Traits Defining Sow Lifetime Maternal Performance
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Preparation
Trait Definitions
2.2. The Analyses of the Maternal Performance Traits
2.3. Estimation of Variance Components and Correlations
3. Results
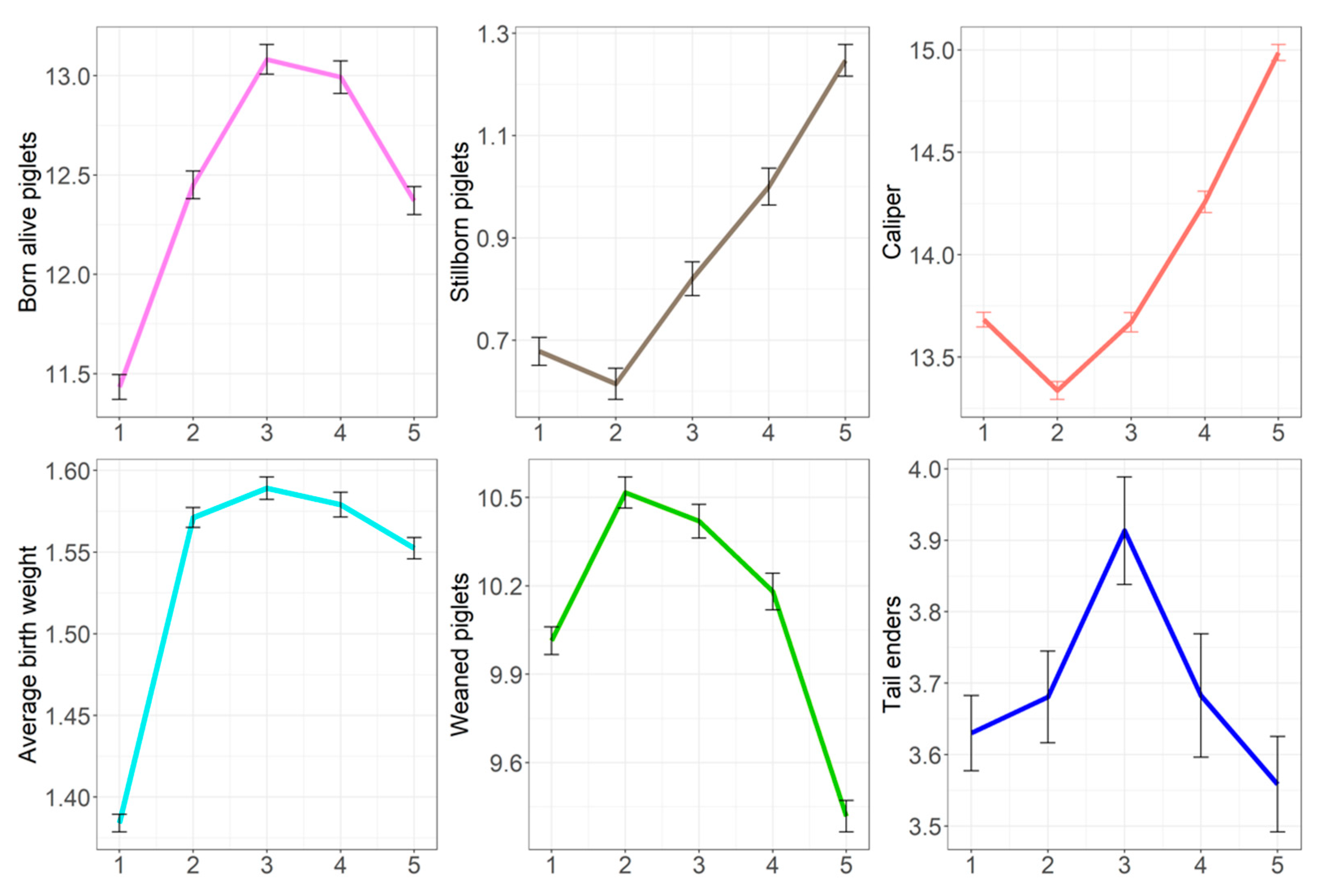
3.1. Phenotypic Data
3.2. Variance Components
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trevisi, P.; Luise, D.; Won, S.; Salcedo, J.; Bertocchi, M.; Barile, D.; Bosi, P. Variations in porcine colostrum oligosaccharide composition between breeds and in association with sow maternal performance. J. Anim. Sci. Biotechnol. 2020, 11, 21. [Google Scholar] [CrossRef]
- Merks, J.W.M.; Mathur, P.K.; Knol, E.F. New phenotypes for new breeding goals in pigs. Animal 2012, 6, 535–543. [Google Scholar] [CrossRef]
- Kanis, E.; Belt, H.V.D.; Groen, A.F.; Schakel, J.; de Greef, K.H. Breeding for improved welfare in pigs: A conceptual framework and its use in practice. Anim. Sci. 2004, 78, 315–329. [Google Scholar] [CrossRef]
- Pedersen, L.J. Chapter 1—Overview of commercial pig production systems and their main welfare challenges. In Advances in Pig Welfare; Špinka, M., Ed.; Woodhead Publishing: Kidlington, UK, 2018; pp. 3–25. [Google Scholar]
- Sell-Kubiak, E.; Knol, E.F.; Mulder, H.A. Selecting for changes in average “parity curve” pattern of litter size in Large White pigs. J. Anim. Breed. Genet. 2019, 136, 134–148. [Google Scholar] [CrossRef]
- Knol, E.F.; Nielsen, B.; Knap, P.W. Genomic selection in commercial pig breeding. Anim. Front. 2016, 6, 15–22. [Google Scholar] [CrossRef]
- Zak, L.J.; Gaustad, A.H.; Bolarin, A.; Broekhuijse, M.L.; Walling, G.A.; Knol, E.F. Genetic control of complex traits, with a focus on reproduction in pigs. Mol. Reprod. Dev. 2017, 84, 1004–1011. [Google Scholar] [CrossRef]
- Sell-Kubiak, E.; Wang, S.; Knol, E.F.; Mulder, H. Genetic analysis of within-litter variation in piglets’ birth weight using genomic or pedigree relationship matrices1. J. Anim. Sci. 2015, 93, 1471–1480. [Google Scholar] [CrossRef]
- Rutherford, K.M.D.; Baxter, E.M.; D’Eath, R.B.; Turner, S.P.; Arnott, G.; Roehe, R.; Ask, B.; Sandøe, P.; Moustsen, V.A.; Thorup, F.; et al. The welfare implications of large litter size in the domestic pig I: Biological factors. Anim. Welf. 2013, 22, 199–218. [Google Scholar] [CrossRef]
- Sell-Kubiak, E.; Bijma, P.; Knol, E.F.; Mulder, H.A. Comparison of methods to study uniformity of traits: Application to birth weight in pigs. J. Anim. Sci. 2015, 93, 900. [Google Scholar] [CrossRef]
- Damgaard, L.H.; Rydhmer, L.; Løvendahl, P.; Grandinson, K. Genetic parameters for within-litter variation in piglet birth weight and change in within-litter variation during suckling. J. Anim. Sci. 2003, 81, 604–610. [Google Scholar] [CrossRef]
- Dobrzański, J.; Mulder, H.A.; Knol, E.F.; Szwaczkowski, T.; Sell-Kubiak, E. Estimation of litter size variability phenotypes in Large White sows. J. Anim. Breed. Genet. 2020, 137, 559–570. [Google Scholar] [CrossRef]
- Lawlor, P.G.; Lynch, P.B. A review of factors influencing litter size in Irish sows. Ir. Vet. J. 2007, 60, 359–366. [Google Scholar] [CrossRef]
- Schukken, Y.; Buurman, J.; Huirne, R.; Willemse, A.H.; Vernooy, J.; Broek, J.V.D.; Verheijden, J. Evaluation of optimal age at first conception in gilts from data collected in commercial swine herds1. J. Anim. Sci. 1994, 72, 1387–1392. [Google Scholar] [CrossRef]
- Koketsu, Y.; Dial, G. Factors influencing the postweaning reproductive performance of sows on commercial farms. Theriogenology 1997, 47, 1445–1461. [Google Scholar] [CrossRef]
- Quiniou, N.; Dagorn, J.; Gaudré, D. Variation of piglets’ birth weight and consequences on subsequent performance. Livest. Prod. Sci. 2002, 78, 63–70. [Google Scholar] [CrossRef]
- Koketsu, Y.; Iida, R. Farm data analysis for lifetime performance components of sows and their predictors in breeding herds. Porc. Heal. Manag. 2020, 6, 24. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Knauer, M.T.; Baitinger, D.J. The sow body condition caliper. Appl. Eng. Agric. 2015, 31, 175–178. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Gilmour, A.R.; Gogel, B.J.; Cullis, B.R.; Welham, S.J.; Thompson, R. ASReml User Guide Release 4.1 Functional Specification; VSN International Ltd.: Hemel Hempstead, UK, 2015. [Google Scholar]
- Knol, E.; Leenhouwers, J.; van der Lende, T. Genetic aspects of piglet survival. Livest. Prod. Sci. 2002, 78, 47–55. [Google Scholar] [CrossRef]
- Harper, J.; Bunter, K.L.; Hermesch, S. Genetic paratemer estimates for pre- and post-weaning piglet mortality. In Proceedings of the Association for the Advancement of Animal Breeding and Genetics, Armidale, NSW, Australia, 27 October–1 November 2019. [Google Scholar]
- Davis, C. 2020 Pig Cost of Production in Selected Countries; AHDB: Warwickshire, UK, 2020. [Google Scholar]
- Smit, M.N.; Spencer, J.D.; Almeida, F.R.C.L.; Patterson, J.L.; Chiarini-Garcia, H.; Dyck, M.K.; Foxcroft, G.R. Consequences of a low litter birth weight phenotype for postnatal lean growth performance and neonatal testicular morphology in the pig. Animal 2013, 7, 1681–1689. [Google Scholar] [CrossRef]
- Rozeboom, D.W. Conditioning of the gilt for optimal reproductive performance. In The Gestating and Lactating Sow; Farmer, C., Ed.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2015; pp. 13–26. [Google Scholar]
- Lewis, C.; Bunter, K. Body development in sows, feed intake and maternal capacity. Part 2: Gilt body condition before and after lactation, reproductive performance and correlations with lactation feed intake. Animal 2011, 5, 1855–1867. [Google Scholar] [CrossRef] [Green Version]
- Vargovic, L.; Hermesch, S.; Athorn, R.Z.; Bunter, K.L. Feed intake and feeding behaviour traits of gestating sows are associated with undesirable outcomes. Livest. Sci. 2021, 249, 104526. [Google Scholar] [CrossRef]
- Bunter, K.L.; Vargovic, L.; Athorn, R.Z.; Henman, D.; Luxford, B.L. The influence of feed delivery and feeding patterns during gestation on reproductive outcomes for sows. In Proceedings of the 11th World Congress on Genetics Applied to Livestock Production, Auckland, New Zealand, 10–15 February 2018. [Google Scholar]
- Muro, B.B.; Carnevale, R.F.; Leal, D.F.; Almond, G.W.; Monteiro, M.S.; Poor, A.P.; Gerbossa, C.A.P. The importance of optimal body condition to maximise reproductive health and perinatal outcomes in pigs. Nutr. Res. Rev. 2022, 22, 1–21. [Google Scholar] [CrossRef]
- Freyer, G. Maximum number of total born piglets in a parity and individual ranges in litter size expressed as specific characteristics of sows. J. Anim. Sci. Technol. 2018, 60, 13. [Google Scholar] [CrossRef]
- Sell-Kubiak, E. Selection for litter size and litter birthweight in Large White pigs: Maximum, mean and variability of reproduction traits. Animal 2021, 15, 100352. [Google Scholar] [CrossRef]
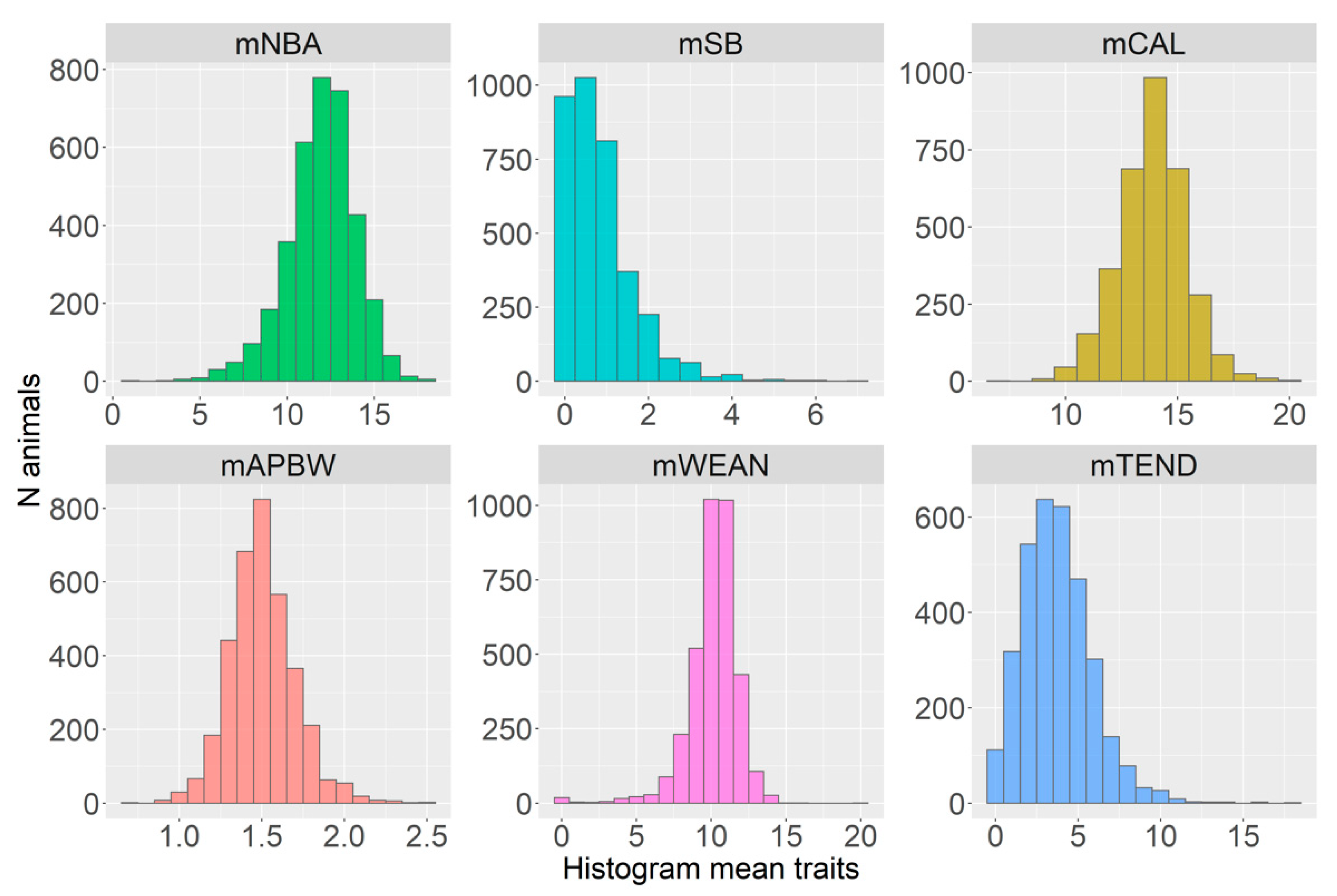


| Trait 1 | N Records | Mean (SD) 2 | Min–Max | IQR 0.25–0.75 3 | CV 4 |
|---|---|---|---|---|---|
| Traits by parity | |||||
| NBA | 13,976 | 12.2 (3.00) | 0–23 | 10–14 | 24.6 |
| SB | 13,976 | 0.89 (1.31) | 0–18 | 0–1 | 1.48 |
| CAL | 9033 | 14.1 (2.13) | 6–22 | 13–16 | 15.1 |
| APBW | 8495 | 1.52 (0.24) | 0.69–2.48 | 1.36–1.65 | 15.6 |
| WEAN | 13,209 | 10.2 (2.21) | 0–26 | 9–12 | 21.7 |
| TEND | 6462 | 3.89 (2.56) | 0–18 | 2–5 | 65.9 |
| Across parity traits | |||||
| mNBA | 3592 | 12.0 (2.00) | 1–18.3 | 11–13.3 | 16.6 |
| mSB | 3592 | 0.84 (0.82) | 0–7 | 0.25–1.14 | 98.3 |
| mCAL | 3338 | 13.9 (1.50) | 7–20 | 13–15 | 10.7 |
| mAPBW | 3532 | 1.51 (0.20) | 0.74–2.48 | 1.38–1.62 | 13.1 |
| mWEAN | 3538 | 10.2 (1.71) | 0–20 | 9.5 – 11.2 | 16.7 |
| mTEND | 3299 | 3.82 (2.10) | 0–18 | 2.25 – 5.00 | 54.9 |
| sdNBA | 2922 | 2.47 (1.29) | 0–10.6 | 1.53–3.20 | 52.3 |
| sdSB | 2922 | 0.94 (0.77) | 0–8.96 | 0.52–1.26 | 81.9 |
| sdCAL | 2468 | 1.51 (0.86) | 0–5.66 | 0.89–2.06 | 56.8 |
| sdAPBW | 2291 | 0.18 (0.11) | 0–0.86 | 0.096–0.227 | 63.2 |
| sdWEAN | 2854 | 1.76 (1.17) | 0–9.90 | 1.00–2.12 | 66.7 |
| sdTEND | 1733 | 2.06 (1.38) | 0–9.90 | 1.00–2.83 | 67.1 |
| NBAsl | 2922 | 0.006 (0.157) | −0.686–0.663 | −0.083–0.095 | NA |
| SBsl | 2922 | 0.016 (0.098) | −0.145–0.751 | −0.044–0.052 | NA |
| CALsl | 2466 | 0.053 (0.210) | −0.634–0.779 | −0.076–0.187 | NA |
| APBWsl | 2291 | 0.003 (0.016) | −0.057–0.062 | −0.006–0.012 | NA |
| WEANsl | 2854 | −0.011 (0.075) | −0.484–0.313 | −0.042–0.033 | NA |
| TENDsl | 1733 | −0.003 (0.033) | −0.173–0.163 | −0.020–0.015 | NA |
| Trait 1 | Additive Genetic Variance | Residual Variance | Heritability |
|---|---|---|---|
| mNBA | 0.44 (0.11) | 3.38 (0.11) | 0.12 (0.03) |
| mSB | 0.07 (0.01) | 0.61 (0.02) | 0.10 (0.03) |
| mCAL | 0.53 (0.09) | 1.53 (0.07) | 0.26 (0.04) |
| mAPBW | 0.01 (0.002) | 0.02 (0.001) | 0.35 (0.04) |
| mWEAN | 0.23 (0.07) | 2.58 (0.07) | 0.08 (0.02) |
| mTEND | 0.25 (0.10) | 3.75 (0.12) | 0.06 (0.02) |
| sdNBA | 3.9 × 10−8 (0.00) | 1.63 (0.04) | 0.00 (0.00) |
| sdSB | 5.3 × 10−5 (0.0053) | 0.56 (0.02) | 0.0001 (0.01) |
| sdCAL | 0.04 (0.02) | 0.68 (0.03) | 0.05 (0.03) |
| sdAPBW | 2.8 × 10−4 (2.8 × 10−4) | 0.01 (4.2 × 10−4) | 0.03 (0.02) |
| sdWEAN | 1.4 × 10−6 (0.00) | 1.36 (0.04) | 0.00 (0.00) |
| sdTEND | 0.11 (0.06) | 1.67 (0.08) | 0.06 (0.04) |
| NBAsl | 3.1 × 10−3 (7.7 × 10−4) | 2.2 × 10−2 (8.2 × 10−4) | 0.13 (0.03) |
| SBsl | 1.3 × 10−3 (3.0 × 10−4) | 8.0 × 10−3 (3.1 × 10−4) | 0.14 (0.03) |
| CALsl | 1.1 × 10−2 (1.9 × 10−2) | 2.8 × 10−2 (1.5 × 10−3) | 0.27 (0.04) |
| APBWsl | 9.1 × 10−5 (1.3 × 10−5) | 1.5 × 10−4 (9.4 × 10−6) | 0.38 (0.05) |
| WEANsl | 5.4 × 10−4 (1.5 × 10−4) | 4.8 × 10−3 (1.8 × 10−4) | 0.10 (0.03) |
| TENDsl | 1.6 × 10−4 (4.7 × 10−5) | 9.4 × 10−4 (4.9 × 10−5) | 0.14 (0.04) |
| Traits 1 | Correlation | mTEND | sdTEND | TENDsl |
|---|---|---|---|---|
| mNBA | rA | 0.71 (0.15) | 0.46 (0.26) | 0.37 (0.18) |
| rP | 0.34 (0.02) | 0.13 (0.03) | 0.07 (0.03) | |
| mSB | rA | NC | 0.45 (0.29) | −0.30 (0.19) |
| rP | 0.04 (0.03) | −0.17 (0.03) | ||
| mAPBW | rA | NC | −0.10 (0.21) | NC |
| rP | −0.04 (0.03) | |||
| mWEAN | rA | 0.20 (0.24) | −0.36 (0.31) | 0.03 (0.21) |
| rP | 0.09 (0.02) | −0.02 (0.03) | 0.06 (0.03) | |
| mTEND | rA | NA | 0.17 (0.37) | 0.64 (0.15) |
| rP | 0.49 (0.02) | 0.76 (0.01) | ||
| mCAL | rA | −0.57 (0.16) | −0.13 (0.24) | −0.29 (0.16) |
| rP | −0.03 (0.02) | 0.04 (0.03) | −0.03 (0.03) | |
| sdTEND | rA | 0.17 (0.37) | NA | 0.25 (0.28) |
| rP | 0.49 (0.02) | NA | 0.27 (0.03) | |
| sdCAL | rA | −0.38 (0.30) | −0.42 (0.41) | 0.12 (0.28) |
| rP | −0.04 (0.02) | −0.01 (0.03) | 0.009 (0.03) | |
| NBAsl | rA | NC | 0.31 (0.26) | 0.31 (0.18) |
| rP | 0.08 (0.02) | 0.08 (0.03) | ||
| SBsl | rA | 0.43 (0.20) | 0.47 (0.26) | −0.26 (0.18) |
| rP | −0.0008 (0.02) | 0.03 (0.02) | −0.14 (0.02) | |
| APBWsl | rA | −0.83 (0.15) | −0.10 (0.21) | NC |
| rP | −0.09 (0.02) | −0.008 (0.03) | ||
| WEANsl | rA | 0.32 (0.23) | −0.10 (0.28) | 0.28 (0.21) |
| rP | 0.04 (0.03) | −0.03 (0.02) | 0.05 (0.02) | |
| CALsl | rA | −0.52 (0.17) | −0.28 (0.26) | −0.29 (0.17) |
| rP | −0.03 (0.02) | 0.03 (0.03) | −0.02 (0.03) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargovic, L.; Harper, J.-A.; Bunter, K.L. Traits Defining Sow Lifetime Maternal Performance. Animals 2022, 12, 2451. https://doi.org/10.3390/ani12182451
Vargovic L, Harper J-A, Bunter KL. Traits Defining Sow Lifetime Maternal Performance. Animals. 2022; 12(18):2451. https://doi.org/10.3390/ani12182451
Chicago/Turabian StyleVargovic, Laura, Jo-Anne Harper, and Kim L. Bunter. 2022. "Traits Defining Sow Lifetime Maternal Performance" Animals 12, no. 18: 2451. https://doi.org/10.3390/ani12182451
APA StyleVargovic, L., Harper, J.-A., & Bunter, K. L. (2022). Traits Defining Sow Lifetime Maternal Performance. Animals, 12(18), 2451. https://doi.org/10.3390/ani12182451





