemother. 2015, 59, 5135–5144. [Google Scholar] [CrossRef]
- Luque-Ortega, J.R.; Saugar, J.M.; Chiva, C.; Andreu, D.; Rivas, L. Identification of new leishmanicidal peptide lead structures by automated real-time monitoring of changes in intracellular ATP. Biochem. J. 2003, 375, 221–230. [Google Scholar] [CrossRef]
- Osorio, Y.; Travi, B.L.; Renslo, A.R.; Peniche, A.G.; Melby, P.C. Identification of small molecule lead compounds for visceral leishmaniasis using a novel ex vivo splenic explant model system. PLoS Negl. Trop. Dis. 2011, 5, e962. [Google Scholar] [CrossRef][Green Version]
- Peniche, A.G.; Osorio, Y.; Renslo, A.R.; Frantz, D.E.; Melby, P.C.; Travi, B.L. Development of an ex vivo lymph node explant model for identification of novel molecules active against Leishmania major. Antimicrob. Agents Chemother. 2014, 58, 78–87. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bassanini, I.; Parapini, S.; Ferrandi, E.E.; Gabriele, E.; Basilico, N.; Taramelli, D.; Sparatore, A. Design, Synthesis and In Vitro Investigation of Novel Basic Celastrol Carboxamides as Bio-Inspired Leishmanicidal Agents Endowed with Inhibitory Activity against. Biomolecules 2021, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Goto-Koshino, Y.; Ohno, K.; Nakajima, M.; Mochizuki, H.; Kanemoto, H.; Tsujimoto, H. A rapid and simple method to obtain canine peripheral blood-derived macrophages. J. Vet. Med. Sci. 2011, 73, 773–778. [Google Scholar] [CrossRef]
- Sundar, S.; Singh, A. Chemotherapeutics of visceral leishmaniasis: Present and future developments. Parasitology 2018, 145, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, J.; Brzezinski, B. Structures and properties of naturally occurring polyether antibiotics. Biomed. Res. Int. 2013, 2013, 162513. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; de Oliveira, D.M.P.; Walker, M.J. The antimicrobial and immunomodulatory effects of Ionophores for the treatment of human infection. J. Inorg. Biochem. 2022, 227, 111661. [Google Scholar] [CrossRef] [PubMed]
- Escobar, P.; Matu, S.; Marques, C.; Croft, S.L. Sensitivities of Leishmania species to hexadecylphosphocholine (miltefosine), ET-18-OCH(3) (edelfosine) and amphotericin B. Acta Trop. 2002, 81, 151–157. [Google Scholar] [CrossRef]
- Croft, S.L.; Yardley, V.; Kendrick, H. Drug sensitivity of Leishmania species: Some unresolved problems. Trans. R Soc. Trop. Med. Hyg. 2002, 96 (Suppl. S1), S127–S129. [Google Scholar] [CrossRef]
- Steverding, D.; Sexton, D. Trypanocidal activity of salinomycin is due to sodium influx followed by cell swelling. Parasites Vectors 2013, 6, 78. [Google Scholar] [CrossRef]
- Rajendran, V.; Ilamathi, H.S.; Dutt, S.; Lakshminarayana, T.S.; Ghosh, P.C. Chemotherapeutic Potential of Monensin as an Anti-microbial Agent. Curr. Top. Med. Chem. 2018, 18, 1976–1986. [Google Scholar] [CrossRef]
- Maia, C.; Rolão, N.; Nunes, M.; Gonçalves, L.; Campino, L. Infectivity of five different types of macrophages by Leishmania infantum. Acta Trop. 2007, 103, 150–155. [Google Scholar] [CrossRef]
- Ueno, N.; Wilson, M.E. Receptor-mediated phagocytosis of Leishmania: Implications for intracellular survival. Trends Parasitol. 2012, 28, 335–344. [Google Scholar] [CrossRef]
- Forrester, M.A.; Wassall, H.J.; Hall, L.S.; Cao, H.; Wilson, H.M.; Barker, R.N.; Vickers, M.A. Similarities and differences in surface receptor expression by THP-1 monocytes and differentiated macrophages polarized using seven different conditioning regimens. Cell Immunol. 2018, 332, 58–76. [Google Scholar] [CrossRef]
- Herrmann, I.; Gotovina, J.; Fazekas-Singer, J.; Fischer, M.B.; Hufnagl, K.; Bianchini, R.; Jensen-Jarolim, E. Canine macrophages can like human macrophages be in vitro activated toward the M2a subtype relevant in allergy. Dev. Comp. Immunol. 2018, 82, 118–127. [Google Scholar] [CrossRef]
- Kart, A.; Bilgil, A. Ionophore Antibiotics: Toxicity, Mode of Action and Neurotoxic Aspect of Carboxylic Ionophores. J. Anim. Vet. Adv. 2008, 7, 748–751. [Google Scholar]
- Oehme, F.W.; Pickrell, J.A. An analysis of the chronic oral toxicity of polyether ionophore antibiotics in animals. Vet. Hum. Toxicol. 1999, 41, 251–257. [Google Scholar] [PubMed]
- Novilla, M.N. The veterinary importance of the toxic syndrome induced by ionophores. Vet. Hum. Toxicol. 1992, 34, 66–70. [Google Scholar] [PubMed]
- Miró, G.; López-Vélez, R. Clinical management of canine leishmaniosis versus human leishmaniasis due to Leishmania infantum: Putting “One Health” principles into practice. Vet. Parasitol. 2018, 254, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Digby, Z.; Tourlomousis, P.; Rooney, J.; Boyle, J.P.; Bibo-Verdugo, B.; Pickering, R.J.; Webster, S.J.; Monie, T.P.; Hopkins, L.J.; Kayagaki, N.; et al. Evolutionary loss of inflammasomes in the Carnivora and implications for the carriage of zoonotic infections. Cell Rep. 2021, 36, 109614. [Google Scholar] [CrossRef]
- Qi, D.; Liu, Y.; Li, J.; Huang, J.H.; Hu, X.; Wu, E. Salinomycin as a potent anticancer stem cell agent: State of the art and future directions. Med. Res. Rev. 2022, 42, 1037–1063. [Google Scholar] [CrossRef]
- Solano-Gálvez, S.G.; Álvarez-Hernández, D.A.; Gutiérrez-Kobeh, L.; Vázquez-López, R. Leishmania: Manipulation of signaling pathways to inhibit host cell apoptosis. Ther. Adv. Infect. Dis. 2021, 8, 20499361211014977. [Google Scholar] [CrossRef]
- Olivier, M.; Gregory, D.J.; Forget, G. Subversion mechanisms by which Leishmania parasites can escape the host immune response: A signaling point of view. Clin. Microbiol. Rev. 2005, 18, 293–305. [Google Scholar] [CrossRef]



| IC50 (µM ± SD) | |||
|---|---|---|---|
| L. infantum | L. braziliensis | L. tropica | |
| Salinomycin | 7.98 ± 0.64 | 5.08 ± 1.74 | 4.53 ± 0.60 |
| Monensin | 0.63 ± 0.11 | 0.27 ± 0.13 | 0.35 ± 0.17 |
| Nigericin | 0.37 ± 0.04 | 0.78 ± 0.08 | 0.23 ± 0.05 |
| Amphotericin B | 0.12 ± 0.02 | 0.08 ± 0.02 | 0.10 ± 0.02 |
| CC50 (μM ± SD) | ||
|---|---|---|
| dTHP-1 | HDF | |
| Salinomycin | 32.1 ± 5.3 | >60 |
| Monensin | >60 | >60 |
| Nigericin | >60 | >60 |
| Amphotericin B | >20 | ND |
| Amastigotes IC50 (μM ± SD) | SI | |
|---|---|---|
| Salinomycin | 1.93 ± 0.1 | 16.6 |
| Monensin | 1.67 ± 0.43 | >35.9 |
| Nigericin | 1.79 ± 0.83 | >33.5 |
| Amphotericin B | 0.18 ± 0.03 | >111.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvo Alvarez, E.; D’Alessandro, S.; Proverbio, D.; Spada, E.; Perego, R.; Taramelli, D.; Basilico, N.; Parapini, S. In Vitro Antiparasitic Activities of Monovalent Ionophore Compounds for Human and Canine Leishmaniases. Animals 2022, 12, 2337. https://doi.org/10.3390/ani12182337
Calvo Alvarez E, D’Alessandro S, Proverbio D, Spada E, Perego R, Taramelli D, Basilico N, Parapini S. In Vitro Antiparasitic Activities of Monovalent Ionophore Compounds for Human and Canine Leishmaniases. Animals. 2022; 12(18):2337. https://doi.org/10.3390/ani12182337
Chicago/Turabian StyleCalvo Alvarez, Estefanía, Sarah D’Alessandro, Daniela Proverbio, Eva Spada, Roberta Perego, Donatella Taramelli, Nicoletta Basilico, and Silvia Parapini. 2022. "In Vitro Antiparasitic Activities of Monovalent Ionophore Compounds for Human and Canine Leishmaniases" Animals 12, no. 18: 2337. https://doi.org/10.3390/ani12182337
APA StyleCalvo Alvarez, E., D’Alessandro, S., Proverbio, D., Spada, E., Perego, R., Taramelli, D., Basilico, N., & Parapini, S. (2022). In Vitro Antiparasitic Activities of Monovalent Ionophore Compounds for Human and Canine Leishmaniases. Animals, 12(18), 2337. https://doi.org/10.3390/ani12182337









