PRAMEY: A Bovid-Specific Y-Chromosome Multicopy Gene Is Highly Related to Postnatal Testicular Growth in Hu Sheep
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample and Testicular Traits Collection
2.2. DNA, Total RNA Extraction and cDNA Synthesis
2.3. Full-Length cDNA Cloning of Ovine PRAMEY
2.4. Bioinformatic Analysis
2.5. Estimation of mRNA Expression Levels and CNV of Ovine PRAMEY
2.6. Association and Statistical Analysis
3. Results
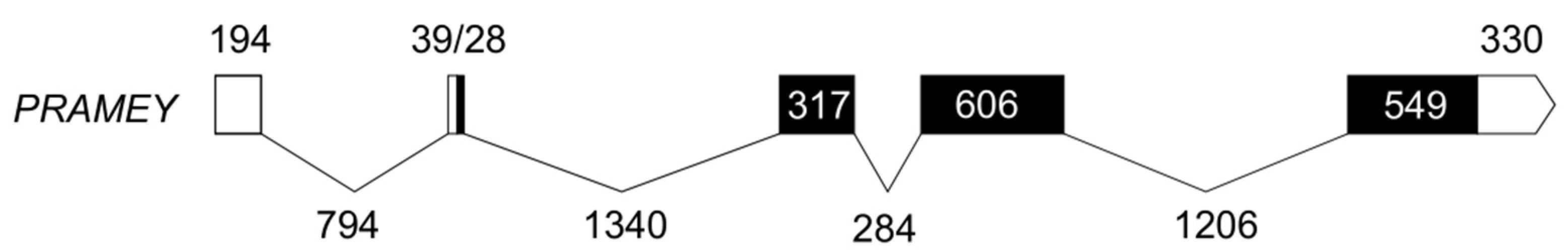
3.1. Molecular Characteristics of Ovine PRAMEY cDNA
3.2. Homology and Phylogenetic Analysis
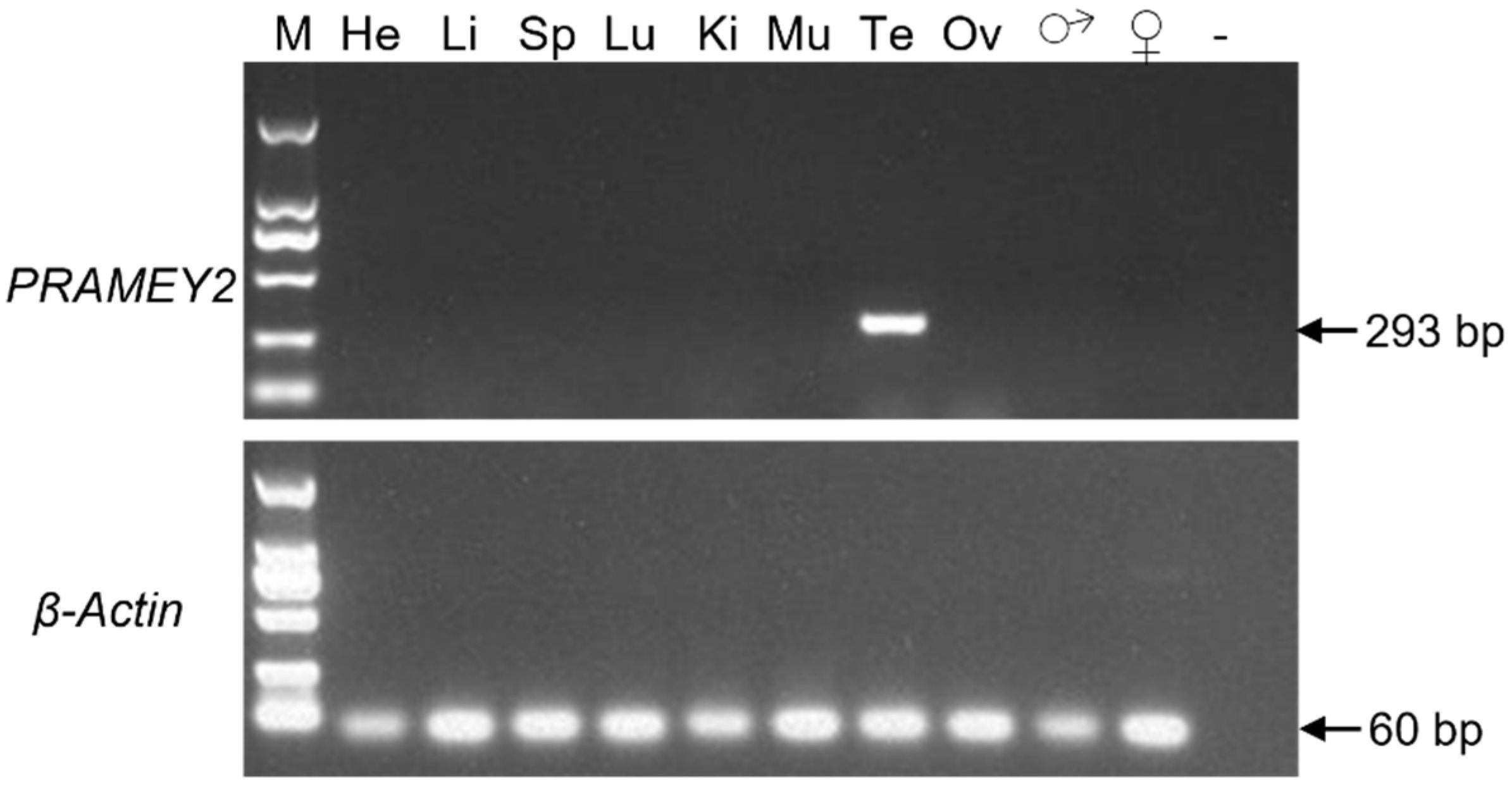
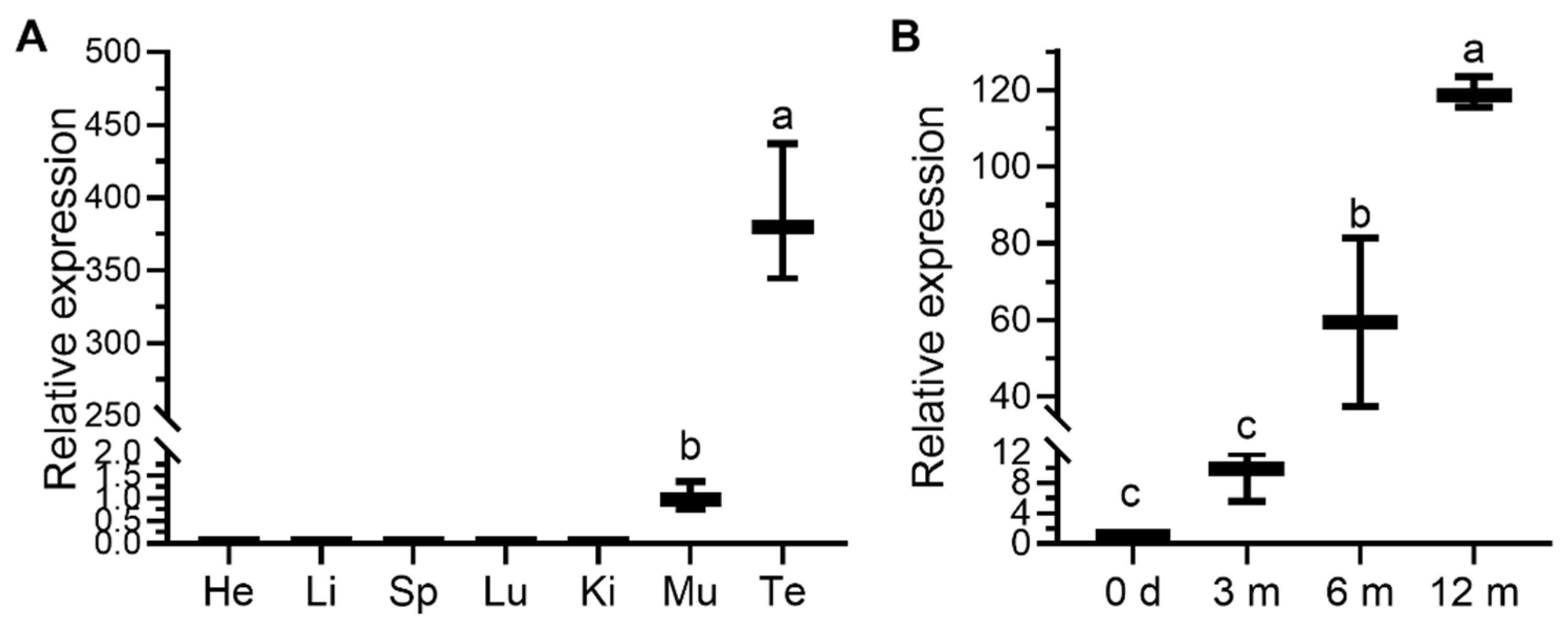
3.3. Expression Profile of the Ovine PRAMEY
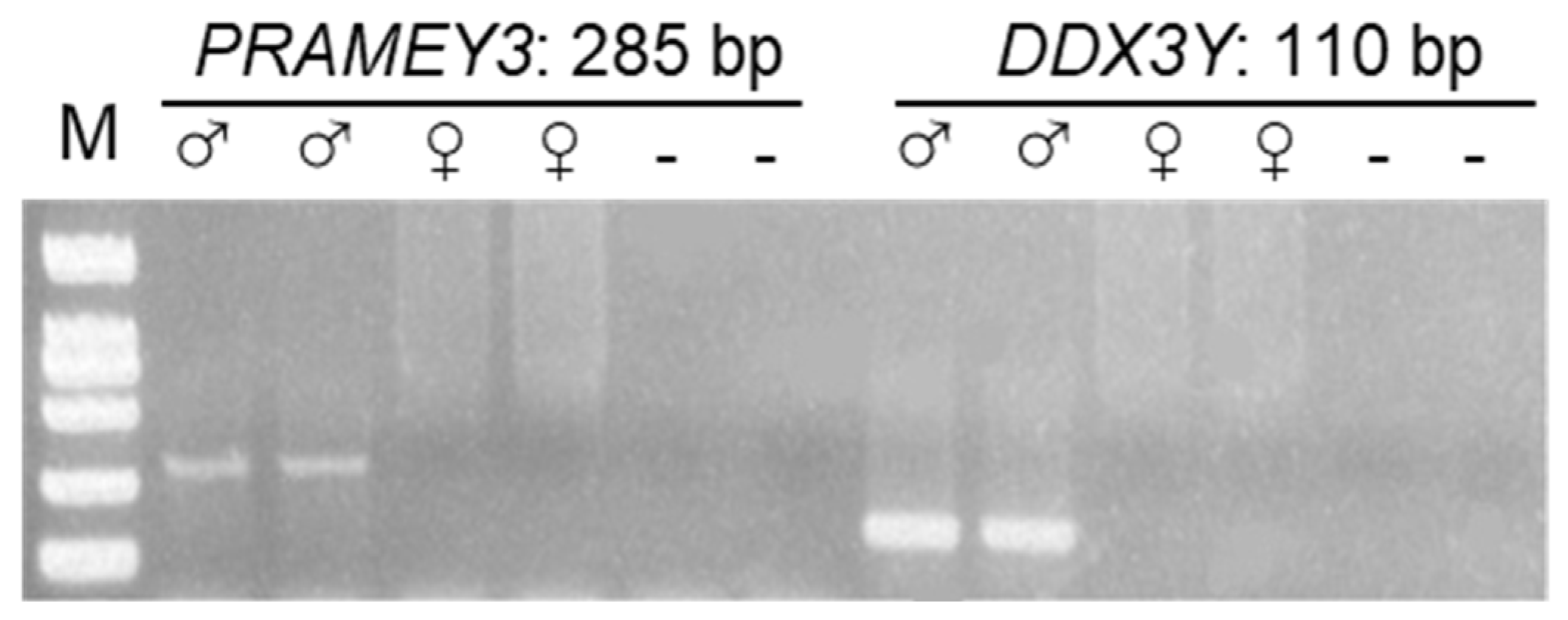
3.4. CNV of PRAMEY across Sheep Breeds
3.5. The Relationship among PRAMEY CNV, mRNA Expression in Testis, and Testicular Size
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gebreselassie, G.; Berihulay, H.; Jiang, L.; Ma, Y. Review on Genomic Regions and Candidate Genes Associated with Economically Important Production and Reproduction Traits in Sheep (Ovies aries). Animals 2020, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Notter, D.R. Genetic aspects of reproduction in sheep. Reprod. Domest. Anim. 2008, 43, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.; Ramón, M.; Calvo, J.; Jiménez, M.; Freire, F.; Vázquez, J.; Arranz, J. Genome-wide association studies for sperm traits in Assaf sheep breed. Animal 2021, 15, 100065. [Google Scholar] [CrossRef]
- Liu, W.-S. Mammalian Sex Chromosome Structure, Gene Content, and Function in Male Fertility. Annu. Rev. Anim. Biosci. 2019, 7, 103–124. [Google Scholar] [CrossRef] [PubMed]
- Subrini, J.; Turner, J. Y chromosome functions in mammalian spermatogenesis. eLife 2021, 10, e67345. [Google Scholar] [CrossRef]
- Skaletsky, H.; Kuroda-Kawaguchi, T.; Minx, P.J.; Cordum, H.S.; Hillier, L.; Brown, L.G.; Repping, S.; Pyntikova, T.; Ali, J.; Bieri, T.; et al. The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature 2003, 423, 825–837. [Google Scholar] [CrossRef]
- Hughes, J.F.; Skaletsky, H.; Pyntikova, T.; Graves, T.A.; van Daalen, S.K.M.; Minx, P.J.; Fulton, R.S.; McGrath, S.D.; Locke, D.P.; Friedman, C.; et al. Chimpanzee and human Y chromosomes are remarkably divergent in structure and gene content. Nature 2010, 463, 536–539. [Google Scholar] [CrossRef]
- Hughes, J.F.; Skaletsky, H.; Brown, L.G.; Pyntikova, T.; Graves, T.; Fulton, R.S.; Dugan, S.; Ding, Y.; Buhay, C.J.; Kremitzki, C.; et al. Strict evolutionary conservation followed rapid gene loss on human and rhesus Y chromosomes. Nature 2012, 483, 82–86. [Google Scholar] [CrossRef]
- Shirleen Soh, Y.Q.; Alföldi, J.; Pyntikova, T.; Brown, L.G.; Graves, T.; Minx, P.J.; Fulton, R.S.; Kremitzki, C.; Koutseva, N.; Mueller, J.L.; et al. Sequencing the Mouse Y Chromosome Reveals Convergent Gene Acquisition and Amplification on Both Sex Chromosomes. Cell 2014, 159, 800–813. [Google Scholar]
- Chang, T.-C.; Yang, Y.; Retzel, E.F.; Liu, W.-S. Male-specific region of the bovine Y chromosome is gene rich with a high transcriptomic activity in testis development. Proc. Natl. Acad. Sci. USA 2013, 110, 12373–12378. [Google Scholar] [CrossRef]
- Cechova, M.; Vegesna, R.; Tomaszkiewicz, M.; Harris, R.S.; Chen, D.; Rangavittal, S.; Medvedev, P.; Makova, K.D. Dynamic evolution of great ape Y chromosomes. Proc. Natl. Acad. Sci. USA 2020, 117, 26273–26280. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yang, P.; Li, M.; Fang, W.; Jiang, Y. A Hu sheep genome with the first ovine Y chromosome reveal in-trogression history after sheep domestication. Sci. China Life Sci. 2021, 64, 1116–1130. [Google Scholar] [CrossRef] [PubMed]
- Brashear, W.A.; Raudsepp, T.; Murphy, W.J. Evolutionary conservation of Y Chromosome ampliconic gene families despite extensive structural variation. Genome Res. 2018, 28, 1841–1851. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Mallidis, C.; Bhasin, S. The role of Y chromosome deletions in male infertility. Eur. J. Endocrinol. 2000, 142, 418–430. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, X.; Tu, W.; Dong, Q.; Ma, Y.; Liu, Y.; Shen, Y. Copy number variation of functional RBMY1 is associated with sperm motility: An azoospermia factor-linked candidate for asthenozoospermia. Hum. Reprod. 2017, 32, 1521–1531. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Q.; Chu, L.; Chang, C.; Chen, Y.; Bao, Z.; Peng, W.; Zhang, L.; Li, S.; Liu, C.; et al. Comprehensive copy number analysis of Y chro-mosome-linked loci for detection of structural variations and diagnosis of male infertility. J. Hum. Genet. 2022, 67, 107–114. [Google Scholar] [CrossRef]
- Yue, X.; Chang, T.; DeJarnette, J.; Marshall, C.; Lei, C.; Liu, W.-S. Copy number variation of PRAMEY across breeds and its association with male fertility in Holstein sires. J. Dairy Sci. 2013, 96, 8024–8034. [Google Scholar] [CrossRef]
- Yue, X.-P.; Dechow, C.; Chang, T.-C.; DeJarnette, J.M.; Marshall, C.E.; Lei, C.-Z.; Liu, W.-S. Copy number variations of the extensively amplified Y-linked genes, HSFY and ZNF280BY, in cattle and their association with male reproductive traits in Holstein bulls. BMC Genom. 2014, 15, 113. [Google Scholar] [CrossRef]
- Pei, S.; Qin, F.; Li, W.; Li, F.; Yue, X. Copy number variation of ZNF280AY across 21 cattle breeds and its association with the reproductive traits of Holstein and Simmental bulls. J. Dairy Sci. 2019, 102, 7226–7236. [Google Scholar] [CrossRef]
- Simpson, A.J.G.; Caballero, O.L.; Jungbluth, A.; Chen, Y.-T.; Old, L.J. Cancer/testis antigens, gametogenesis and cancer. Nat. Cancer 2005, 5, 615–625. [Google Scholar] [CrossRef]
- Chang, T.-C.; Yang, Y.; Yasue, H.; Bharti, A.K.; Retzel, E.F.; Liu, W.-S. The Expansion of the PRAME Gene Family in Eutheria. PLoS ONE 2011, 6, e16867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mistry, B.; Zhao, Y.; Chang, T.-C.; Yasue, H.; Chiba, M.; Oatley, J.; Díaz, F.; Liu, W.-S. Differential Expression of PRAMEL1, a Cancer/Testis Antigen, during Spermatogenesis in the Mouse. PLoS ONE 2013, 8, e60611. [Google Scholar] [CrossRef]
- Liu, W.-S.; Zhao, Y.; Lu, C.; Ning, G.; Ma, Y.; Diaz, F.; O’Connor, M. A novel testis-specific protein, PRAMEY, is involved in spermatogenesis in cattle. Reproduction 2017, 153, 847–863. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.Z.; Zhang, Y.Y.; Liu, W.S.; Deng, X.M. Highly efficient synchronization of sheep skin fibroblasts at G2/M phase and isolation of sheep Y chromosomes by flow cytometric sorting. Sci. Rep. 2020, 10, 9933. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Russell, D.; Maccallum, P.; Russell, D. Molecular Cloning: A Laboratory Manual, 3rd ed. Immunology 2001, 49, 895–909. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Hamilton, C.K.; Verduzco-Gómez, A.R.; Fa vetta, L.A.; Blondin, P.; King, W.A. Testis-specific protein Y-encoded copy number is correlated to its expression and the field fertility of Canadian Holstein bulls. Sex Dev. 2012, 6, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1975, 67, 215–216. [Google Scholar]
- Justel, A.; Peña, D.; Zamar, R. A multivariate Kolmogorov-Smirnov test of goodness of fit. Stat. Probab. Lett. 1997, 35, 251–259. [Google Scholar] [CrossRef]
- Williamson, D.F.; Parker, R.A.; Kendrick, J.S. The Box Plot: A Simple Visual Method to Interpret Data. Ann. Intern. Med. 1989, 110, 916–921. [Google Scholar] [CrossRef]
- Mann, H.B.; Whitney, D.R. On a Test of Whether one of Two Random Variables is Stochastically Larger than the Other. Ann. Math. Stat. 1947, 18, 50–60. [Google Scholar] [CrossRef]
- Dunn, O.J. Multiple comparisons among means. J. Am. Stat. Assoc. 1961, 56, 52–64. [Google Scholar] [CrossRef]
- Di Meo, G.P.; Perucatti, A.; Floriot, S.; Incarnato, D.; Rullo, R.; Jambrenghi, A.C.; Ferretti, L.; Vonghia, G.; Cribiu, E.; Eggen, A.; et al. Chromosome evolution and improved cytogenetic maps of the Y chromosome in cattle, zebu, river buffalo, sheep and goat. Chromosom. Res. 2005, 13, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Sun, L.; Zhao, J.; Xiang, L.; Cheng, X.; Li, J.; Jia, C.; Jiang, H. Histological analysis and identification of spermato-genesis-related genes in 2-, 6-, and 12-month-old sheep testes. Sci. Nat. 2017, 104, 84. [Google Scholar] [CrossRef]
- Hamilton, C.; Favetta, L.; Di Meo, G.; Floriot, S.; Perucatti, A.; Peippo, J.; Kantanen, J.; Eggen, A.; Iannuzzi, L.; King, W. Copy Number Variation of Testis-Specific Protein, Y-Encoded (TSPY) in 14 Different Breeds of Cattle (Bos taurus). Sex. Dev. 2009, 3, 205–213. [Google Scholar] [CrossRef]
- Epping, M.T.; Wang, L.; Plumb, J.A.; Lieb, M.; Gronemeyer, H.; Brown, R.; Bernards, R. A functional genetic screen identifies retinoic acid signaling as a target of histone deacetylase inhibitors. Proc. Natl. Acad. Sci. USA 2007, 104, 17777–17782. [Google Scholar] [CrossRef]
- Abou-Haila, A.; Tulsiani, D.R. Mammalian Sperm Acrosome: Formation, Contents, and Function. Arch. Biochem. Biophys. 2000, 379, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.F.; Page, D.C. The Biology and Evolution of Mammalian Y Chromosomes. Annu. Rev. Genet. 2015, 49, 507–527. [Google Scholar] [CrossRef]
- Hughes, J.F.; Skaletsky, H.; Koutseva, N.; Pyntikova, T.; Page, D.C. Sex chromosome-to-autosome transposition events counter Y-chromosome gene loss in mammals. Genome Biol. 2015, 16, 104. [Google Scholar] [CrossRef]
- Zhang, F.; Gu, W.; Hurles, M.E.; Lupski, J.R. Copy Number Variation in Human Health, Disease, and Evolution. Annu. Rev. Genom. Hum. Genet. 2009, 10, 451–481. [Google Scholar] [CrossRef]
- Vegesna, R.; Tomaszkiewicz, M.; Medvedev, P.; Makova, K.D. Dosage regulation, and variation in gene ex-pression and copy number of human Y chromosome ampliconic genes. PLoS Genet. 2019, 15, e1008369. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Group | Breed (Full Name) | Short Name | Sample Size | Sample Type | Median Copy Number (Range) |
|---|---|---|---|---|---|
| Group I | Dorper | DP | 20 | Blood | 6 (4–9) |
| East Friesian | EF | 6 | Blood | 7 (4–9) | |
| Suffolk | SK | 26 | Blood | 5 (3–9) | |
| South African Mutton Merino | SMM | 17 | Blood | 5 (4–9) | |
| Tan sheep | TS | 12 | Blood | 3 (2–7) | |
| Texel | TL | 27 | Blood | 5 (2–7) | |
| White Suffolk | WSK | 29 | Blood | 6 (2–25) | |
| Group II | Hu sheep | HS | 573 1 | Testis, (Heart, Liver, Spleen, Lung, Kidney, Muscle) 2 | 4 (2–12) |
| Group III | Hu sheep | HS | 12 | Testis |
| Primer Name | Primer Sequence (5′-3′) | PCR Product Length (bp) | Annealing Temperature (°C) | Usage |
|---|---|---|---|---|
| PRAMEY1 | F:CGGAGTAGGTTCACGATGGG | 1112 | 62 | RT-PCR |
| R:TCTAGGTCCTGTAGGGTGGC | ||||
| 5′GSP | GATTACGCCAAGCTTTCTAGGTCCTGTAGGGTGGC | - | - | 5′-RACE |
| 3′GSP | GATTACGCCAAGCTTCGCATCTGGGACGGATGGGA | - | - | 3′-RACE |
| PRAMEY2 | F:TGGAGGTGAACTGCATCTGG | 293 | 58 | sqRT-PCR and qPCR 1 |
| R:TGAAAGCAGGCAGTTGGTGA | ||||
| β-actin | F:CCTGCGGCATTCACGAA | 60 | 60 | |
| R:GCGGATGTCGACGTCCACA | ||||
| PRAMEY3 | F:TGCCCTCATTTCGTTACCCT | 285 | 60 | qPCR 2 |
| R:TTTTCGCTTAGTTATCTGCTATCAT | ||||
| DDX3Y | F:TCGCCGCTTGCTTACGTACACT | 110 | 69 | |
| R:ACACCCTCTGGTTAACGGCCAT |
| Testicular Trait 1 | Mean ± SD | PRAMEY Copy Number | PRAMEY mRNA | ||
|---|---|---|---|---|---|
| r | p | r | p | ||
| TI | 5.29 × 10−3 ± 2.06 × 10−3 | 0.018 | 0.676 | 0.602 | <0.0001 |
| TTW (g) | 238.79 ± 92.67 | 0.012 | 0.766 | 0.609 | <0.0001 |
| LTW (g) | 119.56 ± 46.75 | 0.002 | 0.966 | 0.608 | <0.0001 |
| LTL (mm) | 81.05 ± 12.54 | −0.056 | 0.248 | 0.269 | 0.008 |
| LTWI (mm) | 57.73 ± 8.96 | −0.050 | 0.305 | 0.323 | 0.001 |
| LEW (g) | 18.15 ± 4.45 | −0.013 | 0.750 | 0.551 | <0.0001 |
| RTW (g) | 119.23 ± 46.64 | 0.023 | 0.583 | 0.621 | <0.0001 |
| RTL (mm) | 80.56 ± 12.53 | −0.017 | 0.731 | 0.299 | 0.003 |
| RTWI (mm) | 57.78 ± 8.90 | −0.027 | 0.577 | 0.325 | 0.001 |
| REW (g) | 17.95 ± 4.44 | 0.018 | 0.674 | 0.532 | <0.0001 |
| TEW (g) | 36.05 ± 8.64 | 0.007 | 0.865 | 0.499 | <0.0001 |
| LTV (mL) | 104.97 ± 45.70 | 0.056 | 0.184 | - | - |
| RTV (mL) | 103.56 ± 43.77 | 0.056 | 0.178 | - | - |
| TTV (mL) | 208.53 ± 88.65 | 0.056 | 0.180 | - | - |
| SC | 23.13 ± 3.34 | 0.029 | 0.495 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, S.; Qin, F.; Wang, L.; Li, W.; Li, F.; Yue, X. PRAMEY: A Bovid-Specific Y-Chromosome Multicopy Gene Is Highly Related to Postnatal Testicular Growth in Hu Sheep. Animals 2022, 12, 2380. https://doi.org/10.3390/ani12182380
Pei S, Qin F, Wang L, Li W, Li F, Yue X. PRAMEY: A Bovid-Specific Y-Chromosome Multicopy Gene Is Highly Related to Postnatal Testicular Growth in Hu Sheep. Animals. 2022; 12(18):2380. https://doi.org/10.3390/ani12182380
Chicago/Turabian StylePei, Shengwei, Fang Qin, Li Wang, Wanhong Li, Fadi Li, and Xiangpeng Yue. 2022. "PRAMEY: A Bovid-Specific Y-Chromosome Multicopy Gene Is Highly Related to Postnatal Testicular Growth in Hu Sheep" Animals 12, no. 18: 2380. https://doi.org/10.3390/ani12182380




