In Vivo Ultrasound Prediction of the Fillet Volume in Senegalese Sole (Solea senegalensis)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Fish and Experimental Procedures
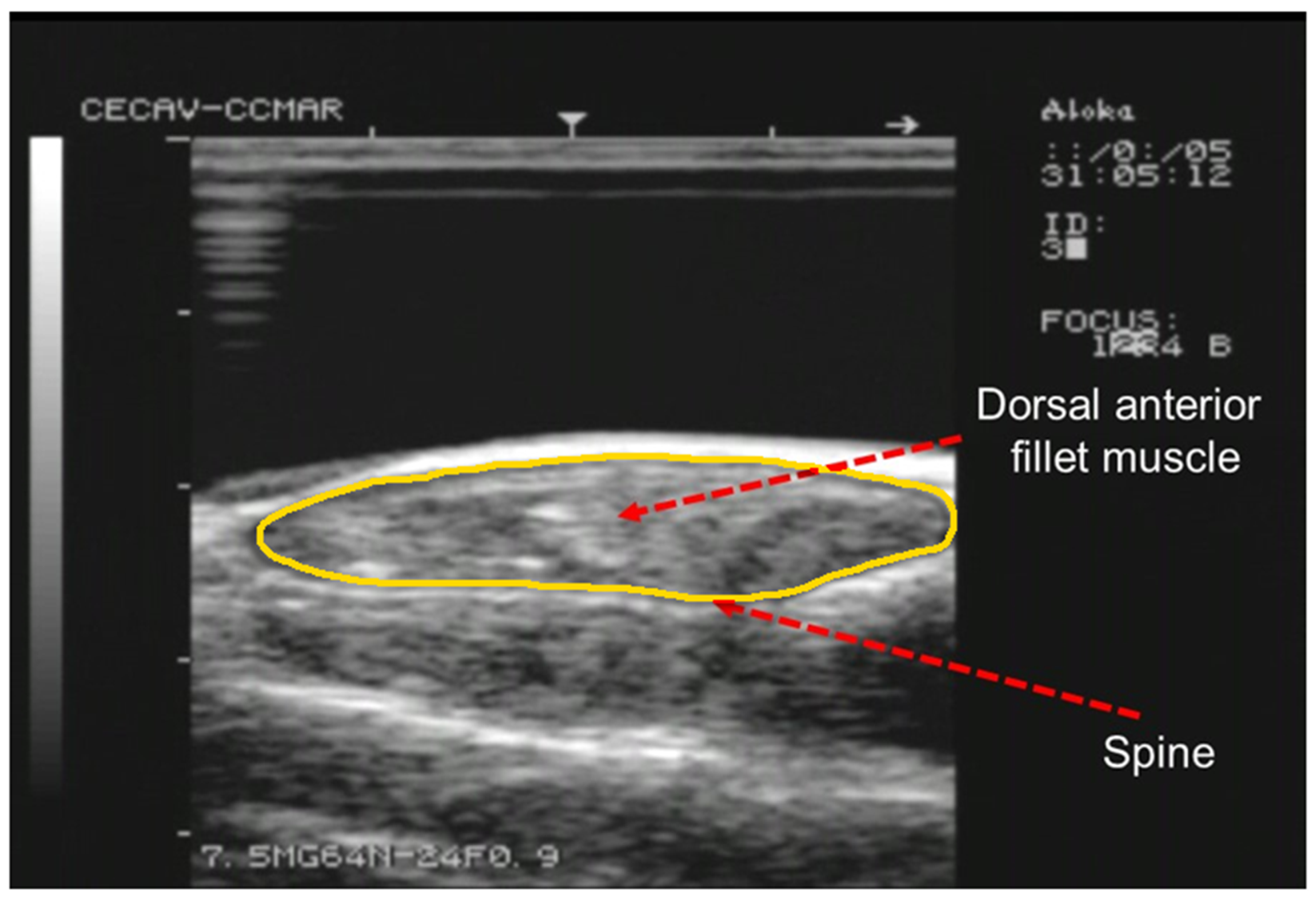
2.2. Ultrasound Procedure
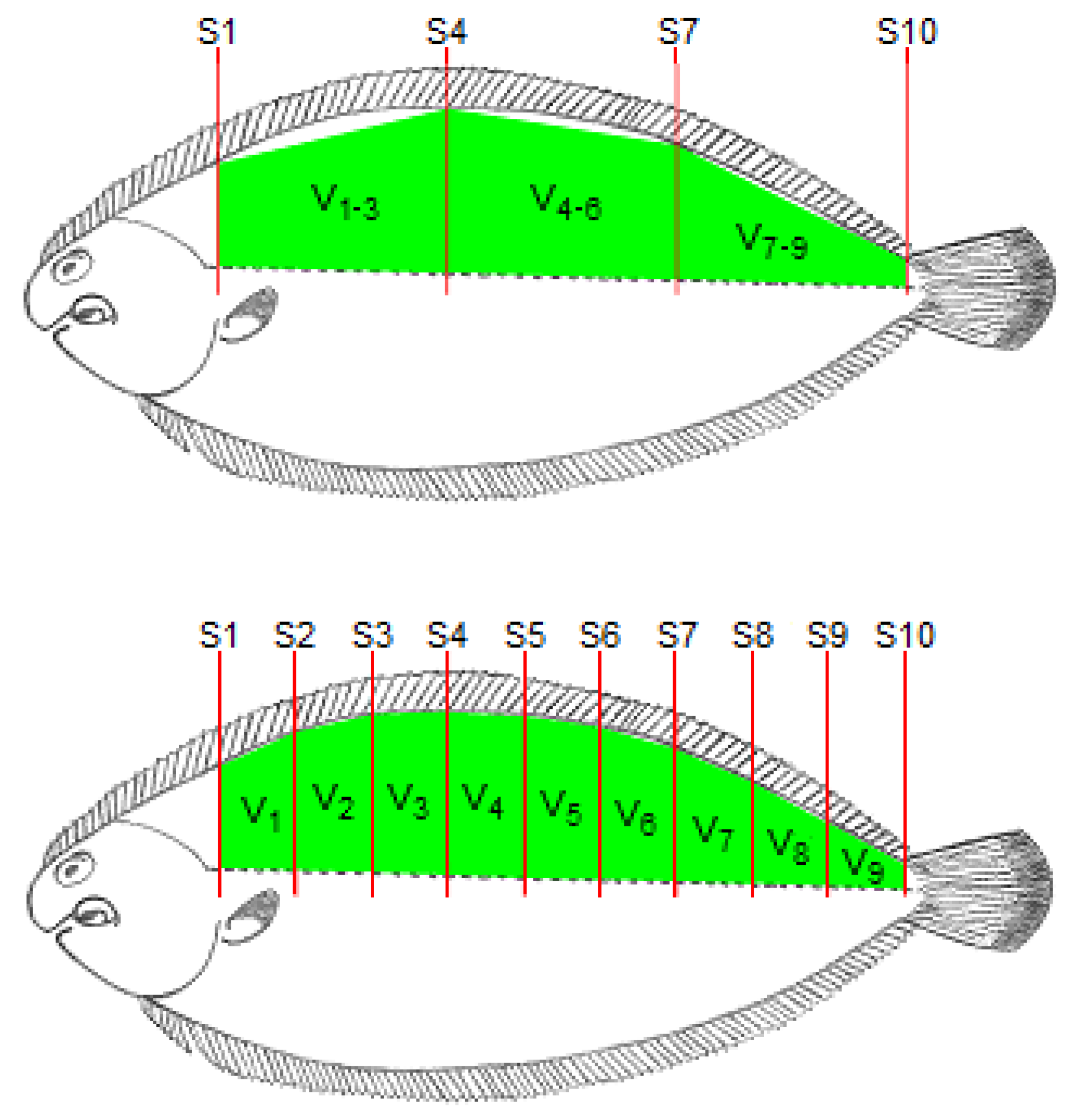
2.3. Image Acquisition, Analysis and Volume Calculation
2.4. Carcass Dissection and Fillet Volume Determination

2.5. Statistical Analysis
3. Results
3.1. Descriptive Statistics
3.2. Prediction of Fillet Volume from RTU Area(s) and Volume(s)
3.3. Prediction of Fillet Volume Using Stepwise Multiple Linear Regression
3.4. Correlations between Fillet Yields and RTU Slice Area(s) and Volume(s)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guillen, J.; Asche, F.; Carvalho, N.; Polanco, J.M.F.; Llorente, I.; Nielsen, R.; Nielsen, M.; Villasante, S. Aquaculture subsidies in the European Union: Evolution, impact and future potential for growth. Mar. Policy 2019, 104, 19–28. [Google Scholar] [CrossRef]
- Imsland, A.K.; Foss, A.; Conceição, L.E.C.; Dinis, M.T.; Delbare, D.; Schram, E.; Kamstra, A.; Rema, P.; White, P. A review of the culture potential of Solea solea and S. senegalensis. Rev. Fish Biol. Fish. 2003, 13, 379–408. [Google Scholar] [CrossRef]
- Veliyulin, E.; van der Zwaag, C.; Burk, W.; Erikson, U. In vivo determination of fat content in Atlantic salmon (Salmo salar) with a mobile NMR spectrometer. J. Sci. Food Agric. 2005, 85, 1299–1304. [Google Scholar] [CrossRef]
- Prchal, M.; Bugeon, J.; Vandeputte, M.; Kause, A.; Vergnet, A.; Zhao, J.; Gela, D.; Genestout, L.; Bestin, A.; Haffray, P.; et al. Potential for Genetic Improvement of the Main Slaughter Yields in Common Carp with in vivo Morphological Predictors. Front. Genet. 2018, 9, 283. [Google Scholar] [CrossRef]
- Haffray, P.; Bugeon, J.; Rivard, Q.; Quittet, B.; Puyo, S.; Allamelou, J.M.; Vandeputte, M.; Dupont-Nivet, M. Genetic parameters of in-vivo prediction of carcass, head and fillet yields by internal ultrasound and 2D external imagery in large rainbow trout (Oncorhynchus mykiss). Aquaculture 2013, 410–411, 236–244. [Google Scholar] [CrossRef]
- Prchal, M.; Kocour, M.; Vandeputte, M.; Kause, A.; Vergnet, A.; Zhao, J.; Gela, D.; Kašpar, V.; Genestout, L.; Bestin, A.; et al. Morphological predictors of slaughter yields using 3D digitizer and their use in a common carp breeding program. Aquaculture 2020, 520, 734993. [Google Scholar] [CrossRef]
- Hancz, C.; Romvári, R.; Szabó, A.; Molnár, T.; Magyary, I.; Horn, P. Measurement of total body composition changes of common carp by computer tomography. Aquac. Res. 2003, 34, 991–997. [Google Scholar] [CrossRef]
- Kolstad, K.; Vegusdal, A.; Baeverfjord, G.; Einen, O. Quantification of fat deposits and fat distribution in Atlantic halibut (Hippoglossus hippoglossus L.) using computerised X-ray tomography (CT). Aquaculture 2004, 229, 255–264. [Google Scholar] [CrossRef]
- Maas, P.; Grzegrzółka, B.; Kreß, P.; Oberle, M.; Judas, M.; Kremer-Rücker, P.V. Prediction of body composition in mirror carp (Cyprinus carpio) by using linear measurements in vivo and computed tomography post-mortem. Arch. Anim. Breed. 2020, 63, 69–80. [Google Scholar] [CrossRef]
- Wu, J.-L.; Zhang, J.-L.; Du, X.-X.; Shen, Y.-J.; Lao, X.; Zhang, M.-L.; Chen, L.-Q.; Du, Z.-Y. Evaluation of the distribution of adipose tissues in fish using magnetic resonance imaging (MRI). Aquaculture 2015, 448, 112–122. [Google Scholar] [CrossRef]
- Scholz, A.; Bünger, L.; Kongsro, J.; Baulain, U.; Mitchell, A. Non-invasive methods for the determination of body and carcass composition in livestock: Dual-energy X-ray absorptiometry, computed tomography, magnetic resonance imaging and ultrasound: Invited review. Animal 2015, 9, 1250–1264. [Google Scholar] [CrossRef]
- Silva, S.; Guedes, C.; Rodrigues, S.; Teixeira, A. Non-Destructive Imaging and Spectroscopic Techniques for Assessment of Carcass and Meat Quality in Sheep and Goats: A Review. Foods 2020, 9, 1074. [Google Scholar] [CrossRef]
- Sanchez, P.D.C.; Arogancia, H.B.T.; Boyles, K.M.; Pontillo, A.J.B.; Ali, M.M. Emerging nondestructive techniques for the quality and safety evaluation of pork and beef: Recent advances, challenges, and future perspectives. Appl. Food Res. 2022, 2, 100147. [Google Scholar] [CrossRef]
- Silva, S.R.; Cadavez, V.P. Real-time ultrasound (RTU) imaging methods for quality control of meats. In Computer Vision Technology in the Food and Beverage Industries; Sun, D.-W., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing Limited: Sawston, UK, 2012. [Google Scholar]
- Perazza, C.A.; Pinaffi, F.L.V.; Silva, L.A.; Hilsdorf, A.W.S. Evaluation of ultrasound imaging to predict loin eye area in tambaqui. Bol. Inst. Pesca 2015, 41, 803–809. [Google Scholar] [CrossRef]
- Silva, S.R.; Guedes, C.M.; Rema, P.; Batista, A.C.; Rodrigues, V.; Loureiro, N.; Dias, J. In vivo assessment of fat composition in Senegalese sole (Solea senegalensis) by real-time ultrasonography and image analysis of subcutaneous fat. Aquaculture 2016, 456, 76–82. [Google Scholar] [CrossRef]
- Gonçalves, C.; Rema, P.; Guedes, C.; Basto, S.; Silva, S.R. Using real-time ultrasound to predict fillet volume of tilapia (Oreochromis niloticus, Linnaeus). Rev. Port. Zootec. 2018, 13, 188–194. [Google Scholar]
- Maas, P.; Grzegrzółka, B.; Kreß, P.; Oberle, M.; Kremer-Rücker, P.V. In vivo–determination of the fat content in mirror carps (Cyprinus carpio) using ultrasound, microwave and linear measurements. Aquaculture 2019, 512, 734359. [Google Scholar] [CrossRef]
- Prchal, M.; Zhao, J.; Gela, D.; Kašpar, J.; Lepič, P.; Kašpar, V.; Kocour, M. Simplified method for genetic slaughter yields improvement in common carp under European pond conditions. Aquac. Rep. 2021, 21, 100832. [Google Scholar] [CrossRef]
- Vandeputte, M.; Puledda, A.; Tyran, A.S.; Bestin, A.; Coulombet, C.; Bajek, A.; Baldit, G.; Vergnet, A.; Allal, F.; Bugeon, J.; et al. Investigation of morphological predictors of fillet and carcass yield in European sea bass (Dicentrarchus labrax) for application in selective breeding. Aquaculture 2017, 470, 40–49. [Google Scholar] [CrossRef]
- Rasband, W.S. ImageJ; U.S. National Institutes of Health: Bethesda, MD, USA, 2018. Available online: https://imagej.nih.gov/ij/ (accessed on 20 August 2022).
- Katz, V. A History of Mathematics: An Introduction, 2nd ed.; Addison-Wesley: Boston, MA, USA, 1998; p. 477. [Google Scholar]
- Bosworth, B.G.; Holland, M.; Brazil, B.L. Evaluation of ultrasound imagery and body shape to predict carcass and fillet yield in farm-raised catfish. J. Anim. Sci. 2001, 79, 1483–1490. [Google Scholar] [CrossRef]
- Silva, S. Use of ultrasonographic examination for in vivo evaluation of body composition and for prediction of carcass quality of sheep. Small Rumin. Res. 2017, 152, 144–157. [Google Scholar] [CrossRef]
- Alempijevic, A.; Vidal-Calleja, T.; Falque, R.; Quin, P.; Toohey, E.; Walmsley, B.; McPhee, M. Lean meat yield estimation using a prototype 3D imaging approach. Meat Sci. 2021, 181, 108470. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.; Pabiou, T.; Evans, R.D.; Beder, C.; Daly, A. Predicting carcass cut yields in cattle from digital images using artificial intelligence. Meat Sci. 2022, 184, 108671. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.R.; Almeida, M.; Condotta, I.; Arantes, A.; Guedes, C.; Santos, V. Assessing the Feasibility of Using Kinect 3D Images to Predict Light Lamb Carcasses Composition from Leg Volume. Animals 2021, 11, 3595. [Google Scholar] [CrossRef]
- Bünger, L.; Clelland, N.; Moore, K.; McLean, K.; Kongsro, J.; Lambe, N. Integrating Computed tomography (CT) into commercial sheep breeding in the UK: Cost and value. In Proceedings of the Farm Animal Imaging III, Copenhagen, Denmark, 1 September 2014; Maltin, C., Craigie, C., Bünger, J., Eds.; FAIM: Copenhagen, Denmark, 2014; pp. 22–27. [Google Scholar]
- Flick, G.J.; Barua, M.A.; Enriquez, L.G. Processing finfish. In The Seafood Industry; Martin, R.E., Flick, G.J., Eds.; Van Nostrand Reinhold: New York, NY, USA, 1990; pp. 117–164. [Google Scholar]
- Sang, N.V.; Thomassen, M.; Klemetsdal, G.; Gjøen, H.M. Prediction of fillet weight, fillet yield, and fillet fat for live river catfish (Pangasianodon hypophthalmus). Aquaculture 2009, 288, 166–171. [Google Scholar] [CrossRef]
- Vandeputte, M.; Bugeon, J.; Bestin, A.; Desgranges, A.; Allamellou, J.-M.; Tyran, A.-S.; Allal, F.; Dupont-Nivet, M.; Haffray, P. First Evidence of Realized Selection Response on Fillet Yield in Rainbow Trout Oncorhynchus mykiss, Using Sib Selection or Based on Correlated Ultrasound Measurements. Front. Genet. 2019, 10, 1225. [Google Scholar] [CrossRef]
- Rutten, M.J.; Bovenhuis, H.; Komen, H. Modeling fillet traits based on body measurements in three Nile tilapia strains (Oreochromis niloticus L.). Aquaculture 2004, 231, 113–122. [Google Scholar] [CrossRef]
| Trait | Mean | SD | Minimum | Maximum | CV (%) |
|---|---|---|---|---|---|
| Fish | |||||
| Body weight (g) | 298.54 | 87.30 | 178.77 | 456.32 | 29.2 |
| Total volume (cm3) | 283.27 | 80.59 | 172.10 | 412.70 | 28.4 |
| Length (mm) | 203.11 | 36.52 | 162.30 | 299.60 | 18.0 |
| Right dorsal fillet weight (g) | 49.31 | 17.28 | 26.90 | 85.00 | 35.0 |
| Right dorsal fillet volume (cm3) | 49.12 | 17.51 | 28.20 | 88.20 | 35.6 |
| Right dorsal fillet yield (%) | 16.32 | 1.57 | 12.08 | 20.13 | 9.6 |
| RTU traits for the right dorsal fillet | |||||
| Slice area (cm2) * | |||||
| A1 | 3.40 | 0.90 | 2.15 | 5.74 | 26.5 |
| A2 | 3.68 | 1.15 | 2.07 | 6.45 | 31.3 |
| A3 | 3.60 | 0.66 | 1.98 | 4.68 | 18.2 |
| A4 | 2.75 | 0.57 | 1.51 | 4.06 | 20.8 |
| A5 | 2.61 | 0.56 | 1.49 | 3.55 | 21.6 |
| A6 | 2.62 | 0.69 | 0.89 | 4.13 | 26.1 |
| A7 | 1.96 | 0.60 | 0.95 | 3.91 | 30.4 |
| A8 | 1.40 | 0.43 | 0.71 | 3.11 | 30.5 |
| A9 | 1.02 | 0.40 | 0.24 | 2.25 | 39.4 |
| A10 | 0.81 | 0.22 | 0.48 | 1.44 | 27.4 |
| Single volumes (cm3) * | |||||
| V1 | 7.12 | 3.11 | 3.89 | 15.26 | 43.7 |
| V2 | 7.80 | 3.82 | 3.45 | 19.32 | 48.9 |
| V3 | 7.43 | 2.31 | 3.25 | 12.99 | 31.0 |
| V4 | 5.64 | 1.64 | 2.59 | 8.77 | 29.1 |
| V5 | 5.41 | 1.91 | 2.56 | 10.10 | 35.2 |
| V6 | 5.48 | 2.24 | 1.52 | 10.48 | 40.8 |
| V7 | 4.05 | 1.69 | 1.85 | 9.27 | 41.9 |
| V8 | 2.86 | 1.05 | 1.35 | 5.91 | 36.7 |
| V9 | 2.13 | 1.09 | 0.41 | 5.36 | 51.2 |
| V1–5 | 33.39 | 11.75 | 17.31 | 62.54 | 35.2 |
| V6–9 | 14.52 | 5.47 | 7.33 | 29.50 | 37.7 |
| Volume combinations (cm3) | |||||
| V1+2 | 14.92 | 6.74 | 8.43 | 33.61 | 45.2 |
| V3+4 | 13.07 | 3.79 | 5.87 | 21.20 | 29.0 |
| V5+6 | 10.89 | 3.94 | 4.08 | 20.58 | 36.2 |
| V7+8 | 6.91 | 2.52 | 3.75 | 13.79 | 36.5 |
| V1+2+3 | 22.35 | 8.78 | 11.72 | 44.75 | 39.3 |
| V4+5+6 | 16.53 | 5.31 | 8.18 | 28.43 | 32.1 |
| V7+8+9 | 9.03 | 3.41 | 4.74 | 19.15 | 37.8 |
| V1+3+5+7+9 | 26.12 | 8.96 | 13.85 | 50.22 | 34.3 |
| V2+4+6+8 | 21.79 | 7.98 | 10.78 | 41.85 | 36.6 |
| V1–3+4–6+7–9 | 51.98 | 18.65 | 25.18 | 98.31 | 35.9 |
| V1+()+9 | 47.91 | 16.81 | 24.64 | 89.04 | 35.1 |
| Variables | Intercept | Slope | R2 | RMSE | |
|---|---|---|---|---|---|
| Dependent | Independent | ||||
| Fillet volume (cm3) | RTU slice areas (cm2) * | ||||
| A1 | −2.53 | 15.20 | 0.613 | 11.02 | |
| A2 | −1.63 | 13.79 | 0.820 | 7.51 | |
| A3 | −12.50 | 17.13 | 0.411 | 13.60 | |
| A4 | 13.83 | 12.82 | 0.175 | 16.09 | |
| A5 | 2.13 | 18.03 | 0.337 | 14.43 | |
| A6 | −3.43 | 20.05 | 0.615 | 10.99 | |
| A7 | 20.22 | 14.76 | 0.252 | 15.32 | |
| A8 | 38.33 | 7.69 | 0.035 | 17.40 | |
| A9 | 25.85 | 22.90 | 0.274 | 15.10 | |
| A10 | 21.57 | 34.18 | 0.186 | 15.98 | |
| Single RTU volumes (cm3) * | |||||
| V1 | 12.75 | 5.11 | 0.826 | 7.40 | |
| V2 | 15.04 | 4.37 | 0.908 | 5.38 | |
| V3 | −2.75 | 6.98 | 0.845 | 6.98 | |
| V4 | −1.43 | 8.97 | 0.707 | 9.58 | |
| V5 | 7.27 | 7.74 | 0.710 | 9.55 | |
| V6 | 9.02 | 7.31 | 0.872 | 6.35 | |
| V7 | 16.13 | 8.15 | 0.622 | 10.89 | |
| V8 | 19.20 | 10.46 | 0.395 | 13.79 | |
| V9 | 23.63 | 12.00 | 0.556 | 11.80 | |
| V1–5 | 0.13 | 1.47 | 0.970 | 3.08 | |
| V6–9 | 7.49 | 2.87 | 0.802 | 7.88 | |
| Combinations of RTU volumes (cm3) | |||||
| V1+2 | 11.95 | 2.49 | 0.920 | 5.01 | |
| V3+4 | −6.66 | 4.27 | 0.853 | 6.79 | |
| V5+6 | 3.83 | 4.16 | 0.877 | 6.21 | |
| V7+8 | 11.20 | 5.49 | 0.626 | 10.83 | |
| V1+2+3 | 5.57 | 1.95 | 0.956 | 3.73 | |
| V4+5+6 | −2.92 | 3.15 | 0.912 | 5.24 | |
| V7+8+9 | 10.92 | 4.23 | 0.679 | 10.05 | |
| V1+3+5+7+9 | −0.42 | 1.90 | 0.942 | 4.25 | |
| V2+4+6+8 | 2.59 | 2.14 | 0.947 | 4.06 | |
| V1–3+4–6+7–9 | 3.18 | 0.88 | 0.887 | 5.96 | |
| V1+()+9 | 0.23 | 1.02 | 0.960 | 3.55 |
| Stepwise Model | Intercept | Independent Variables | R2 | RMSE | ||||
|---|---|---|---|---|---|---|---|---|
| With RTU slice areas | −22.414 | 7.329 A1 | 7.904 A2 | 6.734 A5 | 0.8830 | 5.9901 | ||
| With RTU single volumes | 1.519 | 0.964 V1 | 1.604 V2 | 1.404 V3 | 1.625 V4 | 1.575 V6 | 0.9715 | 2.9573 |
| With combinations of 2 RTU volumes | 0.317 | 1.331 V1+2 | 1.459 V3+4 | 0.908 V5+6 | 0.9727 | 2.8934 | ||
| With combinations of 3 RTU volumes | 0.867 | 1.296 V1+2+3 | 1.167 V4+5+6 | 0.9725 | 2.9060 | |||
| With V1–5, V6, V7, V8 and V9 | 1.655 | 1.246 V1–5 | 1.592 V6 | −1.008 V8 | 0.9755 | 2.8403 | ||
| With V1, V2, V3, V4, V5 and V6–9 | 1.171 | 1.395 V1 | 1.834 V2 | 1.777 V3 | 1.863 V4 | 0.9653 | 3.2585 | |
| With V1–5 and V6–9 | 0.127 | 1.467 V1–5 | 0.9689 | 3.0832 | ||||
| Independent * | Dependent | |
|---|---|---|
| Fillet Yield (%) # | Fillet Yieldv1–5 (%) ## | |
| RTU slice areas (cm2) | ||
| A1 | 0.601 | 0.450 |
| A2 | 0.529 | 0.480 |
| A3 | 0.253 | 0.305 |
| A4 | 0.055 | 0.099 |
| A5 | 0.176 | 0.308 |
| A6 | 0.345 | 0.199 |
| A7 | 0.164 | 0.298 |
| A8 | 0.152 | 0.044 |
| A9 | 0.187 | 0.174 |
| A10 | 0.095 | 0.133 |
| Single RTU volumes (cm3) | ||
| V1 | 0.637 | 0.590 |
| V2 | 0.597 | 0.595 |
| V3 | 0.495 | 0.516 |
| V4 | 0.377 | 0.408 |
| V5 | 0.431 | 0.514 |
| V6 | 0.517 | 0.433 |
| V7 | 0.399 | 0.494 |
| V8 | 0.288 | 0.346 |
| V9 | 0.381 | 0.359 |
| V1–5 | 0.582 | 0.591 |
| V6–9 | 0.466 | 0.468 |
| Combinations of RTU volumes (cm3) | ||
| V1+2 | 0.633 | 0.609 |
| V3+4 | 0.464 | 0.491 |
| V5+6 | 0.501 | 0.494 |
| V7+8 | 0.387 | 0.475 |
| V1+2+3 | 0.616 | 0.402 |
| V4+5+6 | 0.489 | 0.493 |
| V7+8+9 | 0.409 | 0.466 |
| V1+3+5+7+9 | 0.562 | 0.584 |
| V2+4+6+8 | 0.546 | 0.535 |
| V1–3+4–6+7–9 | 0.555 | 0.561 |
| V1+()+9 | 0.559 | 0.566 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afonso, J.; Guedes, C.; Teixeira, A.; Rema, P.; Silva, S. In Vivo Ultrasound Prediction of the Fillet Volume in Senegalese Sole (Solea senegalensis). Animals 2022, 12, 2357. https://doi.org/10.3390/ani12182357
Afonso J, Guedes C, Teixeira A, Rema P, Silva S. In Vivo Ultrasound Prediction of the Fillet Volume in Senegalese Sole (Solea senegalensis). Animals. 2022; 12(18):2357. https://doi.org/10.3390/ani12182357
Chicago/Turabian StyleAfonso, João, Cristina Guedes, Alfredo Teixeira, Paulo Rema, and Severiano Silva. 2022. "In Vivo Ultrasound Prediction of the Fillet Volume in Senegalese Sole (Solea senegalensis)" Animals 12, no. 18: 2357. https://doi.org/10.3390/ani12182357
APA StyleAfonso, J., Guedes, C., Teixeira, A., Rema, P., & Silva, S. (2022). In Vivo Ultrasound Prediction of the Fillet Volume in Senegalese Sole (Solea senegalensis). Animals, 12(18), 2357. https://doi.org/10.3390/ani12182357







