Effects of Autolyzed Yeast Supplementation in a High-Starch Diet on Rumen Health, Apparent Digestibility, and Production Variables of Lactating Holstein Cows
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care and Housing
2.2. Experimental Design
2.3. Data Collection and Sampling Procedures
2.4. Statistical Analyses
3. Results
3.1. Diet Composition
3.2. DMI, BW, BCS, and Lactation Performance
3.3. Rumen pH, Fecal pH and VFA
3.4. Nitrogen Excretion and AD
3.5. Serum and Plasma Chemistry Profile
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weiss, W.P.; Willett, L.B.; St-Pierre, N.R.; Borger, D.C.; McKelvey, T.R.; Wyatt, D.J. Varying forage type, metabolizable protein concentration, and carbohydrate source affects manure excretion, manure ammonia, and nitrogen metabolism of dairy cows. J. Dairy Sci. 2009, 92, 5607–5619. [Google Scholar] [CrossRef]
- Powell, J.M.; Gourley, C.J.P.; Rotz, C.A.; Weaver, D.M. Nitrogen use efficiency: A potential performance indicator and policy tool for dairy farms. Environ. Sci. Policy 2010, 13, 217–228. [Google Scholar] [CrossRef]
- Broderick, G.A.; Mertens, D.R.; Simons, R. Efficacy of Carbohydrate Sources for Milk Production by Cows Fed Diets Based on Alfalfa Silage. J. Dairy Sci. 2002, 85, 1767–1776. [Google Scholar] [CrossRef]
- Oba, M.; Allen, M.S. Effects of Corn Grain Conservation Method on Feeding Behavior and Productivity of Lactating Dairy Cows at Two Dietary Starch Concentrations. J. Dairy Sci. 2003, 86, 174–183. [Google Scholar] [CrossRef]
- Dann, H.M.; Tucker, H.A.; Cotanch, K.W.; Krawczel, P.D.; Mooney, C.S.; Grant, R.J.; Eguchi, T. Evaluation of lower-starch diets for lactating Holstein dairy cows. J. Dairy Sci. 2014, 97, 7151–7161. [Google Scholar] [CrossRef]
- Penner, G.B.; Beauchemin, K.A.; Mutsvangwa, T. Severity of Ruminal Acidosis in Primiparous Holstein Cows during the Periparturient Period. J. Dairy Sci. 2007, 90, 365–375. [Google Scholar] [CrossRef]
- Dias, A.L.G.; Freitas, J.A.; Micai, B.; Azevedo, R.A.; Greco, L.F.; Santos, J.E.P. Effects of supplementing yeast culture to diets differing in starch content on rumen fermentation and digestion in dairy cow. J. Dairy Sci. 2018, 101, 201–221. [Google Scholar] [CrossRef]
- Shi, W.; Knoblock, C.E.; Murphy, K.V.; Bruinjé, T.C.; Yoon, I.; Ambrose, D.J.; Oba, M. Effects of supplementing a Saccharomyces cerevisiae fermentation product during the periparturient period on performance of dairy cows fed fresh diets differing in starch content. J. Dairy Sci. 2019, 102, 3082–3096. [Google Scholar] [CrossRef]
- Ferraretto, L.; Shaver, R.D.; Espiñeira, M.; Gencoglu, H.; Bertics, S.J. Influence of a reduced-starch diet with or without exogenous amylase on lactation performance by dairy cows. J. Dairy Sci. 2011, 94, 1490–1499. [Google Scholar] [CrossRef]
- EPA (Environmental Protection Agency). Aquatic Life Criteria–Ammonia. 2019. Available online: https://www.epa.gov/wqc/aquatic-life-criteria-ammonia (accessed on 7 April 2020).
- Broadway, P.R.; Carroll, J.A.; Sanchez, N.C.B. Live Yeast and Yeast Cell Wall Supplements Enhance Immune Function and Performance in Food-Producing Livestock: A Review. Microorganisms 2015, 3, 417–427. [Google Scholar] [CrossRef]
- Alugongo, G.M.; Xiao, J.; Wu, Z.; Li, S.; Wang, Y.; Cao, Z. Review: Utilization of yeast of Saccharomyces cerevisiae origin in artificially raised calves. J. Anim. Sci. Biotechnol. 2017, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xing, J.; Li, D.; Wang, X.; Zhao, L.; Lv, S.; Huang, D. Effects of β-glucan extracted from Saccharomyces cerevisiae on humoral and cellular immunity in weaned piglets. Arch. Anim. Nutr. 2005, 59, 303–312. [Google Scholar] [CrossRef]
- Volman, J.J.; Ramakers, J.D.; Plat, J. Dietary modulation of immune function by β-glucans. Physiol. Behav. 2008, 94, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Callaway, E.S.; Martin, S.A. Effects of a Saccharomyces cerevisiae Culture on Ruminal Bacteria that Utilize Lactate and Digest Cellulose. J. Dairy Sci. 1997, 80, 2035–2044. [Google Scholar] [CrossRef]
- Desnoyers, M.; Giger-Reverdin, S.; Bertin, G.; Duvaux-Ponter, C.; Sauvant, D. Meta-analysis of the influence of Saccharomyces cerevisiae supplementation on ruminal parameters and milk production of ruminants. J. Dairy Sci. 2009, 92, 1620–1632. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.L.G.; Freitas, J.A.; Micai, B.; Azevedo, R.A.; Greco, L.F.; Santos, J.E.P. Effects of supplementing yeast culture to diets differing in starch content on performance and feeding behavior of dairy cows. J. Dairy Sci. 2018, 101, 186–200. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, V.; Petri, R.; Humer, E.; Kröger, I.; Mann, E.; Reisinger, N.; Wagner, M.; Zebeli, Q. High-grain diets supplemented with phytogenic compounds or autolyzed yeast modulate ruminal bacterial community and fermentation in dry cows. J. Dairy Sci. 2018, 101, 2335–2349. [Google Scholar] [CrossRef]
- Newbold, C.J.; Wallace, R.J.; Mcintosh, F.M. Mode of action of the yeast Saccharomyces cerevisiae as a feed additive for ruminants. Br. J. Nutr. 1996, 76, 249–261. [Google Scholar] [CrossRef]
- Chaucheyras-Durand, F.; Walker, N.; Bach, A. Effects of active dry yeasts on the rumen microbial ecosystem: Past, present and future. Anim. Feed Sci. Technol. 2008, 145, 5–26. [Google Scholar] [CrossRef]
- Julien, C.; Marden, J.P.; Auclair, E.; Moncoulon, R.; Cauquil, L.; Peyraud, J.L.; Bayourthe, C. Interaction between Live Yeast and Dietary Rumen Degradable Protein Level: Effects on Diet Utilization in Early-Lactating Dairy Cows. Agric. Sci. 2015, 6, 1–13. [Google Scholar] [CrossRef]
- Lascano, G.J.; Heinrichs, A.J.; Tricarico, J.M. Substitution of starch by soluble fiber and Saccharomyces cerevisiae dose response on nutrient digestion and blood metabolites for precision-fed dairy heifers. J. Dairy Sci. 2012, 95, 3298–3309. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Kang, K.; Wang, H.; Wang, Z.; Xue, B.; Wang, L.; Xu, F.; Peng, Q. Effects of dietary supplementation of active dried yeast on fecal methanogenic archaea diversity in dairy cows. Anaerobe 2017, 44, 78–86. [Google Scholar] [CrossRef] [PubMed]
- AOAC International. Official method 934.01. Moisture in Animal Feed. In Official Methods of Analysis, 16th ed.; AOAC International: Arlington, VA, USA, 1995; Volume 2, pp. 23–26. [Google Scholar]
- National Research Council. Nutrient Requirements of Dairy Cattle, 7th ed.; National Academies Press: Washington, DC, USA, 2001; p. 138. [Google Scholar] [CrossRef]
- Kononoff, P.; Heinrichs, A.; Buckmaster, D. Modification of the Penn State Forage and Total Mixed Ration Particle Separator and the Effects of Moisture Content on its Measurements. J. Dairy Sci. 2003, 86, 1858–1863. [Google Scholar] [CrossRef]
- Farmer, E.R.; Tucker, H.A.; Dann, H.M.; Cotanch, K.W.; Mooney, C.S.; Lock, A.L.; Yagi, K.; Grant, R.J. Effect of reducing dietary forage in lower starch diets on performance, ruminal characteristics, and nutrient digestibility in lactating Holstein cows. J. Dairy Sci. 2014, 97, 5742–5753. [Google Scholar] [CrossRef] [PubMed]
- AOAC International. Official Method 972.16. Fat, lactose, protein, and solids in milk. Mid-infrared spectroscopic method. In Official Methods of Analysis, 16th ed.; AOAC International: Arlington, VA, USA, 1995; Volume 2, pp. 2–5. [Google Scholar]
- Chen, X. Excretion of Purine Derivatives by Sheep and Cattle and It Use for Estimation of Absorbed Microbial Protein. PhD Thesis, University of Aberdeen, Aberdeen, UK, 1989. [Google Scholar]
- Valadares, R.F.D.; Broderick, G.A.; Filho, S.V.; Clayton, M.K. Effect of Replacing Alfalfa Silage with High Moisture Corn on Ruminal Protein Synthesis Estimated from Excretion of Total Purine Derivatives. J. Dairy Sci. 1999, 82, 2686–2696. [Google Scholar] [CrossRef]
- Maynard, L.A.; Loosli, J.K.; Hintz, H.F.; Warner, R.G. Digestive processes in different species. In Animal Nutrition; McGraw-Hill Inc.: New York, NY, USA, 1979; pp. 21–46. [Google Scholar]
- Johnson, M.M.; Peters, J.P. Technical note: An improved method to quantify nonesterified fatty acids in bovine plasma. J. Anim. Sci. 1993, 71, 753–756. [Google Scholar] [CrossRef]
- Krause, K.M.; Dhuyvetter, D.V.; Oetzel, G.R. Effect of a low-moisture buffer block on ruminal pH in lactating dairy cattle induced with subacute ruminal acidosis. J. Dairy Sci. 2009, 92, 352–364. [Google Scholar] [CrossRef]
- Ferguson, J.D.; Galligan, D.T.; Thomsen, N. Principal Descriptors of Body Condition Score in Holstein Cows. J. Dairy Sci. 1994, 77, 2695–2703. [Google Scholar] [CrossRef]
- Littell, R.C. Analysis of unbalanced mixed model data: A case study comparison of ANOVA versus REML/GLS. J. Agric. Biol. Environ. Stat. 2002, 7, 472–490. [Google Scholar] [CrossRef]
- Cochran, W.G.; Cox, M.G. Completely Randomized, Randomized Block, and Latin Square Designs. In Experimental Designs; Wiley: New York, NY, USA, 1957; pp. 133–139. [Google Scholar]
- Cardoso, F.C.; Sears, W.; Leblanc, S.J.; Drackley, J.K. Technical note: Comparison of 3 methods for analyzing areas under the curve for glucose and nonesterified fatty acids concentrations following epinephrine challenge in dairy cows. J. Dairy Sci. 2011, 94, 6111–6115. [Google Scholar] [CrossRef]
- Ireland-Perry, R.L.; Stallings, C.C. Fecal Consistency as Related to Dietary Composition in Lactating Holstein Cows. J. Dairy Sci. 1993, 76, 1074–1082. [Google Scholar] [CrossRef]
- Krause, K.M.; Oetzel, G.R. Inducing Subacute Ruminal Acidosis in Lactating Dairy Cows. J. Dairy Sci. 2005, 88, 3633–3639. [Google Scholar] [CrossRef]
- Enemark, J.M.D. The monitoring, prevention and treatment of sub-acute ruminal acidosis (SARA): A review. Vet. J. 2008, 176, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Ferraretto, L.; Shaver, R.D.; Bertics, S.J. Effect of dietary supplementation with live-cell yeast at two dosages on lactation performance, ruminal fermentation, and total-tract nutrient digestibility in dairy cows. J. Dairy Sci. 2012, 95, 4017–4028. [Google Scholar] [CrossRef]
- Miettinen, H.; Huhtanen, P. Effects of the Ratio of Ruminal Propionate to Butyrate on Milk Yield and Blood Metabolites in Dairy Cows. J. Dairy Sci. 1996, 79, 851–861. [Google Scholar] [CrossRef]
- Gencoglu, H.; Shaver, R.D.; Steinberg, W.; Ensink, J.; Ferraretto, L.; Bertics, S.J.; Lopes, J.C.; Akins, M.S. Effect of feeding a reduced-starch diet with or without amylase addition on lactation performance in dairy cows. J. Dairy Sci. 2010, 93, 723–732. [Google Scholar] [CrossRef]
- Harvatine, K.J.; Bauman, D.E. Characterization of the acute lactational response to trans-10, cis-12 conjugated linoleic acid. J. Dairy Sci. 2011, 94, 6047–6056. [Google Scholar] [CrossRef]
- Cabrita, A.R.J.; Bessa, R.J.B.; Alves, S.; Dewhurst, R.J.; Fonseca, A.J.M. Effects of Dietary Protein and Starch on Intake, Milk Production, and Milk Fatty Acid Profiles of Dairy Cows Fed Corn Silage-Based Diets. J. Dairy Sci. 2007, 90, 1429–1439. [Google Scholar] [CrossRef]
- Oba, M.; Allen, M.S. Intraruminal infusion of propionate alters feeding behavior and decreases energy intake of lactating dairy cows. J. Nutr. 2003, 133, 1094–1099. [Google Scholar] [CrossRef]
- Thomas, P.C. Milk protein. Proc. Nutr. Soc. 1983, 42, 407–418. [Google Scholar] [CrossRef]
- Zhao, K.; Liu, H.-Y.; Zhou, M.-M.; Zhao, F.-Q.; Liu, J.-X. Insulin stimulates glucose uptake via a phosphatidylinositide 3-kinase-linked signaling pathway in bovine mammary epithelial cells. J. Dairy Sci. 2014, 97, 3660–3665. [Google Scholar] [CrossRef] [PubMed]
- Radostits, O.M.; Blood, D.C.; Gay, C.C. (Eds.) Acute carbohydrate engorgement of ruminants (rumen overload). In Veterinary Medicine; WB Saunders: Philadelphia, PA, USA, 1994; pp. 262–269. [Google Scholar]
- AlZahal, O.; Dionissopoulos, L.; Laarman, A.; Walker, N.; McBride, B. Active dry Saccharomyces cerevisiae can alleviate the effect of subacute ruminal acidosis in lactating dairy cows. J. Dairy Sci. 2014, 97, 7751–7763. [Google Scholar] [CrossRef] [PubMed]
- Beasom, S.L.; LaPlant, L.; Howard, V.W. Fecal pH of Pronghorn and Sheep as Related to Diet. J. Wildl. Manag. 1982, 46, 1101–1104. [Google Scholar] [CrossRef]
- Gressley, T.F.; Hall, M.B.; Armentano, L.E. Ruminant nutrition symposium: Productivity, digestion, and health responses to hindgut acidosis in ruminants. J. Anim. Sci. 2011, 89, 1120–1130. [Google Scholar] [CrossRef] [PubMed]
- Beauchemin, K.A.; Yang, W.Z. Effects of Physically Effective Fiber on Intake, Chewing Activity, and Ruminal Acidosis for Dairy Cows Fed Diets Based on Corn Silage. J. Dairy Sci. 2005, 88, 2117–2129. [Google Scholar] [CrossRef]
- Seymour, W.M.; Campbell, D.R.; Johnson, Z.B. Relationships between rumen volatile fatty acid concentrations and milk production in dairy cows: A literature study. Anim. Feed Sci. Technol. 2005, 119, 155–169. [Google Scholar] [CrossRef]
- Aschenbach, J.R.; Kristensen, N.B.; Donkin, S.S.; Hammon, H.M.; Penner, G.B. Gluconeogenesis in dairy cows: The secret of making sweet milk from sour dough. IUBMB Life 2010, 62, 869–877. [Google Scholar] [CrossRef]
- Aguerre, M.J.; Wattiaux, M.A.; Powell, J.; Broderick, G.A.; Arndt, C. Effect of forage-to-concentrate ratio in dairy cow diets on emission of methane, carbon dioxide, and ammonia, lactation performance, and manure excretion. J. Dairy Sci. 2011, 94, 3081–3093. [Google Scholar] [CrossRef]
- Moorby, J.M.; Dewhurst, R.J.; Evans, R.T.; Danelón, J.L. Effects of Dairy Cow Diet Forage Proportion on Duodenal Nutrient Supply and Urinary Purine Derivative Excretion. J. Dairy Sci. 2006, 89, 3552–3562. [Google Scholar] [CrossRef]
- Sommerfeldt, J.L.; Lyon, K.A. Ration Digestibilities and Ruminal Characteristics in Steers Fed Chickpeas. J. Dairy Sci. 1988, 71, 843–847. [Google Scholar] [CrossRef]
- Agle, M.; Hristov, A.N.; Zaman, S.; Schneider, C.; Ndegwa, P.M.; Vaddella, V.K. Effect of dietary concentrate on rumen fermentation, digestibility, and nitrogen losses in dairy cows. J. Dairy Sci. 2010, 93, 4211–4222. [Google Scholar] [CrossRef] [PubMed]
- Hristov, A.N.; Varga, G.; Cassidy, T.; Long, M.; Heyler, K.; Karnati, S.K.R.; Corl, B.; Hovde, C.J.; Yoon, I. Effect of Saccharomyces cerevisiae fermentation product on ruminal fermentation and nutrient utilization in dairy cows. J. Dairy Sci. 2010, 93, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Bryant, M.P. Nutritional requirements of the predominant rumen cellulolytic bacteria. Fed. Proc. 1973, 32, 1809–1813. [Google Scholar]
- Hernandez, A.; Kholif, A.E.A.A.; Lugo-Coyote, R.; Elghandour, M.M.Y.; Cipriano, M.; Rodríguez, G.B.; Odongo, N.E.; Salem, A.Z.M. The effect of garlic oil, xylanase enzyme and yeast on biomethane and carbon dioxide production from 60-d old Holstein dairy calves fed a high concentrate diet. J. Clean. Prod. 2016, 142, 2384–2392. [Google Scholar] [CrossRef]
- Mwenya, B.; Santoso, B.; Sar, C.; Pen, B.; Morikawa, R.; Takaura, K.; Umetsu, K.; Kimura, K.; Takahashi, J. Effects of Yeast Culture and Galacto-Oligosaccharides on Ruminal Fermentation in Holstein Cows. J. Dairy Sci. 2005, 88, 1404–1412. [Google Scholar] [CrossRef]
- Wohlt, J.E.; Finkelstein, A.D.; Chung, C.H. Yeast culture to improve intake, and performance by dairy cattle nutrient digestibility, during early lactation. J. Dairy Sci. 1991, 74, 1395–1400. [Google Scholar] [CrossRef]
- Erasmus, L.; Botha, P.; Kistner, A. Effect of Yeast Culture Supplement on Production, Rumen Fermentation, and Duodenal Nitrogen Flow in Dairy Cows. J. Dairy Sci. 1992, 75, 3056–3065. [Google Scholar] [CrossRef]
- Yang, W.Z.; Beauchemin, K.A.; Rode, L.M. Effects of Grain Processing, Forage to Concentrate Ratio, and Forage Particle Size on Rumen pH and Digestion by Dairy Cows. J. Dairy Sci. 2001, 84, 2203–2216. [Google Scholar] [CrossRef]
- Beckman, J.L.; Weiss, W.P. Nutrient Digestibility of Diets with Different Fiber to Starch Ratios when Fed to Lactating Dairy Cows. J. Dairy Sci. 2005, 88, 1015–1023. [Google Scholar] [CrossRef]
- Hatew, B.; Podesta, S.C.; Van Laar, H.; Pellikaan, W.F.; Ellis, J.; Dijkstra, J.; Bannink, A. Effects of dietary starch content and rate of fermentation on methane production in lactating dairy cows. J. Dairy Sci. 2015, 98, 486–499. [Google Scholar] [CrossRef]
- Firkins, J.L.; Eastridge, M.L.; St-Pierre, N.R.; Noftsger, S.M. Effects of grain variability and processing on starch utilization by lactating dairy cows. J. Anim. Sci. 2001, 79 (Suppl. E), E218–E238. [Google Scholar] [CrossRef]
- Herdt, T.H. Variability Characteristics and Test Selection in Herdlevel Nutritional and Metabolic Profile Testing. Vet. Clin. N. Am. Food Anim. Pract. 2000, 16, 387–403. [Google Scholar] [CrossRef]
- Allen, M.S.; Bradford, B.; Oba, M. BOARD-INVITED REVIEW: The hepatic oxidation theory of the control of feed intake and its application to ruminants. J. Anim. Sci. 2009, 87, 3317–3334. [Google Scholar] [CrossRef] [PubMed]
- Russel, A.J.F.; Wright, I.A. The use of blood metabolites in the determination of energy status in beef cows. Anim. Sci. 1983, 37, 335–343. [Google Scholar] [CrossRef]
- Cozzi, G.; Ravarotto, L.; Gottardo, F.; Stefani, A.L.; Contiero, B.; Moro, L.; Brscic, M.; Dalvit, P. Short communication: Reference values for blood parameters in Holstein dairy cows: Effects of parity, stage of lactation, and season of production. J. Dairy Sci. 2011, 94, 3895–3901. [Google Scholar] [CrossRef]
- Piccioli-Cappelli, F.; Loor, J.; Seal, C.J.; Minuti, A.; Trevisi, E. Effect of dietary starch level and high rumen-undegradable protein on endocrine-metabolic status, milk yield, and milk composition in dairy cows during early and late lactation. J. Dairy Sci. 2014, 97, 7788–7803. [Google Scholar] [CrossRef]
- Merck Manuals. Global Medical Knowledge. 2020. Available online: https://www.merckmanuals.com/professional/resourcespages/global-medical-knowledge-2020 (accessed on 6 April 2020).
- Cao, Y.; Zhang, J.; Yang, W.; Xia, C.; Zhang, H.Y.; Wang, Y.H.; Xu, C. Predictive value of plasma parameters in the risk of postpartum ketosis in dairy cows. J. Vet. Res. 2017, 61, 91–95. [Google Scholar] [CrossRef]
- ECLINPATH, Cornell University College of Veterinary Medicine. Available online: http://eclinpath.com/chemistry/proteins/acute-phase-proteins/.2013 (accessed on 7 April 2020).
- Bossaert, P.; Trevisi, E.; Opsomer, G.; Bertoni, G.; De Vliegher, S.; Leroy, J.L. The association between indicators of inflammation and liver variables during the transition period in high-yielding dairy cows: An observational study. Vet. J. 2012, 192, 222–225. [Google Scholar] [CrossRef]
- Burke, C.R.; Meier, S.; McDougall, S.; Compton, C.; Mitchell, M.; Roche, J. Relationships between endometritis and metabolic state during the transition period in pasture-grazed dairy cows. J. Dairy Sci. 2010, 93, 5363–5373. [Google Scholar] [CrossRef]
- Baghshani, H.; Nazifi, S.; Saeb, M.; Saeb, S. Influence of road transportation on plasma concentrations of acute phase proteins, including fibrinogen, haptoglobin, serum amyloid A, and ceruloplasmin, in dromedary camels (Camelus dromedarius). Comp. Clin. Pathol. 2010, 19, 193–198. [Google Scholar] [CrossRef]
- Cannizzo, C.; Gianesella, M.; Giudice, E.; Messina, V.; Piccione, G.; Morgante, M. Serum acute phase proteins in cows with SARA (Subacute Ruminal Acidosis) suspect. Med. Vet. Zootec. 2012, 64, 15–22. [Google Scholar] [CrossRef]
- Moolchandani, A.; Sareen, M. A Review: Oxidative Stress during Lactation in Dairy Cattle. J. Dairy Vet. Sci. 2018, 5, 555669. [Google Scholar] [CrossRef]
- Castillo, C.; Hernandez, J.; Bravo, A.; Lopez-Alonso, M.; Pereira, V.; Benedito, J.L. Oxidative status during late pregnancy and early lactation in dairy cows. Vet. J. 2005, 169, 286–292. [Google Scholar] [CrossRef]
- Abaker, J.A.; Xu, T.L.; Jin, D.; Chang, G.J.; Zhang, K.; Shen, X.Z. Lipopolysaccharide derived from the digestive tract provokes oxidative stress in the liver of dairy cows fed a high-grain diet. J. Dairy Sci. 2017, 100, 666–678. [Google Scholar] [CrossRef] [PubMed]

| Diets | ||
|---|---|---|
| Ingredient, % of DM | LS | HS |
| Corn silage 1 | 49.77 | 30.36 |
| Alfalfa hay | 16.02 | 17.38 |
| Soybean meal | 13.14 | 13.38 |
| Dry ground corn grain | 6.41 | 23.23 |
| Canola meal | 4.65 | 5.01 |
| Corn gluten feed | 2.69 | 2.91 |
| Soy hulls | 1.87 | 2.02 |
| Dried molasses | 1.38 | 1.49 |
| Bypass fat 2 | 1.03 | 1.11 |
| Dicalcium phosphate | 0.40 | 0.44 |
| Trace mineral 3 | 0.06 | 0.07 |
| Rumen protected lysine 4 | 0.06 | 0.07 |
| Rumen protected methionine 5 | 0.04 | 0.04 |
| Potassium carbonate | 0.13 | 0.13 |
| Sodium bicarbonate | 0.66 | 0.66 |
| Calcium carbonate | 0.65 | 0.65 |
| Potassium chloride | 0.17 | 0.17 |
| Urea 46% | 0.15 | 0.15 |
| Salt, white | 0.07 | 0.07 |
| Magnesium oxide 54% | 0.07 | 0.07 |
| Vitamin and mineral mix 6 | 0.58 | 0.58 |
| Item | LS | HS | ||
|---|---|---|---|---|
| Mean 1 | SD | Mean 1 | SD | |
| DM, % | 43.1 | 2.36 | 51.9 | 3.22 |
| CP, % of DM | 17.8 | 0.63 | 17.2 | 0.49 |
| ADF, % of DM | 21.4 | 1.37 | 18.6 | 1.06 |
| NDF, % of DM | 31.8 | 0.94 | 28.7 | 1.24 |
| Lignin, % of DM | 3.6 | 0.41 | 3.2 | 0.56 |
| NFC, % of DM | 37.5 | 1.89 | 42.5 | 1.52 |
| Starch, % of DM | 22.8 | 0.70 | 31.2 | 4.22 |
| Crude fat, % of DM | 3.9 | 0.29 | 3.8 | 0.26 |
| Ash, % of DM | 8.96 | 1.73 | 7.81 | 1.02 |
| NEL, Mcal/kg of DM 2 | 1.63 | 0.04 | 1.69 | 0.04 |
| Ca, % of DM | 1.41 | 0.76 | 1.07 | 0.37 |
| P, % of DM | 0.44 | 0.01 | 0.45 | 0.01 |
| Mg, % of DM | 0.28 | 0.02 | 0.27 | 0.01 |
| K, % of DM | 1.57 | 0.09 | 1.47 | 0.04 |
| Na, % of DM | 0.33 | 0.05 | 0.31 | 0.01 |
| S, % of DM | 0.24 | 0.01 | 0.23 | 0.01 |
| Fe, mg/kg | 402 | 157.98 | 354 | 110.30 |
| Zn, mg/kg | 107 | 14.41 | 101 | 2.47 |
| Cu, mg/kg | 16 | 1.85 | 14 | 0.33 |
| Mn, mg/kg | 101 | 23.16 | 88 | 13.75 |
| Mo, mg/kg | 1.1 | 0.25 | 1.1 | 0.01 |
| Treatment 1 | p-Value Contrasts 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | LS0 | HS0 | HS15 | HS30 | HS45 | SEM 3 | LS0 vs. HS0 | HS0 vs. HS15, 30, 45 | Linear TRT | Quad TRT |
| DMI, kg/d | 19.90 | 24.88 | 22.72 | 24.95 | 25.56 | 1.08 | <0.001 | 0.61 | 0.25 | 0.09 |
| BW, kg | 665 | 689 | 671 | 681 | 685 | 8.2 | 0.003 | 0.11 | 0.94 | 0.05 |
| DMI, % of BW | 2.91 | 3.51 | 3.46 | 3.53 | 3.71 | 0.25 | <0.001 | 0.69 | 0.20 | 0.31 |
| BCS | 3.41 | 3.48 | 3.47 | 3.41 | 3.44 | 0.06 | 0.29 | 0.46 | 0.39 | 0.68 |
| Milk yield | ||||||||||
| Milk yield, kg/d | 30.50 | 34.51 | 32.42 | 33.93 | 33.75 | 1.38 | <0.001 | 0.21 | 0.82 | 0.22 |
| FCM, kg/d | 31.85 | 34.36 | 32.30 | 34.74 | 33.12 | 3.13 | 0.08 | 0.40 | 0.78 | 0.82 |
| ECM, kg/d | 31.27 | 34.39 | 32.17 | 34.92 | 33.20 | 3.14 | 0.03 | 0.41 | 0.85 | 0.79 |
| Milk composition | ||||||||||
| Fat, % | 3.89 | 3.56 | 3.78 | 3.56 | 3.60 | 0.17 | 0.007 | 0.35 | 0.85 | 0.30 |
| Fat, kg/d | 1.16 | 1.18 | 1.15 | 1.22 | 1.16 | 0.11 | 0.76 | 0.98 | 0.96 | 0.62 |
| Protein, % | 3.13 | 3.23 | 3.23 | 3.29 | 3.24 | 0.04 | <0.001 | 0.16 | 0.11 | 0.17 |
| Protein, kg/d | 0.94 | 1.10 | 1.02 | 1.13 | 1.07 | 0.10 | <0.001 | 0.42 | 0.88 | 0.62 |
| Casein, % | 2.61 | 2.66 | 2.66 | 2.72 | 2.68 | 0.03 | 0.01 | 0.13 | 0.07 | 0.18 |
| Casein, kg/d | 0.32 | 0.43 | 0.37 | 0.41 | 0.41 | 0.06 | 0.002 | 0.28 | 0.81 | 0.31 |
| Casein, % of protein | 82.06 | 82.42 | 82.11 | 82.59 | 82.60 | 0.43 | 0.29 | 0.96 | 0.33 | 0.50 |
| Lactose, % | 4.67 | 4.73 | 4.73 | 4.69 | 4.72 | 0.05 | 0.11 | 0.49 | 0.56 | 0.44 |
| Lactose, kg/d | 1.41 | 1.63 | 1.49 | 1.61 | 1.57 | 0.15 | 0.004 | 0.23 | 0.82 | 0.34 |
| MUN, mg/dL | 14.37 | 13.56 | 14.18 | 13.20 | 13.86 | 0.49 | 0.09 | 0.63 | 0.96 | 0.94 |
| SCC × 1000/mL | 205 | 205 | 175 | 216 | 221 | 113 | 0.99 | 0.95 | 0.18 | 0.24 |
| Milk/DMI | 1.55 | 1.38 | 1.40 | 1.39 | 1.34 | 0.11 | 0.002 | 0.98 | 0.43 | 0.34 |
| FCM/DMI | 1.62 | 1.32 | 1.42 | 1.39 | 1.31 | 0.11 | <0.001 | 0.37 | 0.82 | 0.09 |
| ECM/DMI | 1.64 | 1.32 | 1.41 | 1.39 | 1.31 | 0.11 | <0.001 | 0.34 | 0.86 | 0.07 |
| Treatment 1 | p-Value Contrasts 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | LS0 | HS0 | HS15 | HS30 | HS45 | SEM 3 | LS0 vs. HS0 | HS0 vs. HS15, 30, 45 | Linear TRT | Quad TRT |
| Rumen fluid | ||||||||||
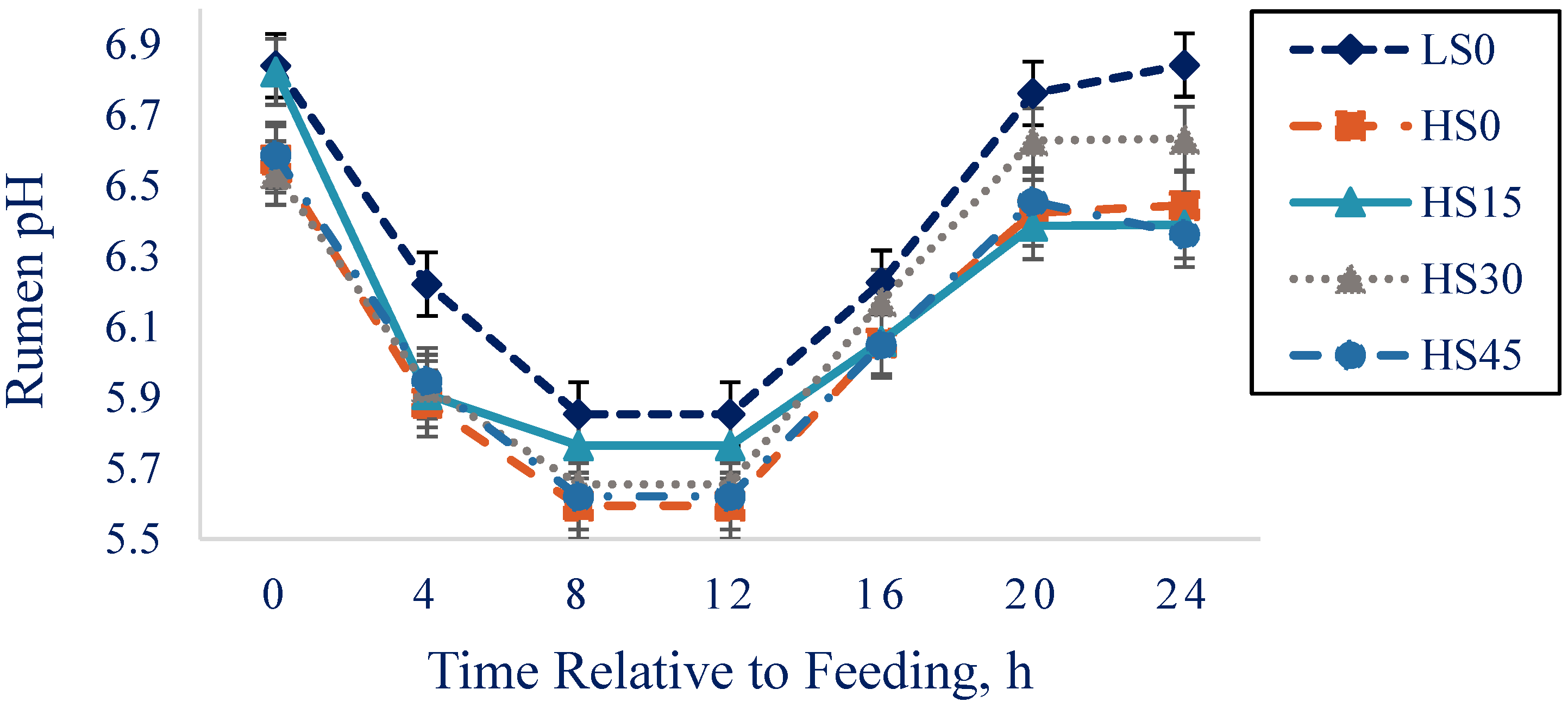
| Ph 4 | 6.38 | 6.10 | 6.15 | 6.18 | 6.12 | 0.05 | <0.001 | 0.17 | 0.54 | 0.08 |
| pH < 5.6, h 5 | 6.28 | 7.15 | 5.97 | 6.62 | 8.08 | 1.50 | 0.62 | 0.84 | 0.49 | 0.23 |
| Nadir pH | 5.74 | 5.53 | 5.55 | 5.57 | 5.57 | 0.03 | <0.001 | 0.007 | 0.002 | 0.43 |
| AUC, pH × h/d 6 | 0.07 | 0.26 | 0.12 | 0.15 | 0.11 | 0.12 | 0.23 | 0.22 | 0.25 | 0.60 |
| Fecal pH | 6.95 | 6.71 | 6.72 | 6.59 | 6.66 | 0.08 | 0.04 | 0.58 | 0.43 | 0.75 |
| Total VFA, mmol/L | 127.63 | 137.42 | 129.75 | 134.08 | 134.43 | 2.96 | <0.001 | 0.02 | 0.57 | 0.03 |
| Individual VFA, mol/100 mol of total VFA 7 | ||||||||||
| Acetate | 82.93 | 85.30 | 80.44 | 82.79 | 82.08 | 1.77 | 0.16 | 0.006 | 0.15 | 0.07 |
| Propionate 8 | 20.75 | 23.87 | 23.74 | 24.63 | 24.79 | 2.60 | <0.001 | 0.78 | 0.03 | 0.02 |
| Butyrate | 13.81 | 14.92 | 13.71 | 14.24 | 14.93 | 0.40 | 0.006 | 0.04 | 0.66 | <0.001 |
| Isobutyrate | 1.05 | 1.04 | 0.98 | 0.99 | 0.95 | 0.03 | 0.41 | <0.001 | <0.001 | 0.44 |
| Valerate | 1.78 | 2.13 | 1.93 | 2.02 | 2.09 | 0.12 | <0.001 | 0.04 | 0.97 | 0.005 |
| Isovalerate | 0.78 | 0.80 | 0.75 | 0.77 | 0.74 | 0.02 | 0.37 | <0.001 | 0.004 | 0.21 |
| Treatment 1 | p-Value Contrasts 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | LS0 | HS0 | HS15 | HS30 | HS45 | SEM 3 | LS0 vs. HS0 | HS0 vs. HS15, 30, 45 | Linear TRT | Quad TRT |
| N intake, g/d | 617 | 717 | 686 | 729 | 738 | 42 | 0.08 | 0.98 | 0.56 | 0.64 |
| Milk protein N 4, g/d | 140.29 | 165.99 | 149.65 | 176.40 | 170.45 | 16.61 | 0.04 | 0.95 | 0.28 | 0.53 |
| Milk protein N, % of N intake | 22.82 | 22.60 | 22.15 | 24.27 | 22.75 | 1.96 | 0.88 | 0.70 | 0.58 | 0.61 |
| Urinary excretion | ||||||||||
| Urine volume 5, L/d | 39.87 | 37.03 | 38.01 | 36.65 | 40.94 | 3.01 | 0.43 | 0.59 | 0.34 | 0.49 |
| Total N, g/d | 244.30 | 263.31 | 250.26 | 245.08 | 270.49 | 15.74 | 0.30 | 0.57 | 0.77 | 0.13 |
| Total N, % of N intake | 41.33 | 35.10 | 42.30 | 34.54 | 38.45 | 3.51 | 0.14 | 0.31 | 0.86 | 0.56 |
| Urea N, g/d | 199.60 | 216.54 | 212.04 | 213.30 | 227.22 | 10.61 | 0.14 | 0.91 | 0.34 | 0.24 |
| Urea N, % of total urinary N | 76.92 | 82.41 | 81.87 | 80.15 | 83.62 | 2.31 | 0.08 | 0.83 | 0.84 | 0.36 |
| Allantoin, mmol/d | 172.87 | 194.81 | 170.21 | 181.85 | 186.47 | 12.14 | 0.07 | 0.11 | 0.72 | 0.08 |
| Uric acid, mmol/d | 66.58 | 74.32 | 60.46 | 73.61 | 78.91 | 5.57 | 0.16 | 0.43 | 0.11 | 0.01 |
| Total PD, mmol/d | 219.22 | 256.28 | 226.62 | 240.16 | 245.12 | 20.33 | 0.03 | 0.15 | 0.69 | 0.14 |
| Microbial N production 6, g/d | 137.97 | 161.30 | 142.63 | 151.15 | 154.27 | 12.79 | 0.03 | 0.16 | 0.81 | 0.22 |
| PUN, mg/dL | 14.90 | 14.22 | 15.54 | 13.93 | 15.27 | 0.57 | 0.38 | 0.26 | 0.53 | 0.99 |
| Fecal N excretion | ||||||||||
| N, g/d | 221.95 | 250.09 | 239.35 | 260.48 | 242.38 | 17.51 | 0.25 | 0.73 | 0.93 | 0.99 |
| N, % of intake | 38.02 | 37.06 | 36.42 | 35.30 | 33.11 | 1.74 | 0.68 | 0.24 | 0.07 | 0.63 |
| Nutrient intakes, kg/d | ||||||||||
| OM | 18.61 | 22.51 | 20.55 | 22.07 | 22.87 | 1.28 | 0.06 | 0.58 | 0.54 | 0.21 |
| CP | 3.66 | 4.50 | 4.08 | 4.59 | 4.61 | 0.30 | 0.05 | 0.76 | 0.48 | 0.38 |
| Starch | 4.71 | 6.53 | 6.32 | 6.95 | 6.94 | 0.42 | 0.002 | 0.65 | 0.30 | 0.81 |
| NDF | 6.15 | 6.54 | 6.03 | 6.11 | 6.45 | 0.36 | 0.88 | 0.97 | 0.99 | 0.96 |
| Apparent digestibility, % | ||||||||||
| OM | 65.41 | 66.23 | 67.66 | 65.95 | 68.19 | 1.22 | 0.63 | 0.44 | 0.43 | 0.75 |
| CP | 61.13 | 62.04 | 64.05 | 63.33 | 66.07 | 1.67 | 0.73 | 0.15 | 0.09 | 0.91 |
| Starch | 95.43 | 94.13 | 94.77 | 93.72 | 94.72 | 0.54 | 0.08 | 0.64 | 0.75 | 0.72 |
| NDF | 52.03 | 47.87 | 51.86 | 45.36 | 50.81 | 1.75 | 0.10 | 0.46 | 0.76 | 0.66 |
| Treatment 1 | p-Value Contrasts 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | LS0 | HS0 | HS15 | HS30 | HS45 | SEM 3 | LS0 vs. HS0 | HS0 vs. HS15, 30, 45 | Linear TRT | Quad TRT |
| Blood 4 | ||||||||||
| Metabolism | ||||||||||
| Glucose, mg/dL | 70.15 | 70.14 | 71.90 | 71.53 | 70.52 | 1.04 | 0.99 | 0.11 | 0.79 | 0.03 |
| GLDH 5, U/L | 32.20 | 31.47 | 33.05 | 32.27 | 32.93 | 3.96 | 0.68 | 0.25 | 0.39 | 0.43 |
| Cholesterol total, mg/dL | 169 | 174 | 171 | 173 | 178 | 27 | 0.12 | 0.92 | 0.19 | 0.17 |
| BHB, mmol/L | 0.58 | 0.52 | 0.52 | 0.48 | 0.47 | 0.03 | 0.12 | 0.17 | 0.08 | 0.77 |
| Triglycerides, mg/dL | 8.18 | 7.81 | 8.18 | 8.43 | 7.83 | 0.59 | 0.36 | 0.28 | 0.80 | 0.08 |
| NEFA 6, µEq/L | 102.2 | 82.6 | 111.8 | 85.1 | 81.3 | 9.3 | 0.02 | 0.57 | 0.71 | 0.19 |
| Insulin, µg/L | 0.74 | 0.93 | 0.86 | 0.95 | 0.92 | 0.09 | 0.03 | 0.77 | 0.84 | 0.76 |
| CPK 7, U/L | 150 | 181 | 143 | 182 | 159 | 25.4 | 0.36 | 0.46 | 0.78 | 0.75 |
| D-Lactate, mM | 0.57 | 0.57 | 0.63 | 0.57 | 0.60 | 0.03 | 0.97 | 0.39 | 0.98 | 0.55 |
| Total protein, g/dL | 7.57 | 7.45 | 7.65 | 7.64 | 7.56 | 0.11 | 0.07 | 0.001 | 0.11 | 0.002 |
| Albumin, g/dL | 3.38 | 3.34 | 3.39 | 3.33 | 3.30 | 0.04 | 0.29 | 0.91 | 0.07 | 0.09 |
| Globulin, g/dL | 4.15 | 4.08 | 4.24 | 4.31 | 4.22 | 0.13 | 0.29 | <0.001 | 0.01 | 0.005 |
| Albumin/Globulin ratio | 0.82 | 0.83 | 0.81 | 0.79 | 0.81 | 0.03 | 0.37 | 0.03 | 0.07 | 0.06 |
| Minerals | ||||||||||
| Calcium, mg/dL | 9.31 | 9.12 | 9.29 | 9.34 | 9.20 | 0.09 | 0.07 | 0.05 | 0.37 | 0.03 |
| Phosphorus, mg/dL | 5.38 | 5.86 | 5.85 | 5.74 | 5.80 | 0.15 | 0.004 | 0.64 | 0.60 | 0.74 |
| Sodium, mmol/L | 136.52 | 135.78 | 136.71 | 136.60 | 134.84 | 0.68 | 0.44 | 0.58 | 0.07 | 0.09 |
| Potassium, mmol/L | 4.39 | 4.38 | 4.44 | 4.41 | 4.40 | 0.06 | 0.94 | 0.53 | 0.89 | 0.50 |
| Na:K Ratio | 31.13 | 31.11 | 30.96 | 31.07 | 30.80 | 0.42 | 0.98 | 0.66 | 0.59 | 0.87 |
| Liver function | ||||||||||
| AST 8, U/L | 72.62 | 69.49 | 68.62 | 69.33 | 66.88 | 4.31 | 0.06 | 0.36 | 0.16 | 0.49 |
| GGT 9, U/L | 24.81 | 26.11 | 26.02 | 25.20 | 25.64 | 1.15 | 0.02 | 0.21 | 0.14 | 0.43 |
| Total bilirubin, mg/dL | 0.14 | 0.12 | 0.12 | 0.11 | 0.12 | 0.009 | 0.01 | 0.71 | 0.67 | 0.38 |
| Alkaline phosphate total, U/L | 46.03 | 46.32 | 43.69 | 46.69 | 45.22 | 2.39 | 0.78 | 0.18 | 0.93 | 0.42 |
| Inflammation | ||||||||||
| SAA 10, µg/mL | 142 | 108 | 161 | 140 | 150 | 25 | 0.16 | 0.03 | 0.16 | 0.19 |
| LBP 11, µg/mL | 20.1 | 21.9 | 20.6 | 21.3 | 20.5 | 2.3 | 0.43 | 0.54 | 0.59 | 0.90 |
| SOD 12, U/mL | 3.67 | 4.08 | 3.77 | 3.60 | 3.50 | 0.32 | 0.19 | 0.07 | 0.05 | 0.64 |
| GSH-Px 13, nmol/min/mL | 86.9 | 84.1 | 87.7 | 81.5 | 88.8 | 4.2 | 0.55 | 0.61 | 0.60 | 0.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knollinger, S.E.; Poczynek, M.; Miller, B.; Mueller, I.; de Almeida, R.; Murphy, M.R.; Cardoso, F.C. Effects of Autolyzed Yeast Supplementation in a High-Starch Diet on Rumen Health, Apparent Digestibility, and Production Variables of Lactating Holstein Cows. Animals 2022, 12, 2445. https://doi.org/10.3390/ani12182445
Knollinger SE, Poczynek M, Miller B, Mueller I, de Almeida R, Murphy MR, Cardoso FC. Effects of Autolyzed Yeast Supplementation in a High-Starch Diet on Rumen Health, Apparent Digestibility, and Production Variables of Lactating Holstein Cows. Animals. 2022; 12(18):2445. https://doi.org/10.3390/ani12182445
Chicago/Turabian StyleKnollinger, Sara E., Milaine Poczynek, Bryan Miller, Isabel Mueller, Rodrigo de Almeida, Michael R. Murphy, and Felipe C. Cardoso. 2022. "Effects of Autolyzed Yeast Supplementation in a High-Starch Diet on Rumen Health, Apparent Digestibility, and Production Variables of Lactating Holstein Cows" Animals 12, no. 18: 2445. https://doi.org/10.3390/ani12182445
APA StyleKnollinger, S. E., Poczynek, M., Miller, B., Mueller, I., de Almeida, R., Murphy, M. R., & Cardoso, F. C. (2022). Effects of Autolyzed Yeast Supplementation in a High-Starch Diet on Rumen Health, Apparent Digestibility, and Production Variables of Lactating Holstein Cows. Animals, 12(18), 2445. https://doi.org/10.3390/ani12182445






