Retrospective Evaluation of a Combination of Carboplatin and Bleomycin for the Treatment of Canine Carcinomas
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
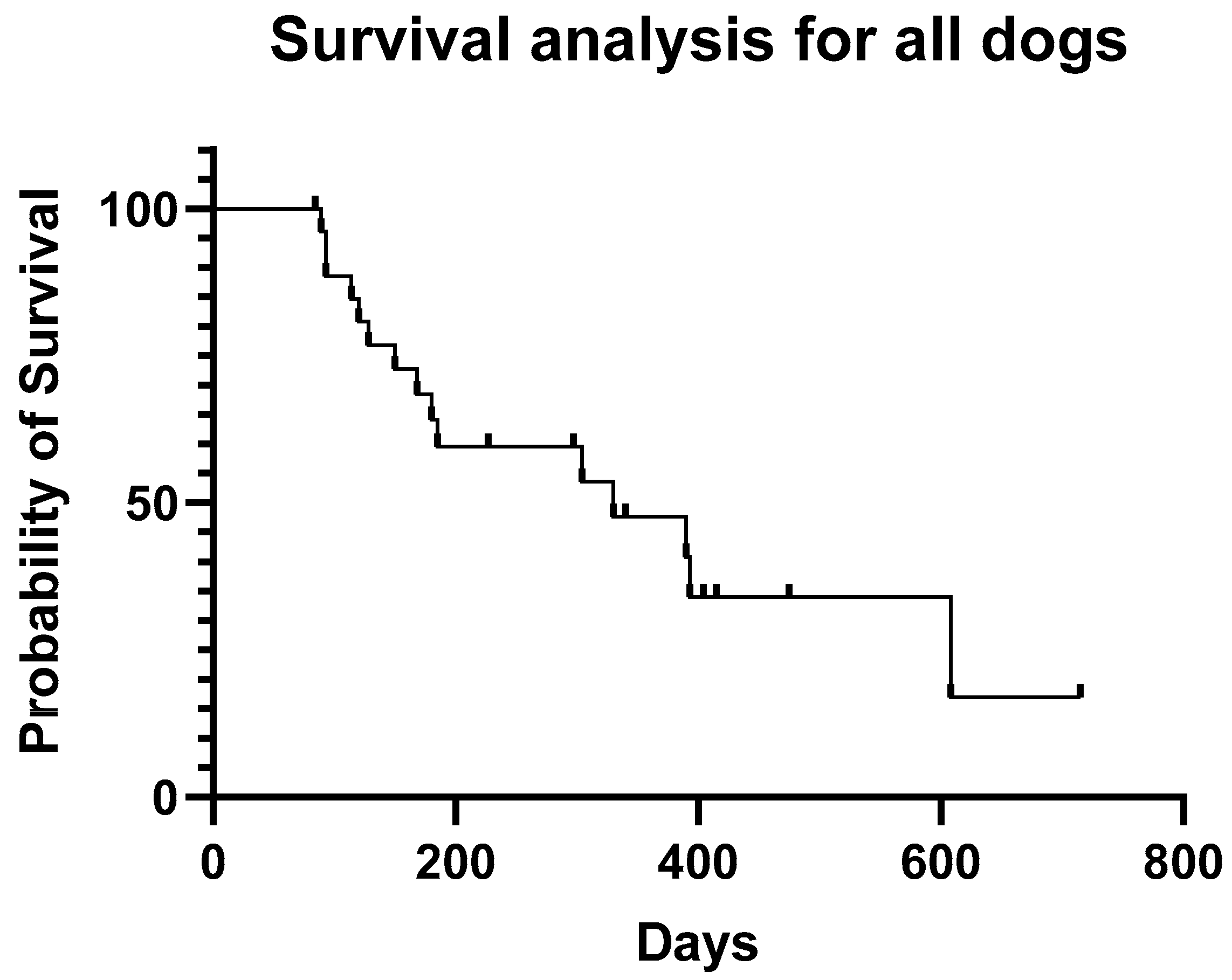
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Umezawa, H.; Ishizuka, M.; Maeda, K.; Takeuchi, T. Studies on bleomycin. Cancer 1967, 20, 891–895. [Google Scholar] [CrossRef]
- Quada, J.C.; Zuber, G.F.; Hecht, S.M. Interaction of bleomycin with DNA. Pure Appl. Chem. 1998, 70, 307–311. [Google Scholar] [CrossRef]
- Hecht, S.M. Bleomycin: New perspectives on the mechanism of action. J. Nat. Prod. 2000, 63, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Murray, V.; Chen, J.K.; Chung, L.H. The interaction of the metallo-glycopeptide anti-tumour drug bleomycin with DNA. Int. J. Mol. Sci. 2018, 19, 1372. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Xia, C.; Krailo, M.; Amatruda, J.F.; Arul, S.G.; Billmire, D.F.; Brady, W.E.; Covens, A.; Gershenson, D.M.; Hale, J.P.; et al. Is carboplatin-based chemotherapy as effective as cisplatin-based chemotherapy in the treatment of advanced-stage dysgerminoma in children, adolescents and young adults? Gynecol. Oncol. 2018, 150, 253–260. [Google Scholar] [CrossRef]
- Einhorn, L.H. Curing metastatic testicular cancer. Proc. Natl. Acad. Sci. USA 2002, 99, 4592–4595. [Google Scholar] [CrossRef]
- Risk, M. Treatment of Disseminated Germ Cell Tumors with Cisplatin, Bleomycin, and Either Vinblastine or Etoposide. In 50 Studies Every Urologist Should Know; Oxford University Press: Oxford, UK, 2021. [Google Scholar]
- Udupa, C.; Koteshwar, P.; Udupa, K. Bleomycin in Hodgkin’s lymphoma—A boon or a bane?—A retrospective study of bleomycin pulmonary toxicity in Hodgkin’s lymphoma. Indian J. Palliat. Care 2019, 25, 523. [Google Scholar] [CrossRef]
- Froudarakis, M.; Hatzimichael, E.; Kyriazopoulou, L.; Lagos, K.; Pappas, P.; Tzakos, A.G.; Karavasilis, V.; Daliani, D.; Papandreou, C.; Briasoulis, E. Revisiting bleomycin from pathophysiology to safe clinical use. Crit. Rev. Oncol. Hematol. 2013, 87, 90–100. [Google Scholar] [CrossRef]
- O’Sullivan, J.M.; Huddart, R.A.; Norman, A.R.; Nicholls, J.; Dearnaley, D.P.; Horwich, A. Predicting the risk of bleomycin lung toxicity in patients with germ-cell tumours. Ann. Oncol. 2003, 14, 91–96. [Google Scholar] [CrossRef]
- Sleijfer, S. Bleomycin-induced pneumonitis. Chest 2001, 120, 617–624. [Google Scholar] [CrossRef] [Green Version]
- Fleischman, R.W.; Baker, J.R.; Thompson, G.R.; Schaeppi, U.H.; Illievski, V.R.; Cooney, D.A.; Davis, R.D. Bleomycin-induced interstitial pneumonia in dogs. Thorax 1971, 26, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.A.D.; Zitnik, R.J.; Matthay, R.A. Mechanisms of drug-induced pulmonary disease. Annu. Rev. Med. 1988, 39, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Saito, F.; Tasaka, S.; Inoue, K.I.; Miyamoto, K.; Nakano, Y.; Ogawa, Y.; Yamada, W.; Shiraishi, Y.; Hasegawa, N.; Fujishima, S.; et al. Role of interleukin-6 in bleomycin-induced lung inflammatory changes in mice. Am. J. Respir. Cell Mol. Biol. 2008, 38, 566–571. [Google Scholar] [CrossRef] [PubMed]
- den Hollander, M.W.; Westerink, N.D.; Lubberts, S.; Bongaerts, A.H.; Wolf, R.F.; Altena, R.; Nuver, J.; Oosting, S.F.; de Vries, E.G.; Walenkamp, A.M.; et al. Bleomycin-Induced Pulmonary Changes on Restaging Computed Tomography Scans in Two Thirds of Testicular Cancer Patients Show No Correlation with Fibrosis Markers. Oncologist 2016, 21, 995–1001. [Google Scholar] [CrossRef]
- Comis, R.L. Bleomycin pulmonary toxicity: Current status and future directions. Semin. Oncol. 1992, 19 (Suppl. 5), 64–70. [Google Scholar]
- Broughton, A.; Strong, J.E.; Holoye, P.Y.; Bedrossian, C.W.M. Clinical pharmacology of bleomycin following intravenous infusion as determined by radioimmunoassay. Cancer 1977, 40, 2772–2778. [Google Scholar] [CrossRef]
- Thompson, G.R.; Baker, J.R.; Fleischman, R.W.; Rosenkrantz, H.; Schaeppi, U.H.; Cooney, D.A.; Davis, R.D. Preclinical toxicologic evaluation of bleomycin (NSC 125 066), a new antitumor antibiotic. Toxicol. Appl. Pharmacol. 1972, 22, 544–555. [Google Scholar] [CrossRef]
- Pron, G.; Mahrour, N.; Orlowski, S.; Tounekti, O.; Poddevin, B.; Belehradek, J.; Mir, L.M. Internalisation of the bleomycin molecules responsible for bleomycin toxicity: A receptor-mediated endocytosis mechanism. Biochem. Pharmacol. 1999, 57, 45–56. [Google Scholar] [CrossRef]
- Poddevin, B.; Orlowski, S.; Belehradek, J.; Mir, L.M. Very high cytotoxicity of bleomycin introduced into the cytosol of cells in culture. Biochem. Pharmacol. 1991, 42 (Suppl. 1), S67–S75. [Google Scholar] [CrossRef]
- Friedman, M.A. A review of the bleomycin experience in the United States. Recent Results Cancer Res. 1978, 63, 152–168. [Google Scholar]
- Valenti, P.; Menicagli, F.; Baldi, A.; Barella, G.; Catalucci, C.; Attorri, V.; Spugnini, E.P. Evaluation of electrochemotherapy in the management of apocrine gland anal sac adenocarcinomas in dogs: A retrospective study. Open Vet. J. 2021, 11, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Simčič, P.; Lowe, R.; Granziera, V.; Pierini, A.; Torrigiani, F.; Lubas, G. Electrochemotherapy in treatment of canine oral non-tonsillar squamous cell carcinoma. A case series report. Vet. Comp. Oncol. 2020, 18, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.M.; Belding, B.A.; Schaefer, A.K. Acanthomatous ameloblastoma in dogs treated with intralesional bleomycin. Vet. Comp. Oncol. 2010, 8, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Batschinski, K.; Dervisis, N.; Kitchell, B.; Newman, R.; Erfourth, T. Combination of bleomycin and cytosine arabinoside chemotherapy for relapsed canine lymphoma. J. Am. Anim. Hosp. Assoc. 2018, 54, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.A.; Lejeune, A.; Kow, K.; Milner, R.J.; Souza, C.H.M. Clinical response and adverse event profile of bleomycin chemotherapy for canine multicentric lymphoma. J. Am. Anim. Hosp. Assoc. 2017, 53, 128–134. [Google Scholar] [CrossRef]
- de Vos, J.P.; Burm, A.G.D.; Focker, B.P.; Boschloo, H.; Karsijns, M. Results of the Combined Treatment with Piroxicam and Carboplatin in Canine Oral Non-Tonsillar Squamous Cell Carcinoma. Vet. Comp. Oncol. 2005, 3, 42–43. [Google Scholar] [CrossRef]
- Phillips, B.; Powers, B.E.; Dernell, W.S.; Straw, R.C.; Khanna, C.; Hogge, G.S.; Vail, D.M. Use of single-agent carboplatin as adjuvant or neoadjuvant therapy in conjunction with amputation for appendicular osteosarcoma in dogs. J. Am. Anim. Hosp. Assoc. 2009, 45, 33–38. [Google Scholar] [CrossRef]
- Chun, R.; Knapp, D.W.; Widmer, W.R.; Del Nicola, D.B.; Glickman, N.W.; Kuczek, T.; Degortari, A.; Han, C.M. Phase II clinical trial of carboplatin in canine transitional cell carcinoma of the urinary bladder. J. Vet. Intern. Med. Am. Coll. Vet. Intern. Med. 1997, 11, 279–283. [Google Scholar] [CrossRef]
- Page, R.L.; McEntee, M.C.; George, S.L.; Williams, P.L.; Heidner, G.L.; Novotney, C.A.; Riviere, J.E.; Dewhirst, M.W.; Thrall, D.E. Pharmacokinetic and Phase I Evaluation of Carboplatin in Dogs. J. Vet. Intern. Med. 1993, 7, 235–240. [Google Scholar] [CrossRef]
- Coffee, C.; Roush, J.K.; Higginbotham, M.L. Carboplatin-induced myelosuppression as related to body weight in dogs. Vet. Comp. Oncol. 2020, 18, 804–810. [Google Scholar] [CrossRef]
- Gaver, R.C.; George, A.M.; Duncan, G.F.; Morris, A.D.; Deeb, G.; Faulkner, H.C.; Farmen, R.H. The disposition of carboplatin in the beagle dog. Cancer Chemother. Pharmacol. 1988, 21, 197–202. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, A.K.; Atherton, M.; Bentley, R.T.; Boudreau, C.E.; Burton, J.H.; Curran, K.M.; Dow, S.; Giuffrida, M.A.; Kellihan, H.B.; Mason, N.J.; et al. Veterinary Cooperative Oncology Group—Common Terminology Criteria for Adverse Events (VCOG-CTCAE v2) following investigational therapy in dogs and cats. Vet. Comp. Oncol. 2021, 19, 311–352. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.M.; Thamm, D.H.; Vail, D.M.; London, C.A. Response evaluation criteria for solid tumours in dogs (v1.0): A Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet. Comp. Oncol. 2015, 13, 176–183. [Google Scholar] [CrossRef]
- Behnia, M.; Foster, R.; Einhorn, L.H.; Donohue, J.; Nichols, C.R. Adjuvant bleomycin, etoposide and cisplatin in pathological stage II non-seminomatous testicular cancer: The Indiana University experience. Eur. J. Cancer 2000, 36, 472–475. [Google Scholar] [CrossRef]
- Loehrer, P.J.; Johnson, D.; Elson, P.; Einhorn, L.H.; Trump, D. Importance of bleomycin in favorable-prognosis disseminated germ cell tumors: An Eastern Cooperative Oncology Group trial. J. Clin. Oncol. 1995, 13, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.T.; Hudson, M.M.; Stokes, D.C.; Krasin, M.J.; Spunt, S.L.; Ness, K.K. Pulmonary outcomes in survivors of childhood cancer: A systematic review. Chest 2011, 140, 881–901. [Google Scholar] [CrossRef] [PubMed]
- Liles, A.; Blatt, J.; Morris, D.; Wardrop, R., 3rd; Sharma, A.; Sznewajs, A.; Goldsby, R. Monitoring pulmonary complications in lnog-term childhood cancer survivors: Guidelines for the primary care physician. Clevel. Clin. J. Med. 2008, 75, 531–539. [Google Scholar] [CrossRef] [PubMed]
- de Vos, J.; Ramos Vega, S.; Noorman, E.; de Vos, P. Primary frontal sinus squamous cell carcinoma in three dogs treated with piroxicam combined with carboplatin or toceranib. Vet. Comp. Oncol. 2012, 10, 206–213. [Google Scholar] [CrossRef]
- de Vos, J.P.; Burm, A.G.D.; Focker, A.P.; Boschloo, H.; Karsijns, M.; van der Waal, I. Piroxicam and carboplatin as a combination treatment of canine oral non-tonsillar squamous cell carcinoma: A pilot study and a literature review of a canine model of human head and neck squamous cell carcinoma. Vet. Comp. Oncol. 2005, 3, 16–24. [Google Scholar] [CrossRef]
- Murphy, S.; Hayes, A.; Adams, V.; Maglennon, G.; Neath, P.; Ladlow, J.; Brearley, M.J. Role of carboplatin in multi-modality treatment of canine tonsillar squamous cell carcinoma—A case series of five dogs. J. Small Anim. Pract. 2006, 47, 216–220. [Google Scholar] [CrossRef]
- Mestrinho, L.A.; Bernardo, E.; Niza, M.; Lloret, A.; Buracco, P. Neoadjuvant chemoradiotherapy and surgery as treatment for oral maxillary squamous cell carcinoma in a dog. Aust. Vet. J. 2012, 90, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.; Dobson, J. Prospective clinical trial of masitinib mesylate treatment for advanced stage III and IV canine malignant melanoma. J. Small Anim. Pract. 2020, 61, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Allstadt, S.D.; Rodriguez, C.O.; Boostrom, B.; Rebhun, R.B.; Skorupski, K.A. Randomized Phase III Trial of Piroxicam in Combination with Mitoxantrone or Carboplatin for First-Line Treatment of Urogenital Tract Transitional Cell Carcinoma in Dogs. J. Vet. Intern. Med. 2015, 29, 261–267. [Google Scholar] [CrossRef]
- Boria, P.A.; Glickman, N.W.; Schmidt, B.R.; Widmer, W.R.; Mutsaers, A.J.; Adams, L.G.; Snyder, P.W.; DiBernardi, L.; De Gortari, A.E.; Bonney, P.L.; et al. Carboplatin and piroxicam therapy in 31 dogs with transitional cell carcinoma of the urinary bladder. Vet. Comp. Oncol. 2005, 3, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Menard, K.; Flesner, B.K.; Glahn, A.; Boudreaux, B.; Bryan, J.N. Concurrent 5-fluorouracil and carboplatin for the treatment of canine carcinomas. Vet. Comp. Oncol. 2018, 16, 590–595. [Google Scholar] [CrossRef]
- Dominguez, P.A.; Dervisis, N.G.; Cadile, C.D.; Sarbu, L.; Kitchell, B.E. Combined gemcitabine and carboplatin therapy for carcinomas in dogs. J. Vet. Intern. Med. 2009, 23, 130–137. [Google Scholar] [CrossRef]
- Woodruff, M.J.; Heading, K.L.; Bennett, P. Canine intranasal tumours treated with alternating carboplatin and doxorubin in conjunction with oral piroxicam: 29 cases. Vet. Comp. Oncol. 2019, 17, 42–48. [Google Scholar] [CrossRef]


| Patient | Breed Sex, Age | Diagnosis | Clinical Response | Objective Response | ST | PFS |
|---|---|---|---|---|---|---|
| 1 | Schnauzer MN-11YO | Nasal carcinoma | Yes | SD | 393 d | 270 d |
| 2 | Shiba Inu MN-11YO | Nasal carcinoma | Yes | NE | 304 d | NE |
| 3 | Chihuahua MN-11YO | Nasal carcinoma | Yes | NE | 340 d c | NE |
| 4 | Bichon Frise FS-13 YO | Nasal carcinoma | No | NE | NE | NE |
| 5 | Golden retriever FS-13YO | Nasal carcinoma | Yes | PR | 390 d | 310 d |
| 6 | Toy poodle MN-14YO | Nasal carcinoma | Yes | PR | 120 d c | 105 d |
| 7 | Toy poodle FS-12YO | Maxillary SCC | Yes | SD | 168 d | 45 d |
| 8 | Mini pinscher MN-16YO | Maxillary SCC | Yes | PR | 404 d a | 387 d |
| 9 | Corgi MN-8YO | lingual SCC | No | PD | 93 d | 21 d |
| 10 | Toy poodle FN-8YO | Nasal carcinoma | Yes | NE | 120 d | NE |
| 11 | Toy poodle MN-15YO | Nasal carcinoma | Yes | NE | 330 d | NE |
| 12 | Golden retriever FS-12YO | Nasal carcinoma | Yes | SD | 150 d c | 56 d |
| 13 | WHWT FN-13YO | Tonsil carcinoma | No | PD | 128 d | 21 d |
| 14 | Schnauzer MN-11YO | Ear canal carcinoma | Yes | PR | 715 d a | 666 d |
| 15 | Mongrel FN-12 YO | Bladder TCC | Yes | SD | 608 d | 437 d |
| 16 | Dachshund MN-9YO | Cutaneous SCC | Yes | PR | 180 d c | 105 d |
| 17 | Pekinese MN-16YO | Nasal carcinoma (SCC) | No | PD | NE | 21 d |
| 18 | Sheltie FN-8YO | Urethra TCC | Yes | SD | 150 d | 60 d |
| 19 | Dachshund MN-11YO | Prostatic carcinoma | Yes | PD | 93 d | 21 d |
| 20 | Corgi MN-14YO | Pulmonary carcinoma | Yes | SD | 180 d | 63 d |
| 21 | Mini collie MN-11YO | Bladder TCC | Yes | SD | 297d c | 242 d |
| 22 | Toy poodle MN-13YO | Bladder TCC | No | SD | 415 d a | 415 d |
| 23 | Scottish perrier MN-12YO | Prostatic TCC | Yes | SD | 185 d | 98 d |
| 24 | Toy poodle FS-9YO | Bladder TCC | Yes | SD | 475 d b | 352 d |
| 25 | Jack Russell FS-14YO | Pulmonary carcinoma | Yes | SD | 227 d a | 227 d |
| 26 | Toy poodle MN-9YO | Prostatic TCC | Yes | SD | 84 d b | 84 d |
| 27 | Corgi FS-10YO | Pulmonary carcinoma | Yes | PD | 114 d | 21 d |
| 28 | Toy poodle FN-6 YO | Mammary carcinoma | No | PD | NE | 21 d |
| 29 | Bichon Frise, MN-12YO | Frontal sinus SCC | Yes | PR | 228 d a | 228 d |
| 30 | Bichon Frise, MN-15YO | Maxillary SCC | Yes | SD | 89 d | 89 d |
| Neutropenia | Number of Dogs | Percentage of Dogs |
|---|---|---|
| G1 | 4 | 13% |
| G2 | 2 | 6% |
| G3 | 1 | 3% |
| G4 | 0 | |
| Trombocytopenia | ||
| G1 | 4 | 13% |
| G2 | 2 | 6% |
| G3 | 0 | |
| G4 | 0 | |
| Anorexia | ||
| G1 | 12 | 40% |
| G2 | 1 | 3% |
| G3 | 0 | |
| G4 | 0 | |
| Vomiting | ||
| G1 | 4 | 13% |
| G2 | 0 | |
| G3 | 0 | |
| G4 | 0 | |
| Diarrhea | ||
| G1 | 3 | 10% |
| G2 | 0 | |
| G3 | 0 | |
| G4 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giuliano, A.; Almendros, A. Retrospective Evaluation of a Combination of Carboplatin and Bleomycin for the Treatment of Canine Carcinomas. Animals 2022, 12, 2340. https://doi.org/10.3390/ani12182340
Giuliano A, Almendros A. Retrospective Evaluation of a Combination of Carboplatin and Bleomycin for the Treatment of Canine Carcinomas. Animals. 2022; 12(18):2340. https://doi.org/10.3390/ani12182340
Chicago/Turabian StyleGiuliano, Antonio, and Angel Almendros. 2022. "Retrospective Evaluation of a Combination of Carboplatin and Bleomycin for the Treatment of Canine Carcinomas" Animals 12, no. 18: 2340. https://doi.org/10.3390/ani12182340
APA StyleGiuliano, A., & Almendros, A. (2022). Retrospective Evaluation of a Combination of Carboplatin and Bleomycin for the Treatment of Canine Carcinomas. Animals, 12(18), 2340. https://doi.org/10.3390/ani12182340





