The Use of Metabolomics as a Tool to Compare the Regulatory Mechanisms in the Cecum, Ileum, and Jejunum in Healthy Rabbits and with Diarrhea
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statements
2.2. Animals, Feeding Management, and Sample Collection
2.3. Morphological Analysis of Intestinal Tissue
2.4. UHPLC–MS/MS Analysis
2.5. Data Processing and Metabolite Identification
3. Results
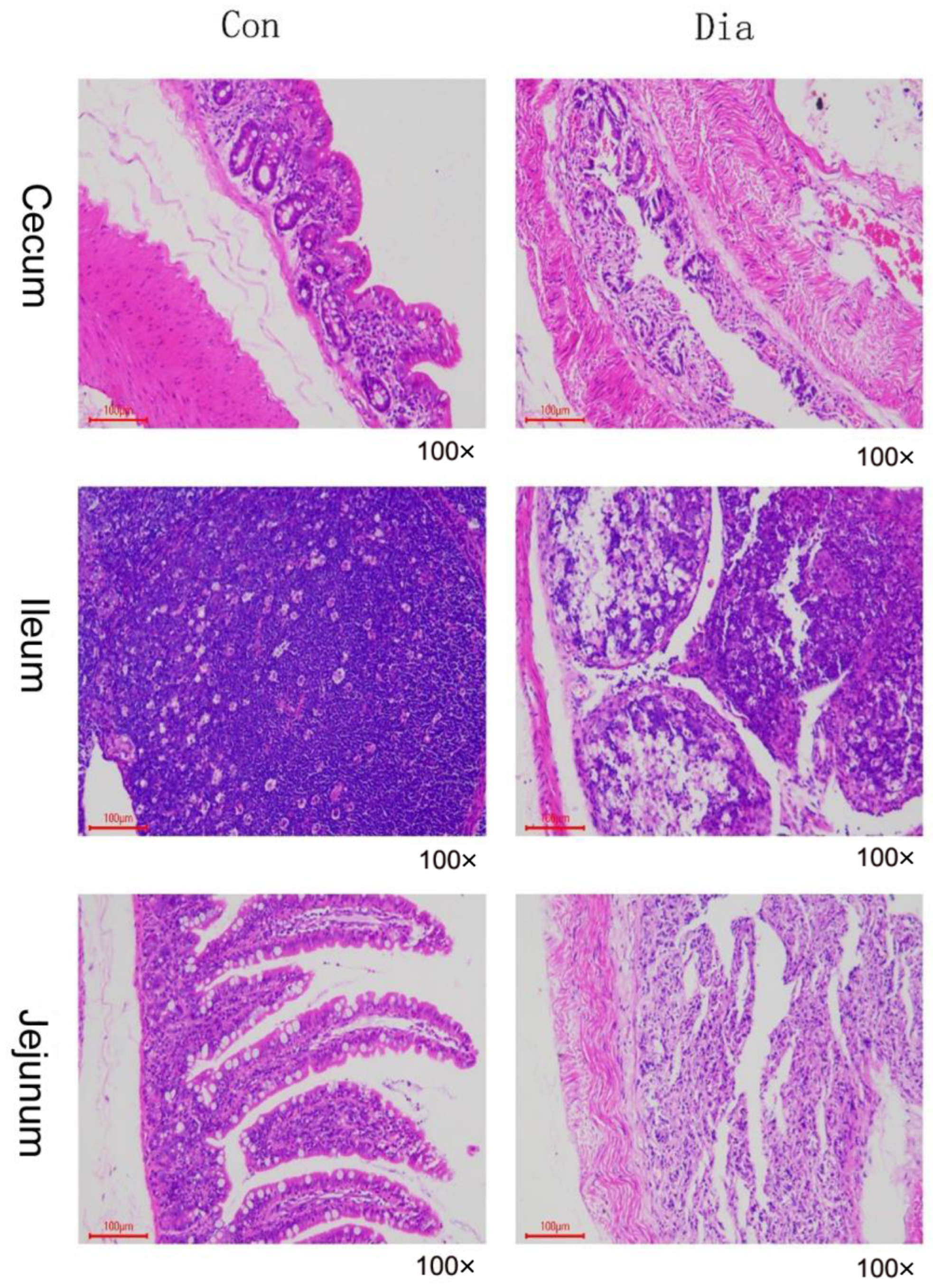
3.1. Intestinal Pathological Features
3.2. Metabolomics Changes in Diarrhea Rabbits
3.3. Differential Metabolite Analysis
3.4. KEGG Pathway Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cromwell, G.L. Why and how antibiotics are used in swine production. Anim. Biotechnol. 2002, 13, 7–27. [Google Scholar] [CrossRef] [PubMed]
- Ianiro, G.; Tilg, H.; Gasbarrini, A. Antibiotics as deep modulators of gut microbiota: Between good and evil. Gut 2016, 65, 1906–1915. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.D. Antibiotic Adjuvants: Rescuing Antibiotics from Resistance. Trends Microbiol. 2016, 24, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Aminov, R.I. The role of antibiotics and antibiotic resistance in nature. Environ. Microbiol. 2009, 11, 2970–2988. [Google Scholar] [CrossRef]
- Cullere, M.; Zotte, A.D. Rabbit meat production and consumption: State of knowledge and future perspectives. Meat Sci. 2018, 143, 137–146. [Google Scholar] [CrossRef]
- Hoop, R.K.; Ehrsam, H.; Keller, B. 10 years of rabbit autopsy—A review of frequent disease and mortality causes. Schweiz Arch. Tierheilkd. 1993, 135, 212–216. [Google Scholar]
- Li, S.; Zeng, W.; Li, R.; Hoffman, L.C.; He, Z.; Sun, Q.; Li, H. Rabbit meat production and processing in China. Meat Sci. 2018, 145, 320–328. [Google Scholar] [CrossRef]
- Rehg, J.E.; Lawton, G.W.; Pakes, S.P. Cryptosporidium cuniculus in the rabbit (Oryctolagus cuniculus). Lab. Anim. Sci. 1979, 29, 656–660. [Google Scholar]
- Warren, M.F.; Hamilton, P.B. Intestinl fragility during ochratoxicosis and aflatoxicosis in broiler chickens. Appl. Environ. Microbiol. 1980, 40, 641–645. [Google Scholar] [CrossRef]
- Gonzalez-Gil, A.M.; Elizondo-Montemayor, L. The Role of Exercise in the Interplay between Myokines, Hepatokines, Osteokines, Adipokines and Modulation of Inflammation for Energy Substrate Redistribution and Fat Mass Loss: A Review. Nutrients 2020, 12, 1899. [Google Scholar] [CrossRef]
- Tang, T.; Li, Y.; Wang, J.; Elzo, M.A.; Shao, J.; Li, Y.; Xia, S.; Fan, H.; Jia, X.; Lai, S. Untargeted Metabolomics Reveals Intestinal Pathogenesis and Self-Repair in Rabbits Fed an Antibiotic-Free Diet. Animals 2021, 11, 1560. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Cai, J.; Allman, E.L.; Smith, P.B.; Patterson, A.D. Quantitative Analysis of Bile Acid with UHPLC-MS/MS. Methods Mol. Biol. 2021, 2194, 291–300. [Google Scholar]
- Xuan, Q.; Hu, C.; Yu, D.; Wang, L.; Zhou, Y.; Zhao, X.; Li, Q.; Hou, X.; Xu, G. Development of a High Coverage Pseudotargeted Lipidomics Method Based on Ultra-High Performance Liquid Chromatography-Mass Spectrometry. Anal. Chem. 2018, 90, 7608–7616. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Ang, I.L.; Lam, M.M.; Wei, R.; Lei, K.M.; Zhou, X.; Lam, H.H.N.; He, Q.; Poon, T.C.W. Susceptibility to false discovery in biomarker research using liquid chromatography-high resolution mass spectrometry based untargeted metabolomics profiling. Clin. Transl. Med. 2021, 11, e469. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.X.; Chen, J. Immune-related intestinal diseases characterized by chronic diarrhea. Chin. J. Pathol. 2021, 50, 428–430. [Google Scholar]
- Pavlov, V.A.; Tracey, K.J. The vagus nerve and the inflammatory reflex—Linking immunity and metabolism. Nat. Rev. Endocrinol. 2012, 8, 743–754. [Google Scholar] [CrossRef]
- Williams, N.C.; O’Neill, L.A.J. A Role for the Krebs Cycle Intermediate Citrate in Metabolic Reprogramming in Innate Immunity and Inflammation. Front. Immunol. 2018, 9, 141. [Google Scholar] [CrossRef]
- Sharif, O.; Brunner, J.S.; Vogel, A.; Schabbauer, G. Macrophage Rewiring by Nutrient Associated PI3K Dependent Pathways. Front. Immunol. 2019, 10, 2002. [Google Scholar] [CrossRef]
- Wei, S.; Han, C.; He, F.; Song, Q.; Kang, B.; Liu, H.; Li, L.; Xu, H.; Zeng, X. Inhibition of PI3K-Akt-mTOR signal pathway dismissed the stimulation of glucose on goose liver cell growth. J. Anim. Physiol. Anim. Nutr. 2017, 101, e133–e143. [Google Scholar] [CrossRef]
- Hu, X.; Xu, Q.; Wan, H.; Hu, Y.; Xing, S.; Yang, H.; Gao, Y.; He, Z. PI3K-Akt-mTOR/PFKFB3 pathway mediated lung fibroblast aerobic glycolysis and collagen synthesis in lipopolysaccharide-induced pulmonary fibrosis. Lab. Investig. 2020, 100, 801–811. [Google Scholar] [CrossRef]
- Zhang, S.; Lachance, B.B.; Mattson, M.P.; Jia, X. Glucose metabolic crosstalk and regulation in brain function and diseases. Prog. Neurobiol. 2021, 204, 102089. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, J.; Wang, P.H.; Yang, N.; Huang, J.; Ou, J.; Xu, T.; Zhao, X.; Liu, T.; Huang, X.; et al. SARS-CoV-2 spike promotes inflammation and apoptosis through autophagy by ROS-suppressed PI3K/AKT/mTOR signaling. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166260. [Google Scholar] [CrossRef] [PubMed]
- Bishnupuri, K.S.; Alvarado, D.M.; Khouri, A.N.; Shabsovich, M.; Chen, B.; Dieckgraefe, B.K.; Ciorba, M.A. IDO1 and Kynurenine Pathway Metabolites Activate PI3K-Akt Signaling in the Neoplastic Colon Epithelium to Promote Cancer Cell Proliferation and Inhibit Apoptosis. Cancer Res. 2019, 79, 1138–1150. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.; Al Rihani, S.B.; Sharma, A.; Weadick, B.; Govindarajan, R.; Khan, S.U.; Sharma, P.R.; Dogra, A.; Nandi, U.; Reddy, C.N.; et al. Crocetin promotes clearance of amyloid-β by inducing autophagy via the STK11/LKB1-mediated AMPK pathway. Autophagy 2021, 17, 3813–3832. [Google Scholar] [CrossRef]
- Wu, C.; Chen, H.; Zhuang, R.; Zhang, H.; Wang, Y.; Hu, X.; Li, J.; Li, Y.; Wang, X.; Xu, H.; et al. Betulinic acid inhibits pyroptosis in spinal cord injury by augmenting autophagy via the AMPK-mTOR-TFEB signaling pathway. Int. J. Biol. Sci. 2021, 17, 1138–1152. [Google Scholar] [CrossRef]
- Foerster, E.G.; Mukherjee, T.; Cabral-Fernandes, L.; Rocha, J.D.; Girardin, S.E.; Philpott, D.J. How autophagy controls the intestinal epithelial barrier. Autophagy 2022, 18, 86–103. [Google Scholar] [CrossRef]
- Wu, W.; Wang, S.; Liu, Q.; Shan, T.; Wang, Y. Metformin Protects against LPS-Induced Intestinal Barrier Dysfunction by Activating AMPK Pathway. Mol. Pharm. 2018, 15, 3272–3284. [Google Scholar] [CrossRef]
- Xu, X.; Gao, Z.; Yang, F.; Yang, Y.; Chen, L.; Han, L.; Zhao, N.; Xu, J.; Wang, X.; Ma, Y.; et al. Antidiabetic Effects of Gegen Qinlian Decoction via the Gut Microbiota Are Attributable to Its Key Ingredient Berberine. Genom. Proteom. Bioinform. 2020, 18, 721–736. [Google Scholar] [CrossRef]
- Li, C.; Ai, G.; Wang, Y.; Lu, Q.; Luo, C.; Tan, L.; Lin, G.; Liu, Y.; Li, Y.; Zeng, H.; et al. Oxyberberine, a novel gut microbiota-mediated metabolite of berberine, possesses superior anti-colitis effect: Impact on intestinal epithelial barrier, gut microbiota profile and TLR4-MyD88-NF-κB pathway. Pharmacol. Res. 2020, 152, 104603. [Google Scholar] [CrossRef]
- Zheng, J.; Xu, H.; Huang, C.; Fan, J.; Mei, Q.; Lu, Y.; Lou, L.; Wang, X.; Zeng, Y. Quercetin protects against intestinal barrier disruption and inflammation in acute necrotizing pancreatitis through TLR4/MyD88/p38 MAPK and ERS inhibition. Pancreatology 2018, 18, 742–752. [Google Scholar]
- Dicarlo, M.; Teti, G.; Verna, G.; Liso, M.; Cavalcanti, E.; Sila, A.; Raveenthiraraj, S.; Mastronardi, M.; Santino, A.; Serino, G.; et al. Quercetin Exposure Suppresses the Inflammatory Pathway in Intestinal Organoids from Winnie Mice. Int. J. Mol. Sci. 2019, 20, 5771. [Google Scholar] [CrossRef] [PubMed]
- Zapata-Pérez, R.; Wanders, R.J.; van Karnebeek, C.D.; Houtkooper, R.H. NAD(+) homeostasis in human health and disease. EMBO Mol. Med. 2021, 13, e13943. [Google Scholar] [CrossRef] [PubMed]
- Gabandé-Rodríguez, E.; de las Heras, M.M.G.; Mittelbrunn, M. Control of Inflammation by Calorie Restriction Mimetics: On the Crossroad of Autophagy and Mitochondria. Cells 2019, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Haq, S.; Grondin, J.; Banskota, S.; Khan, W.I. Autophagy: Roles in intestinal mucosal homeostasis and inflammation. J. Biomed. Sci. 2019, 26, 19. [Google Scholar] [CrossRef] [PubMed]
- Gassen, N.C.; Papies, J.; Bajaj, T.; Emanuel, J.; Dethloff, F.; Chua, R.L.; Trimpert, J.; Heinemann, N.; Niemeyer, C.; Weege, F.; et al. SARS-CoV-2-mediated dysregulation of metabolism and autophagy uncovers host-targeting antivirals. Nat. Commun. 2021, 12, 3818. [Google Scholar] [CrossRef]
- Thomas, M.; Bader, C.; Monet, J.D. Sex steroid hormone modulation of NADPH pathways in MCF-7 cells. Cancer Res. 1990, 50, 1195–1200. [Google Scholar]
- Banihani, S.A. Effect of Coenzyme Q(10) Supplementation on Testosterone. Biomolecules 2018, 8, 172. [Google Scholar] [CrossRef]
- Schmidt, M.; Naumann, H.; Weidler, C.; Schellenberg, M.; Anders, S.; Straub, R.H. Inflammation and sex hormone metabolism. Ann. N. Y. Acad. Sci. 2006, 1069, 236–246. [Google Scholar] [CrossRef]
- Bruscoli, S.; Febo, M.; Riccardi, C.; Migliorati, G. Glucocorticoid Therapy in Inflammatory Bowel Disease: Mechanisms and Clinical Practice. Front. Immunol. 2021, 12, 691480. [Google Scholar] [CrossRef]
- Slominski, A.T.; Mahata, B.; Raman, C.; Bereshchenko, O. Editorial: Steroids and Secosteroids in the Modulation of Inflammation and Immunity. Front. Immunol. 2021, 12, 825577. [Google Scholar] [CrossRef]
- Vidal-Lletjós, S.; Beaumont, M.; Tomé, D.; Benamouzig, R.; Blachier, F.; Lan, A. Dietary Protein and Amino Acid Supplementation in Inflammatory Bowel Disease Course: What Impact on the Colonic Mucosa? Nutrients 2017, 9, 310. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front. Cell. Infect. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Sun-Waterhouse, D.; Cui, C. The therapeutic potential of diet on immune-related diseases: Based on the regulation on tryptophan metabolism. Crit. Rev. Food Sci. Nutr. 2021, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Platten, M.; Nollen, E.A.; Röhrig, U.F.; Fallarino, F.; Opitz, C.A. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discov. 2019, 18, 379–401. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef]
- Campbell, K.; Vowinckel, J.; Keller, M.A.; Ralser, M. Methionine Metabolism Alters Oxidative Stress Resistance via the Pentose Phosphate Pathway. Antioxid. Redox Signal. 2016, 24, 543–547. [Google Scholar] [CrossRef]
- McKell, M.C.; Crowther, R.R.; Schmidt, S.M.; Robillard, M.C.; Cantrell, R.; Lehn, M.A.; Janssen, E.M.; Qualls, J.E. Promotion of Anti-Tuberculosis Macrophage Activity by L-Arginine in the Absence of Nitric Oxide. Front. Immunol. 2021, 12, 653571. [Google Scholar] [CrossRef]
- Wu, M.; Xiao, H.; Shao, F.; Tan, B.; Hu, S. Arginine accelerates intestinal health through cytokines and intestinal microbiota. Int. Immunopharmacol. 2020, 81, 106029. [Google Scholar] [CrossRef]
- Xia, Z.; Huang, L.; Yin, P.; Liu, F.; Liu, Y.; Zhang, Z.; Lin, J.; Zou, W.; Li, C. L-Arginine alleviates heat stress-induced intestinal epithelial barrier damage by promoting expression of tight junction proteins via the AMPK pathway. Mol. Biol. Rep. 2019, 46, 6435–6451. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, C.; Sifuentes-Dominguez, L.; Zarek, C.M.; Propheter, D.C.; Kuang, Z.; Wang, Y.; Pendse, M.; Ruhn, K.A.; Hassell, B.; et al. Small proline-rich protein 2A is a gut bactericidal protein deployed during helminth infection. Science 2021, 374, eabe6723. [Google Scholar] [CrossRef]
- Yang, Z.; Liao, S.F. Physiological Effects of Dietary Amino Acids on Gut Health and Functions of Swine. Front. Vet. Sci. 2019, 6, 169. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Dietary protein intake and human health. Food Funct. 2016, 7, 1251–1265. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.W.; Qiao, S.Y.; Li, D.F. Amino acids and gut function. Amino Acids 2009, 37, 105–110. [Google Scholar] [CrossRef] [PubMed]





| Intestinal Tissue Comparison | Ion Mode | Not Signif. Different 1 | Not Signif. Up 2 | Not Signif. Down 3 |
|---|---|---|---|---|
| Dia_cecum vs. Con_cecum | Positive | 485 | 307 | 178 |
| Negative | 167 | 102 | 65 | |
| Sum | 652 | 409 | 243 | |
| Dia_ileum vs. Con_ileum | Positive | 399 | 244 | 155 |
| Negative | 220 | 155 | 65 | |
| Sum | 619 | 399 | 220 | |
| Dia_jejunum vs. Con_jejunum | Positive | 459 | 154 | 305 |
| Negative | 172 | 65 | 107 | |
| Sum | 631 | 219 | 412 | |
| All intestinal tissue comparisons | Total | 1902 | 1027 | 875 |
| Tisue | Map ID | Map Title | p-Value | N | Meta IDs |
|---|---|---|---|---|---|
| Cecum | map04976 | Bile secretion | 0.073658105 | 105 | Salicylic acid, Deoxycholic acid, Lithocholic acid, and Chenoaeoxychohc acid. |
| map00520 | Amino sugar and nucletide sugar metabolism | 0.098581105 | 105 | L-FucoseN-Acetylneuraminic acid, and N-Acetyl-alpha-D-glucosamine 1-phospnate. | |
| map00380 | Trptophan metabolism | 0.244398105 | 105 | Picolinic acid, and Quinolinic acid. | |
| map01523 | Antifolate resistance | 0.072913182 | 182 | Folic acid, Guanosine monophosphate and Adenosine 5′-monophosphate. | |
| Ileum | map00140 | Steroid hormone biosynthesis | 0.144317115 | 115 | Androsterone and Aldosterone. |
| map00400 | Phenylalanine, tyrosine, and tryptophan biosynthesis | 0.155651115 | 115 | D-Erythrose 4-phosphate, Phenylpyruvic acid, and 5-Phosphonbosyl 1-pyrophosphate | |
| map00250 | Alanine, aspartate, and glutamate metabolism | 0.050281 | 150 | L-Glutamic acidzD-Glucosamine 6-phosphate and L-Asparagine. | |
| map00230 | Purine metabolism | 0.102723 | 150 | 2′-Deoxyadenosine, dAMP, Deoxyadenosine and Uric acid. | |
| map00430 | Taurine and hypotaurine metabolism | 0.137807 | 150 | L-Glutamic acid and L-Glutamicacid. | |
| Jejunum | map00562 | Inositol phosphate metabolism | 0.010489 | 103 | D-Glucose 6-phosphate, D-myo-Inositol 1,4-bisphosphate, Inositol and Glyceraldehyde 3-phpsphate. |
| map00030 | Pentose phosphate pathway | 0.103877 | 103 | D-Erythrose 4-phosphate, D-Sedoheptulose 7-phosphate, Glyceraldehyde 3-phosphate. | |
| map00052 | Galactose metabolism | 0.103877 | 103 | Dulcitol and Glyceraldehyde 3-phosphate. | |
| map00130 | Ubiquinone and other terpenoid-quinone biosynthesis | 0.058071 | 163 | Menaquinone, Phylloquinone, gamma-Tocopherol and Shikonin. | |
| map04152 | AMPK signaling pathway | 0.135346 | 163 | Berberine, Quercetin, and D-Fructose 6-phosphate. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Shao, J.; Lai, S.; Wang, J.; Zhao, K.; Tang, T.; Wang, M. The Use of Metabolomics as a Tool to Compare the Regulatory Mechanisms in the Cecum, Ileum, and Jejunum in Healthy Rabbits and with Diarrhea. Animals 2022, 12, 2438. https://doi.org/10.3390/ani12182438
Liu Z, Shao J, Lai S, Wang J, Zhao K, Tang T, Wang M. The Use of Metabolomics as a Tool to Compare the Regulatory Mechanisms in the Cecum, Ileum, and Jejunum in Healthy Rabbits and with Diarrhea. Animals. 2022; 12(18):2438. https://doi.org/10.3390/ani12182438
Chicago/Turabian StyleLiu, Zheliang, Jiahao Shao, Songjia Lai, Jie Wang, Kaisen Zhao, Tao Tang, and Meigui Wang. 2022. "The Use of Metabolomics as a Tool to Compare the Regulatory Mechanisms in the Cecum, Ileum, and Jejunum in Healthy Rabbits and with Diarrhea" Animals 12, no. 18: 2438. https://doi.org/10.3390/ani12182438
APA StyleLiu, Z., Shao, J., Lai, S., Wang, J., Zhao, K., Tang, T., & Wang, M. (2022). The Use of Metabolomics as a Tool to Compare the Regulatory Mechanisms in the Cecum, Ileum, and Jejunum in Healthy Rabbits and with Diarrhea. Animals, 12(18), 2438. https://doi.org/10.3390/ani12182438






