The Complex and Well-Developed Morphological and Histological Structures of the Gastrointestinal Tract of the Plateau Zokor Improve Its Digestive Adaptability to High-Fiber Foods
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Their Habitats
2.2. Experimental Design
2.3. Determination of Plant Nutrient Composition and Stomach Contents
2.4. Gross Anatomy of the Experimental Animals
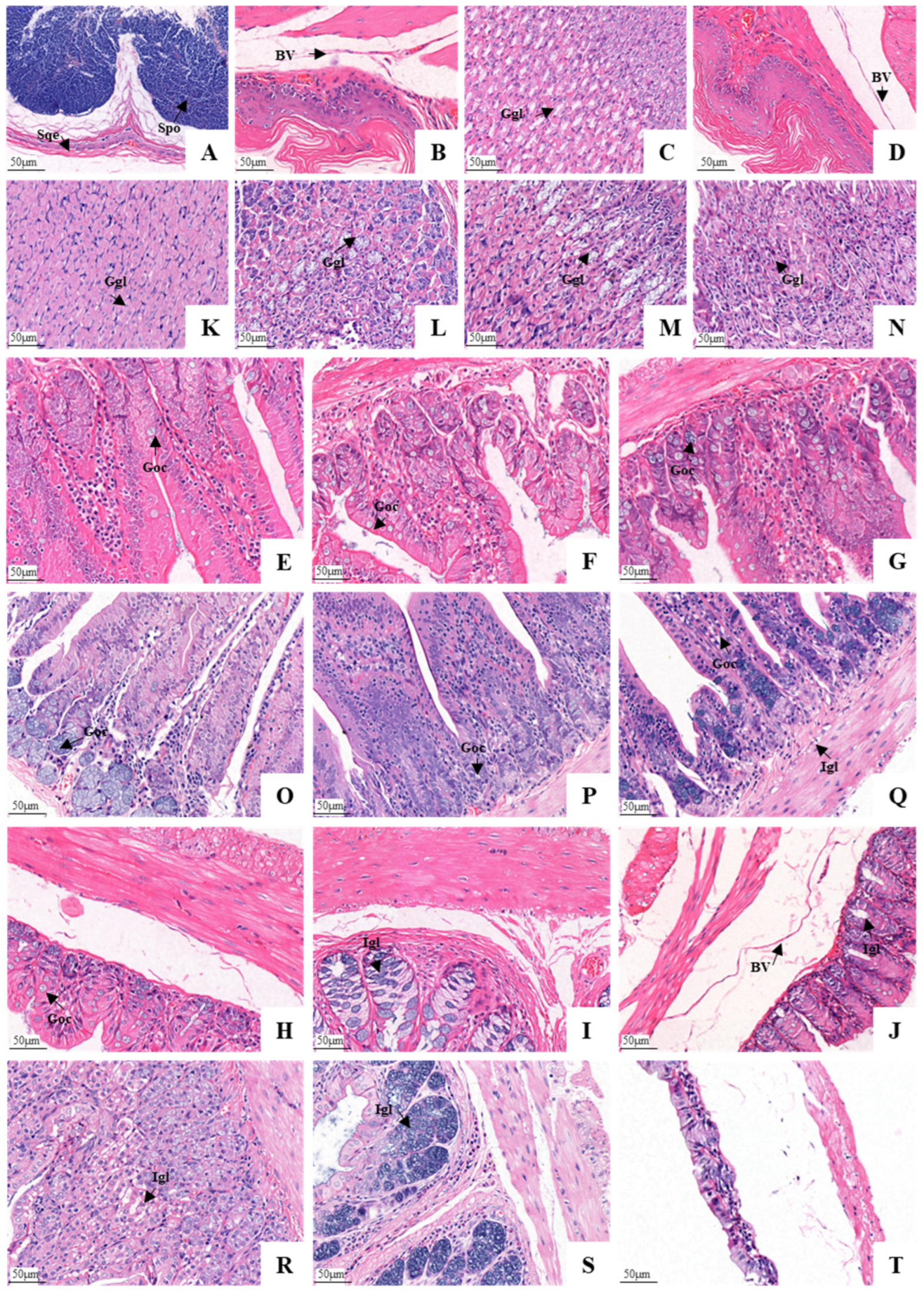
2.5. Determination of the Gastrointestinal Histology
2.5.1. Microstructure
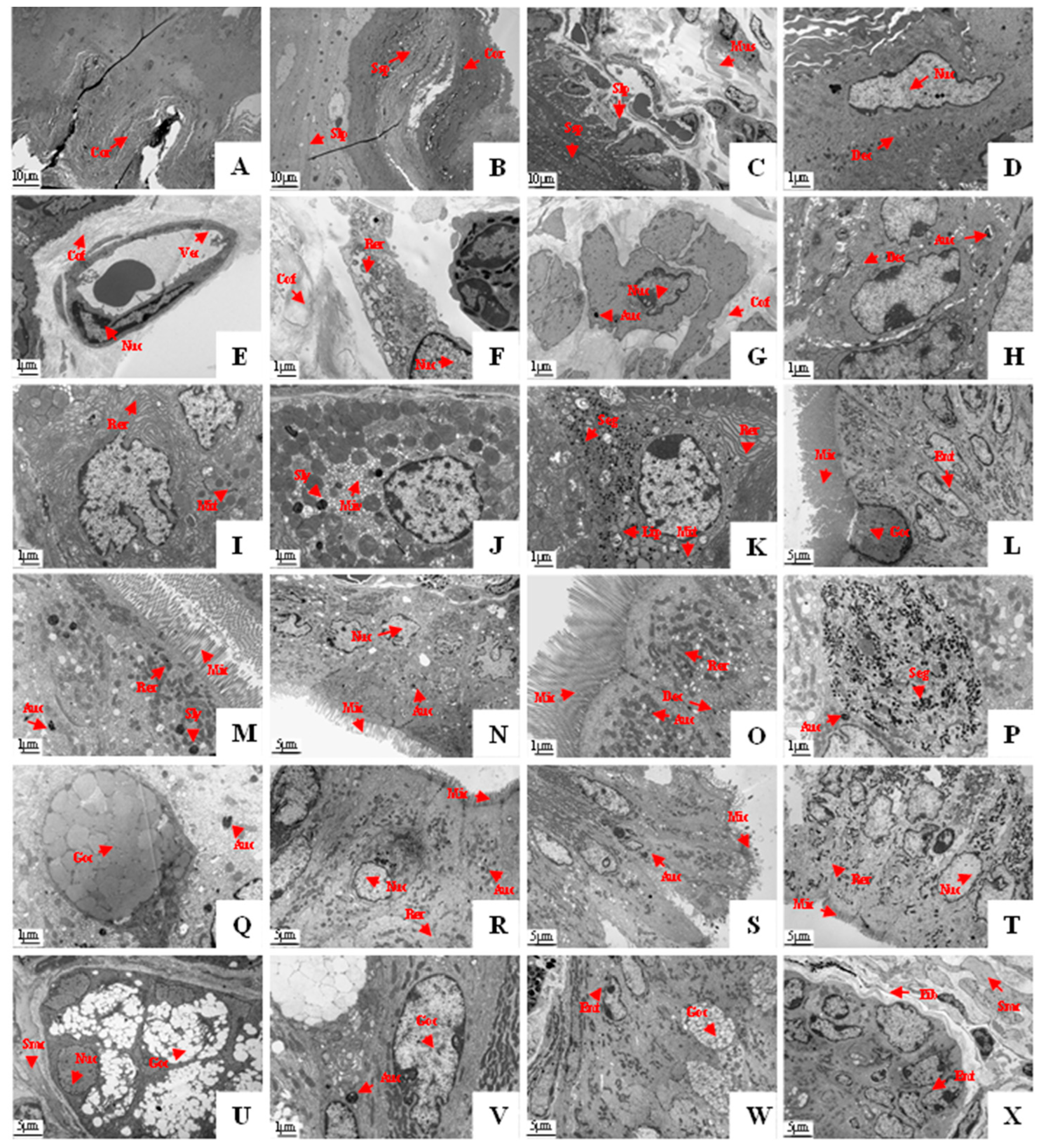
2.5.2. Ultrastructure
2.6. Data Analysis
3. Results
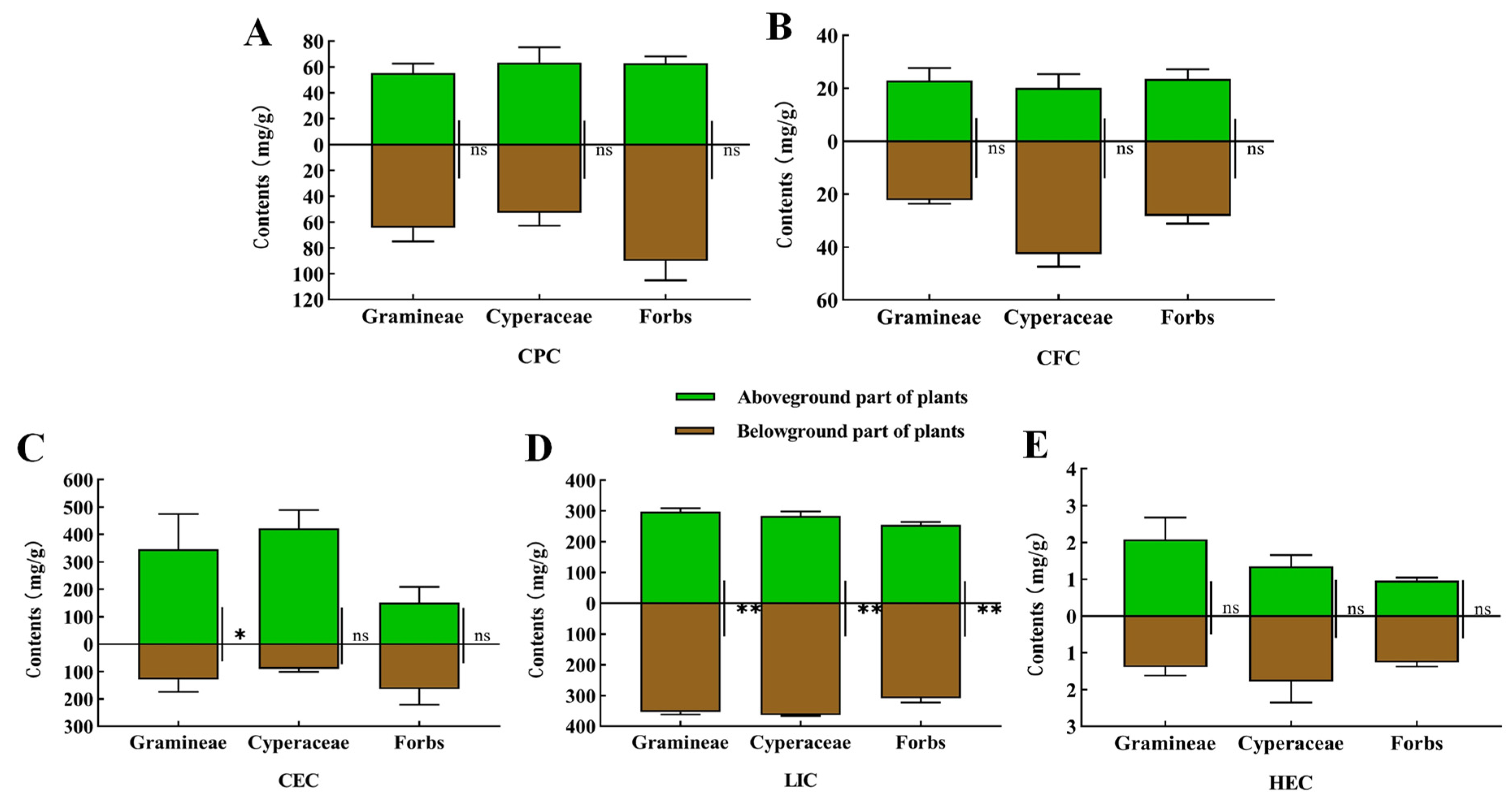
3.1. Analysis and Determination of Plant Nutrient Composition in the Habitats of the Plateau Zokor and Plateau Pika and in Animal Stomach Contents
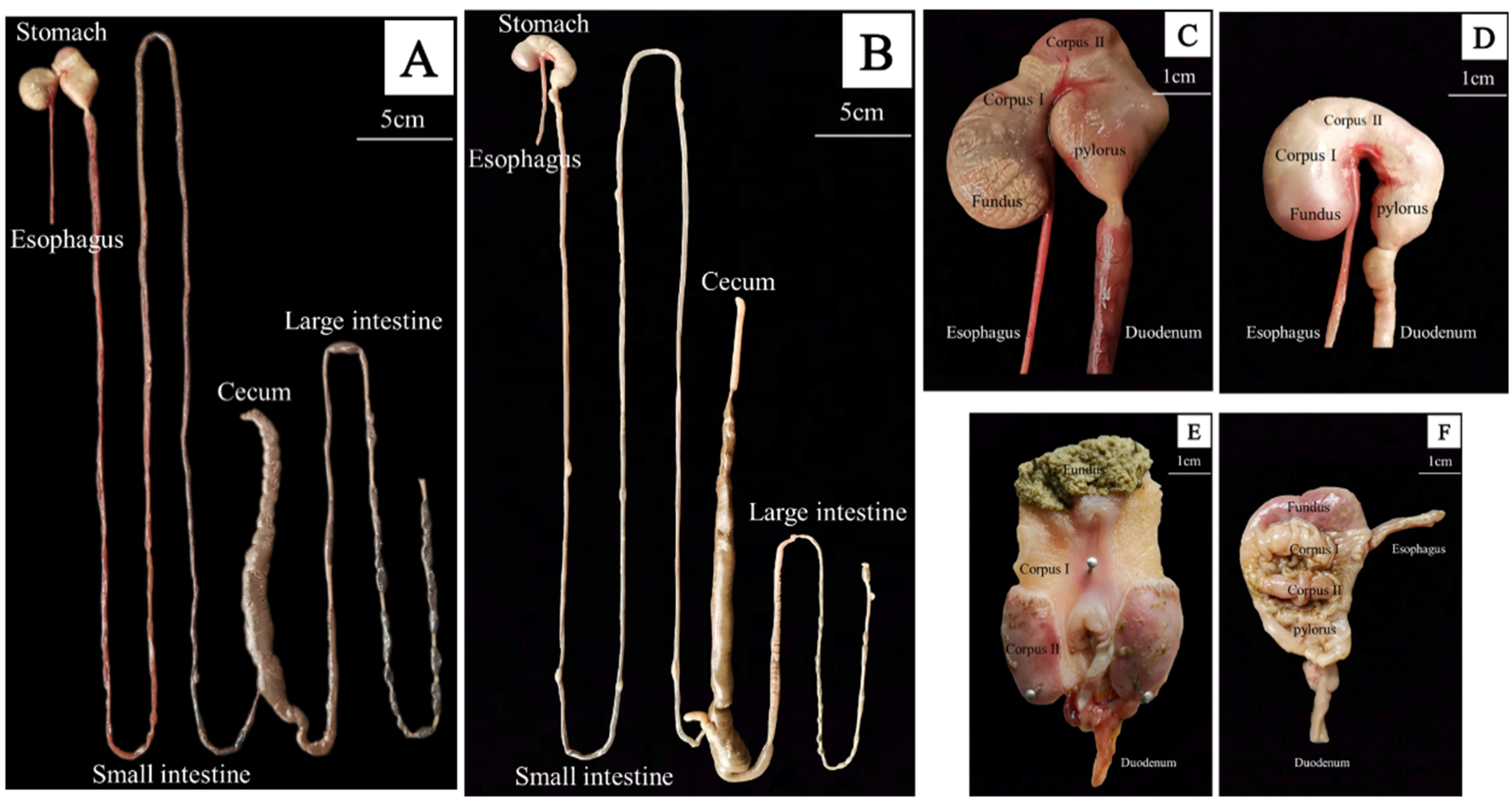
3.2. Gross Morphology of the GIT
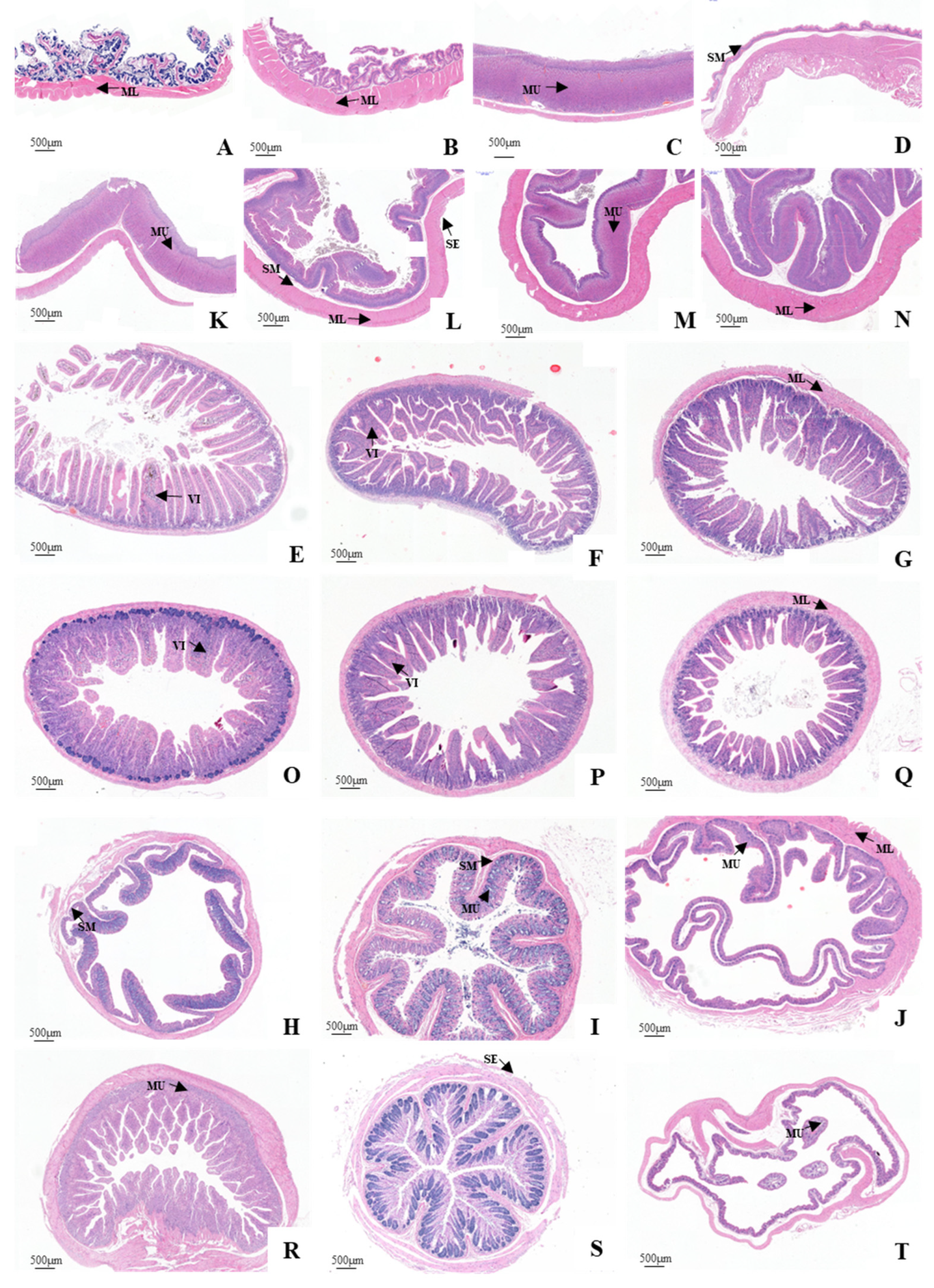
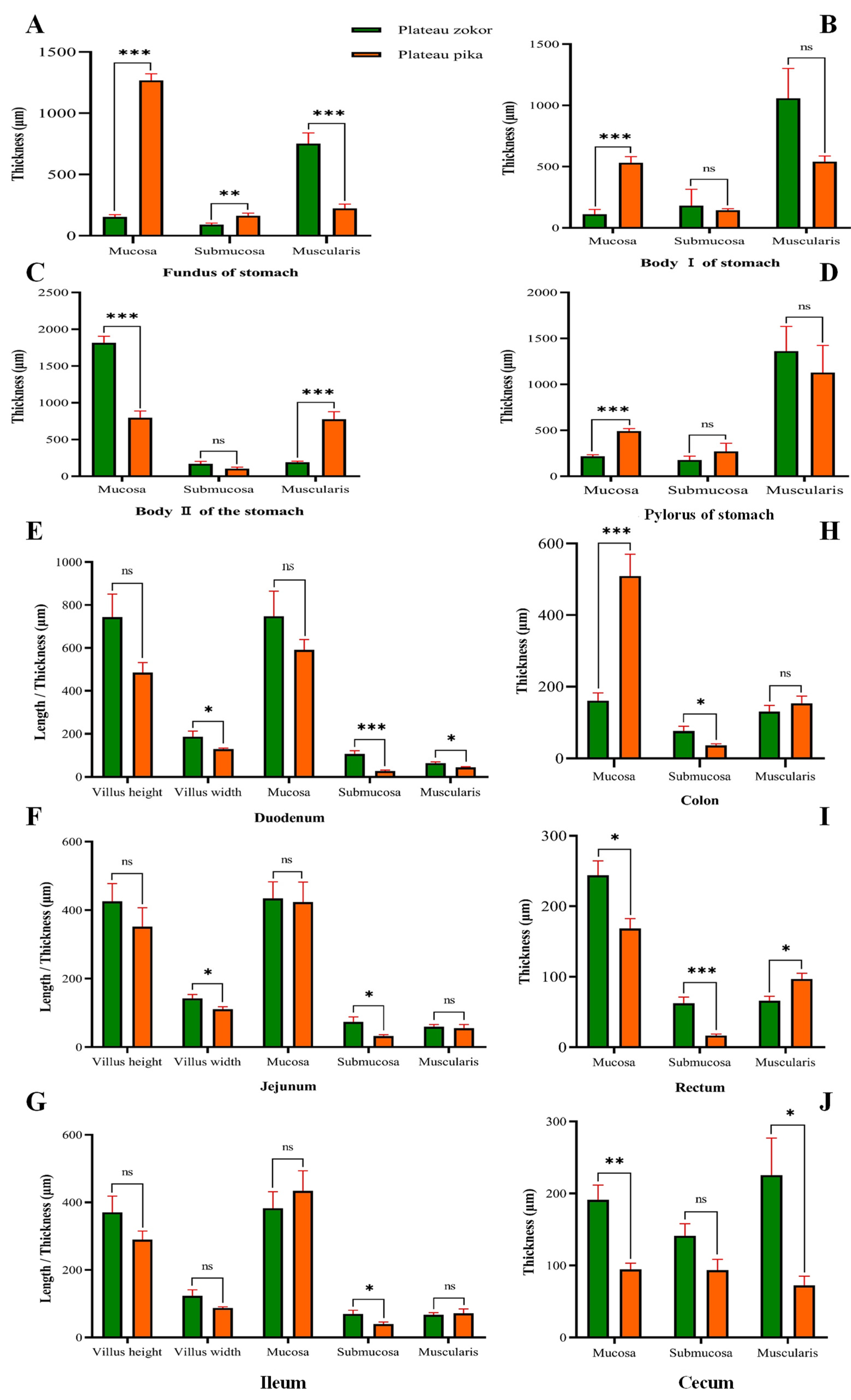
3.3. Histological Structure of the GIT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fišer, C. Adaptation: Morphological; Elsevier: Amsterdam, The Netherlands, 2019; pp. 33–39. [Google Scholar]
- Phillips, A.G.; Töpfer, T.; Böhning-Gaese, K.; Fritz, S.A. Evidence for distinct evolutionary optima in the morphology of migratory and resident birds. J. Avian Biol. 2018, 49, e01807. [Google Scholar] [CrossRef]
- Moura, R.F.; Tizo-Pedroso, E.; Del-Claro, K. Can morphological and behavioral traits predict the foraging and feeding dynamics of social arachnids? Curr. Zool. 2021, 67, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Karasov, W.H.; Martinez del Rio, C.; Caviedes-Vidal, E. Ecological physiology of diet and digestive systems. Annu. Rev. Physiol. 2011, 73, 69–93. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.E.; Wang, Z.; Wunder, B.A. Effects of food quality and energy needs: Changes in gut morphology and capacity of Microtus ochrogaster. J. Mammal. 1985, 66, 661–667. [Google Scholar] [CrossRef]
- Walters, J.; Marais, S.; Johnson, O.; Bennett, N.C.; Alagaili, A.N.; Mohammed, O.B.; Kotze, S.H. The comparative gastrointestinal morphology of five species of muroid rodents found in Saudi Arabia. J. Morphol. 2014, 275, 980–990. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Jiang, Z.; Chen, A. Rodents Zoology; Shanghai Jiao Tong University Press: Shanghai, China, 2008; pp. 168–188. [Google Scholar]
- Begall, S.; Burda, H.; Schleich, C.E. Subterranean rodents: News from underground. In Subterranean Rodents; Begall, S., Burda, H., Schleich, C.E., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 3–9. [Google Scholar]
- Xu, X.J.; Lv, J.W.; Xie, Z.L.; Ji, S.B.; Zhou, S.T.; Wang, C.H. Digestive tract morphology of Apodemus agrarius mantchuricus and Cricetulus barabensis manchu = ricus in the plantation of lower Nenjiang Valley. Chin. J. Zool. 2008, 43, 131–136. [Google Scholar]
- Del Valle, J.C.; López Mañanes, A.A. Digestive flexibility in females of the subterranean rodent ctenomys talarum in their natural habitat. J. Exp. Zool. Part A 2011, 315A, 141–148. [Google Scholar] [CrossRef]
- Zou, Y.; Xu, M.; Ren, S.E.; Liang, N.N.; Han, C.X.; Nan, X.N.; Shi, M.N. Taxonomy and phylogenetic relationship of zokors. J. Genet. 2020, 99, 38. [Google Scholar] [CrossRef]
- Chu, B.; Ji, C.P.; Zhou, J.W.; Zhou, Y.S.; Hua, L.M. Why does the plateau zokor (Myospalax fontanieri: Rodentia: Spalacidae) move on the ground in summer in the eastern Qilian Mountains? J. Mammal. 2021, 102, 346–357. [Google Scholar] [CrossRef]
- Hu, L.; Zi, H.B.; Ade, L.; Lerdau, M.; Wang, C.T. Effects of zokors (Myospalax baileyi) on plant, on abiotic and biotic soil characteristic of an alpine meadow. Ecol. Eng. 2017, 103, 95–105. [Google Scholar] [CrossRef]
- Niu, Y.J.; Yang, S.W.; Zhu, H.M.; Zhou, J.W.; Chu, B.; Ma, S.J.; Hua, R.; Hua, L.M. Cyclic formation of zokor mounds promotes plant diversity and renews plant communities in alpine meadows on the Tibetan Plateau. Plant Soil 2020, 446, 65–79. [Google Scholar] [CrossRef]
- An, K.; Yao, B.; Kang, Y.; Bao, M.; Tan, Y.; Pu, Q.; Su, J. Seasonal Expression of Gonadotropin Genes in the Pituitary and Testes of Male Plateau Zokor (Eospalax baileyi). Animals 2022, 12, 725. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Li, W.; Nevo, E.; Su, J.; Zhang, T. Adaptive evolution of flaky thumb claw and elongated compulsory arousal duration in the subterranean rodent plateau zokor. Ethol. Ecol. Evol. 2011, 23, 77–80. [Google Scholar] [CrossRef]
- Shao, Y.; Li, J.X.; Ge, R.L.; Zhong, L.; Irwin, D.M.; Murphy, R.W.; Zhang, Y.P. Genetic adaptations of the plateau zokor in high-elevation burrows. Sci. Rep. 2015, 5, 17262. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, S.; Cai, Z.Y.; Kuang, Z.R.; Wan, N.; Wang, Y.J.; Mao, L.Y.; An, X.N.; Li, F.; Feng, T.; et al. Genomic insights into zokors’ phylogeny and speciation in China. Proc. Natl. Acad. Sci. USA 2022, 119, e2121819119. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.H.; Wang, Z.W. Seasonal variations in gastrointestinal tract morphology in plateau zokor (Myospalax baileyi). Acta Theriol. Sin. 2000, 20, 270–276. [Google Scholar]
- Fišer, Ž. Adaptation to low food. In Encyclopedia of Caves; White, W.B., Culver, D.C., Pipan, T., Eds.; Elsevier: Amsterdam, The Netherlands; Academic Press: London, UK, 2019; pp. 1–7. [Google Scholar]
- Naumova, E.I.; Chistova, T.Y.; Zharova, G.K.; Kam, M.; Khokhlova, I.S.; Krasnov, B.R.; Degen, A.A. Energy requirements, length of digestive tract compartments and body mass in six gerbilline rodents of the Negev Desert. Zoology 2019, 137, 125715. [Google Scholar] [CrossRef]
- Peña-Villalobos, I.; Casanova-Maldonado, I.; Lois, P.; Sabat, P.; Palma, V. Adaptive physiological and morphological adjustments mediated by intestinal stem cells in response to food availability in mice. Front. Physiol. 2019, 9, 1821. [Google Scholar] [CrossRef]
- Schieck, J.O.; Millar, J.S. Alimentary tract measurements as indicators of diets of small mammals. Mammalia 1985, 49, 93–104. [Google Scholar] [CrossRef]
- Langer, P.; Clauss, M. Morphological adaptation of the eutherian gastrointestinal tract to diet. Vertebr. Zool. 2018, 68, 237–252. [Google Scholar]
- Ye, G.H.; Chu, B.; Zhou, R.; Zhang, F.Y.; Hua, X.Z.; Hua, R.; Wang, T.; Dong, K.C.; Hua, L.M. Relationship between plant functional group distribution and soil properties under the disturbance of plateau pika (Ochotona curzoniae). Chin. J. Ecol. 2019, 38, 2382–2388. [Google Scholar]
- Zhang, J.G.; Jiang, Y.M.; Yao, T.; Gao, Y.M.; Li, H.Y.; Lan, X.J.; Tian, Y.L.; Li, J.H.; Zhang, Y. Effects of different management strategies on the number of soil nitrogen microorganism groups in an alpine meadow. Acta Prataculturae Sin. 2017, 26, 65–73. [Google Scholar]
- Zhang, C.J.; Yao, B.H.; Wang, X.Y.; Sun, X.M.; Su, J.H. Effects of grassland sowing on the feeding habits of Eospalax baileyi in the Gannan alpine meadow. Pratacultural Sci. 2021, 38, 967–975. [Google Scholar]
- Hou, C.; Shang, G.Z.; Wu, X.Q.; Cao, Y.F.; Chen, J.H.; Bian, J.H. Comparative studies on food habit analysis of gastric contents and colon contents of plateau pika (Ochotona curzoniae). Acta Theriol. Sin. 2019, 39, 662–669. [Google Scholar]
- Sáez-Plaza, P.; Michałowski, T.; Navas, M.J.; Asuero, A.G.; Wybraniec, S. An overview of the Kjeldahl method of nitrogen determination. Part I. Early history, chemistry of the procedure, and titrimetric finish. Crit. Rev. Anal. Chem. 2013, 43, 178–223. [Google Scholar] [CrossRef]
- Jensen, W.B. The origin of the soxhlet extractor. J. Chem. Educ. 2007, 84, 1913–1914. [Google Scholar] [CrossRef]
- Janshekar, H.; Brown, C.; Fiechter, A. Determination of biodegraded lignin by ultraviolet spectrophotometry. Anal. Chim. Acta 1981, 130, 81–91. [Google Scholar] [CrossRef]
- Li, X.L.; Sun, C.J.; Zhou, B.X.; He, Y. Determination of hemicellulose, cellulose and lignin in moso bamboo by near infrared spectroscopy. Sci. Rep. 2015, 5, 17210. [Google Scholar] [CrossRef]
- Tardieu, L.; Sundaram, V.; Adogwa, A.O.; Garcia, G.W. Anatomy and histology of the gastrointestinal tract of the neo--tropical opossum (Didelphis marsupialis insularis, Allen 1902). Acta Zool. 2020, 101, 384–391. [Google Scholar] [CrossRef]
- Eto, T.; Ozaki, R.; Kato, G.A.; Sakamoto, S.H.; Koshimoto, C.; Morita, T. Flexibility of digestive tract morphology in response to environmental conditions in the large Japanese field mouse apodemus speciosus. Mamm. Study 2016, 41, 71–76. [Google Scholar] [CrossRef]
- Bai, C.; Liu, T.G.; Xu, J.N.; Ma, X.Y.; Huang, L.; Liu, S.Y.; Yu, H.; Chen, J.X.; Gu, X.H. Effect of high calorie diet on intestinal flora in LPS-induced pneumonia rats. Sci. Rep. 2020, 10, 1701. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.Z.; Fang, J.; Peng, X.; Cui, H.M.; He, M.; Zuo, Z.C.; Zhou, Y.; Yang, Z.Z. Study on the morphology, histology and enzymatic activity of the digestive tract of Gymnocypris eckloni Herzenstein. Fish Physiol. Biochem. 2017, 43, 1175–1185. [Google Scholar] [CrossRef]
- Naya, D.E. Gut size flexibility in rodents: What we know, and don’t know, after a century of research. Rev. Chil. Hist. Nat. 2008, 81, 599–612. [Google Scholar] [CrossRef]
- Zhang, C.J.; Wang, X.Y.; Yao, B.H.; Wang, C.; Kang, Y.K.; Zhang, Q.; Su, J.H. Diet composition and trophic niche characteristics of three rodents in Gannan meadow. Acta Agrestia Sin. 2021, 29, 1484–1490. [Google Scholar]
- Wang, D.H.; Pei, Y.X.; Yang, J.C.; Wang, Z.W. Digestive tract morphology and food habits in six species of rodents. Folia Zool. 2003, 52, 51–55. [Google Scholar]
- Kotzé, S.H.; Van Der Merwe, E.L.; Bennett, N.C.; O’Riain, M.J. The comparative anatomy of the abdominal gastrointestinal tract of six species of african mole—Rats (Rodentia, Bathyergidae). J. Morphol. 2010, 271, 50–60. [Google Scholar] [CrossRef]
- Del Valle, J.C.; Busch, C.; Mañanes, A.A.L. Phenotypic plasticity in response to low quality diet in the South American omnivorous rodent Akodon azarae (Rodentia: Sigmodontinae). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2006, 145, 397–405. [Google Scholar] [CrossRef]
- Heroldova, M.; Janova, E. Feeding strategy of two rodent species in a set-aside field and its influence on alimentary tract morphometry. Mammalia 2019, 83, 34–40. [Google Scholar] [CrossRef]
- Zhu, W.; Mu, Y.; Liu, J.; Wang, Z. Energy requirements during lactation in female Apodemus chevrieri (Mammalia: Rodentia: Muridae) in the Hengduan Mountain region. Ital. J. Zool. 2015, 82, 165–171. [Google Scholar]
- Langer, P. The digestive tract and life history of small mammals. Mammal Rev. 2002, 32, 107–131. [Google Scholar] [CrossRef]
- Kohl, K.D.; Stengel, A.; Samuni-Blank, M.; Dearing, M.D. Effects of a natomy and d iet on g astrointestinal pH in rodents. J. Exp. Zool. Part A 2013, 319, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Kohl, K.D.; Miller, A.W.; Marvin, J.E.; Mackie, R.; Dearing, M.D. Herbivorous rodents (Neotoma spp.) harbour abundant and active foregut microbiota. Environ. Microbiol. 2014, 16, 2869–2878. [Google Scholar] [CrossRef]
- Sahd, L.; Pereira, D.L.; Bennett, N.C.; Kotzé, S.H. Comparative gastrointestinal morphology of Tachyoryctes splendens (rüppell, 1835) and Heliophobius emini, (noack, 1894) two species of East African mole—Rats. J. Morphol. 2017, 278, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Treuting, P.; Arends, M.; Dintzis, S. Upper gastrointestinal tract. In Comparative Anatomy and Histology, a Mouse and Human Atlas; Treuting, P., Dintzis, S., Eds.; Elsevier: Amsterdam, The Netherlands; Academic Press: London, UK, 2012; pp. 191–211. [Google Scholar]
- Yang, C.; Du, Y.; Lin, G.; Su, J.; Zhang, T. Histological structure comparison of the small intestine between gansu zokor (Myospalax cansus) and plateau zokor (Myospalax baileyi). Acta Theriol. Sin. 2013, 33, 172–177. [Google Scholar]


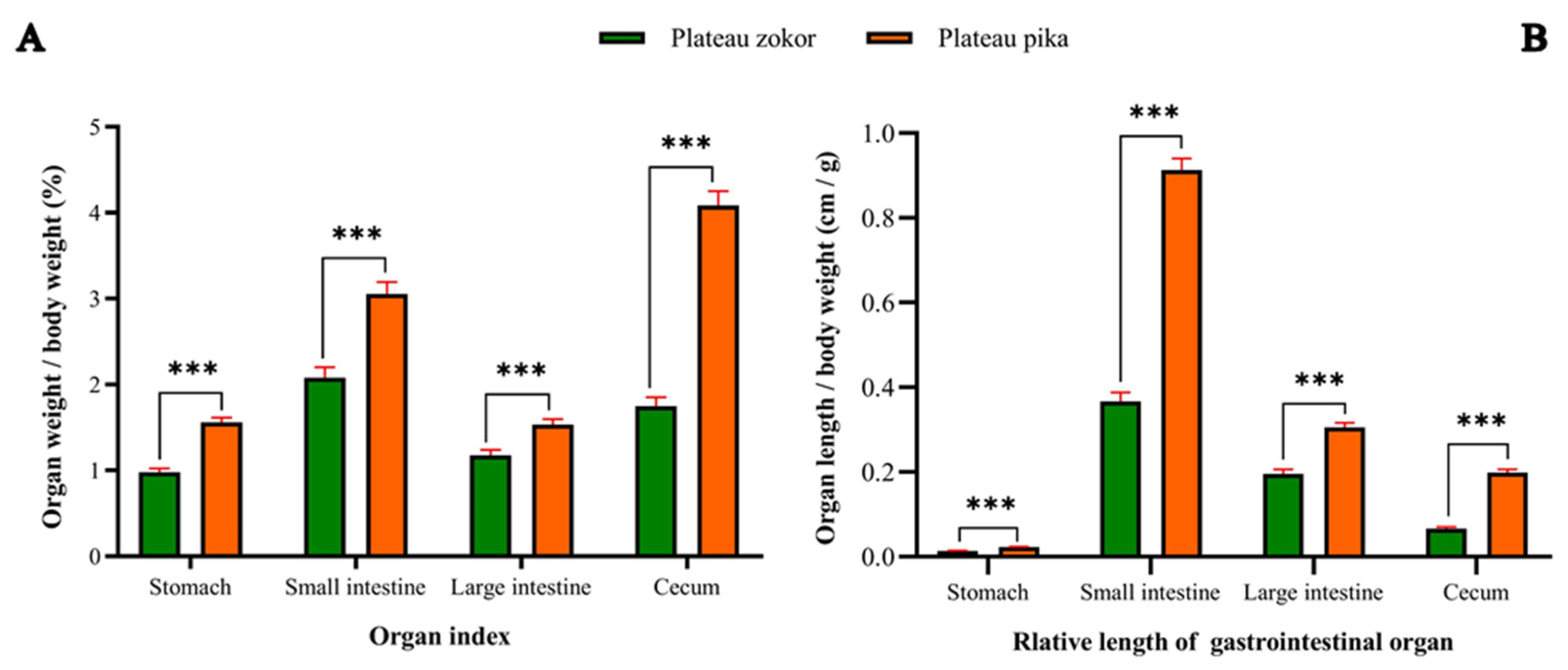
| Body Weight (g) | Weight of Gross GIT (g) | Length of Gross GIT (cm) | Organ Index of Gross GIT (%) | Relative Length of Gross GIT (cm/g) | |
|---|---|---|---|---|---|
| Plateau zokor (Eospalax baileyi) | 335.49 ± 18.78 (n = 22) | 19.30 ± 0.72 (n = 15) | 205.47 ± 6.29 (n = 15) | 5.99 ± 0.26 (n = 15) | 0.64 ± 0.03 (n = 15) |
| Plateau pika (Ochotona curzoniae) | 155.24 ± 5.41 n = 21 | 14.82 ± 0.45 (n = 15) | 207.50 ± 2.86 (n = 15) | 10.24 ± 0.33 (n = 15) | 1.44 ± 0.04 (n = 15) |
| Significances | *** | *** | ns | *** | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, X.; Bao, D.; Ye, G.; Chu, B.; Tang, Z.; Hua, R.; Hua, L. The Complex and Well-Developed Morphological and Histological Structures of the Gastrointestinal Tract of the Plateau Zokor Improve Its Digestive Adaptability to High-Fiber Foods. Animals 2022, 12, 2447. https://doi.org/10.3390/ani12182447
Cai X, Bao D, Ye G, Chu B, Tang Z, Hua R, Hua L. The Complex and Well-Developed Morphological and Histological Structures of the Gastrointestinal Tract of the Plateau Zokor Improve Its Digestive Adaptability to High-Fiber Foods. Animals. 2022; 12(18):2447. https://doi.org/10.3390/ani12182447
Chicago/Turabian StyleCai, Xincheng, Darhan Bao, Guohui Ye, Bin Chu, Zhuangsheng Tang, Rui Hua, and Limin Hua. 2022. "The Complex and Well-Developed Morphological and Histological Structures of the Gastrointestinal Tract of the Plateau Zokor Improve Its Digestive Adaptability to High-Fiber Foods" Animals 12, no. 18: 2447. https://doi.org/10.3390/ani12182447
APA StyleCai, X., Bao, D., Ye, G., Chu, B., Tang, Z., Hua, R., & Hua, L. (2022). The Complex and Well-Developed Morphological and Histological Structures of the Gastrointestinal Tract of the Plateau Zokor Improve Its Digestive Adaptability to High-Fiber Foods. Animals, 12(18), 2447. https://doi.org/10.3390/ani12182447





