The Efficacy of a 3β-Hydroxysteroid Dehydrogenase Inhibitor for the Termination of Mid-Term Pregnancies in Dogs
Abstract
:Simple Summary
Abstract
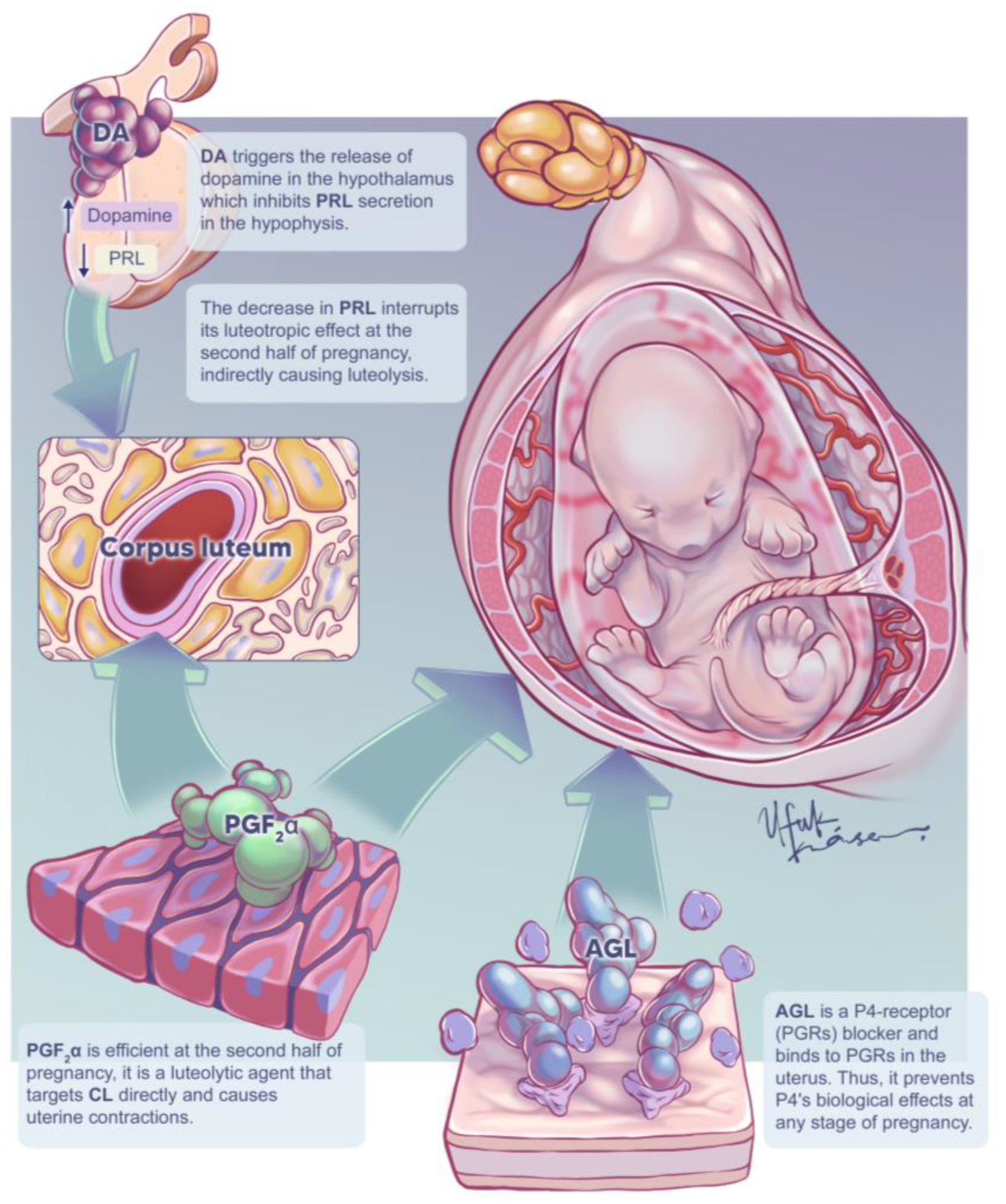
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.3. Medical Induction of Pregnancy Termination
2.4. Ovariohysterectomy and Tissue Sampling
2.5. CBC, Serum Biochemistry, and Progesterone Analyses
2.6. Immunohistochemistry
2.7. Statistical Analysis
3. Results
3.1. Study Groups and Pregnancy Termination Rate
3.2. Clinical Findings and Side Effects
3.3. Fetal Distress
3.4. Plasma Progesterone Concentrations
3.5. Expression of StAR and 3β-HSD in Canine Corpora Lutea
4. Discussion
4.1. Routine Clinical Findings
4.2. Fetal Heart Rate
4.3. Pregnancy Terminations and Progesterone Concentrations
4.4. Expression of StAR and 3β-HSD in Canine Corpora Lutea
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kowalewski, M.P.; Pereira, M.T.; Papa, P.; Gram, A. Progesterone receptor blockers: Historical perspective, mode of function and insights into clinical and scientific applications. Tierarztl Prax Ausg K Kleintiere Heimtiere 2020, 48, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.; Kustritz, M.; Olson, P. (Eds.) Prevention and termination of canine pregnancy. In Canine and Feline Theriogenology, 1st ed.; Saunders: Philadelphia, PA, USA, 2001; pp. 168–192. [Google Scholar]
- Gogny, A.; Fieni, F. Aglepristone: A review on its clinical use in animal. Theriogenology 2016, 85, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Eilts, B.E. Pregnancy termination in the bitch and queen. Clin. Tech. Small Anim. Pract. 2002, 17, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, S. Practical use of hormones in small animal reproduction. Rev. Bras. Reprod. Anim. Belo Horiz. 2017, 41, 59–67. [Google Scholar]
- Ağaoğlu, A.; Aslan, S.; Emre, B.; Korkmaz, O.; Özdemir Salcı, E.; Kocamüftüoğlu, M.; Seyrek-İntaş, K.; Schäfer-Somi, S. Clinical evaluation of different applications of misoprostol and aglepristone for induction of abortion in bitches. Theriogenology 2014, 81, 947–951. [Google Scholar] [CrossRef]
- Romagnoli, S.E.; Cela, M.; Camillo, F. Use of prostaglandin F2α for early pregnancy termination in the mismated bitch. Vet. Clin. North Am. Small Anim. Pract. 1991, 21, 487–499. [Google Scholar] [CrossRef]
- Ay, S.; Önyay, F.; Saral, G.; Kaya, D.; Aslan, A.; Fındık, M. The efficacy of alone or combined treatment of aglepristone and cabergoline on termination of mid-term pregnancy in cats. Kafkas Univ. Vet. Fak. Derg. 2018, 24, 491–496. [Google Scholar]
- Post, K.; Evans, L.E.; Jochle, W. Effects of prolactin suppression with cabergoline on the pregnancy of the bitch. Theriogenology 1988, 29, 1239–1243. [Google Scholar] [CrossRef]
- Corrada, Y.; Garcia, P.; De La Sota, P.E.; Huzman, M.; Landoni, M.F.; Gobello, C. Decrease of body temperature after aglepristone treatment in bitches. Anim. Reprod. Sci. 2005, 87, 295–299. [Google Scholar] [CrossRef]
- Fieni, F.; Martal, P.G.; Siliart, B.; Bernard, F.; Riou, M.; Bruyas, J.F.; Tainturier, D. Hormonal variation in bitches after early or mid-pregnancy termination with aglepristone (RU534). J. Reprod. Fertil. Suppl. 2001, 57, 243–248. [Google Scholar]
- Kowalewski, M.; Beceriklisoy, H.; Aslan, S.; Ağaoğlu, A.; Hoffmann, B. Time related changes in luteal prostaglandin synthesis and steroidogenic capacity during pregnancy, normal and antiprogestin induced luteolysis in the bitch. Anim. Reprod. Sci. 2009, 116, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Kowalewski, M. Luteal regression vs. prepartum luteolysis: Regulatory mechanisms governing canine corpus luteum function. Reprod. Biol. 2014, 14, 89–102. [Google Scholar] [CrossRef] [PubMed]
- King, S.R.; Stocco, D.M. Steroidogenic acute regulatory protein expression in the central nervous system. Front. Endocrinol. 2011, 2, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramsey, I.K. Trilostane in dogs. Vet. Clin. North Am. Small Anim. Pract. 2010, 40, 269–283. [Google Scholar] [CrossRef] [PubMed]
- De Bosschere, H.; Ducatelle, R.; Tshamala, M.; Coryn, M. The use of epostane in an attempt to reproduce cystic endometrial hyperplasia in the bitch. Vet. Rec. Commun. 2003, 27, 527–538. [Google Scholar] [CrossRef] [PubMed]
- De Gier, J.; Wolthers, C.; Galac, S.; Okkens, A.; Kooistra, H. Effects of the 3beta hydroxysteroid dehydrogenase inhibitor trilostane on luteal progesterone production in the dog. Theriogenology 2011, 75, 1271–1279. [Google Scholar] [CrossRef]
- Jurczak, A.; Janowki, T.; Zdunczyk, S.; Failing, K.; Schuler, G.; Hoffmann, B. Attempts to downregulate ovarian function in the bitch by applying a GnRH agonist implant in combination with a 3β-hydroxysteroid–dehydrogenase blocker; a pilot study. Theriogenology 2020, 145, 176–180. [Google Scholar] [CrossRef]
- Taylor, M.; Webb, R.; Mitchell, M.; Robinson, J. Effect of progesterone withdrawal in sheep during late pregnancy. J. Endocrinol. 1982, 92, 85–93. [Google Scholar] [CrossRef]
- Weems, Y.; Vincent, D.; Lemme, C.; Weems, C. Trilostane but not prostaglandin F2α (PGF2α) or cortisol aborts 90-day-pregnant lutectomized sheep. Prostaglandins Other Lipid Mediat. 1999, 58, 77–86. [Google Scholar] [CrossRef]
- Schutzer, W.E.; Kerby, J.L.; Holtan, D.W. Differential effect of trilostane on the progestin milieu in the pregnant mare. J. Reprod. Fertil. 1996, 107, 241–248. [Google Scholar] [CrossRef]
- Yeager, A.; Mohammed, H.; Meyers-Wallen, V.; Vannerson, L.; Concannon, P. Ultrasonographic appearance of the uterus, placenta, fetus, and fetal membranes throughout accurately timed pregnancy in beagles. Am. J. Vet. Res. 1992, 53, 342–351. [Google Scholar] [PubMed]
- Giannico, A.T.; Gil, E.M.U.; Garcia, D.A.A.; Froes, T.R. The use of Doppler evaluation of the canine umbilical artery in prediction of delivery time and fetal distress. Anim. Reprod. Sci. 2015, 154, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Kaya, D.; Küçükaslan, İ.; Ağaoğlu, A.; Ay, S.; Schäfer-Somi, S.; Emre, B.; Bal, Y.; Einspanier, A.; Gürcan, İ.; Gültiken, N. The effects of aglepristone alone and in combination with cloprostenol on hormonal values during termination of mid–term pregnancy in bitches. Anim. Reprod. Sci. 2014, 146, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, C.; Tidholm, A. Safety and efficacy of mid-term pregnancy termination using aglepristone in dogs. J. Small Anim. Pract. 2009, 50, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Seguin, B. Ovariectomy and Ovariohysterectomy. In Small Animal Soft Tissue Surgery; Monnent, E., Ed.; Willey: Hoboken, NJ, USA, 2013; pp. 651–658. [Google Scholar]
- Kowalewski, M.P.; Fox, B.; Gram, A.; Boos, A.; Reichler, I. Prostaglandin E2 functions as a luteotrophic factor in the dog. Reproduction 2013, 145, 213–226. [Google Scholar] [CrossRef]
- Fieni, F.; Martal, J.; Marnet, P.G.; Siliart, B.; Guittot, F. Clinical, biological and hormonal study of mid-pregnancy termination in cats with aglepristone. Theriogenology 2006, 66, 1721–1728. [Google Scholar] [CrossRef]
- Lemetayer, J.; Blois, S. Update on the use of trilostane in dogs. Can. Vet. J. 2018, 59, 397–407. [Google Scholar]
- Behrend, E.N. Canine hyperadrenocorticism. In Canine & Feline Endocrinology, 4th ed.; Feldman, E.C., Nelson, R.W., Reusch, C., Scott–Moncrieff, J.C., Behrend, E.N., Eds.; Elsevier: St. Louis, MO, USA, 2015; pp. 377–451. [Google Scholar]
- Nelson, R.W. Endocrine, metabolic, and lipid disorders. In Small Animal Clinical Diagnosis by Laboratory Methods, 5th ed.; Willard, M.D., Tvedten, H., Eds.; Elsevier: St. Louis, MO, USA, 2012; pp. 156–190. [Google Scholar]
- Burkhardt, T.; Schmidt, N.O.; Vettorazzi, E.; Aberle, J.; Menhel, M.; Flitsch, J. DHEA(S)—A novel marker in Cushing’s diseases. Acta Neurochir. 2013, 155, 379–484. [Google Scholar] [CrossRef]
- Reine, N.J. Medical management of pituitary-dependent hyperadrenocorticism: Mitotane versus trilostane. Clin. Tech. Small Anim. Pract. 2007, 22, 18–25. [Google Scholar] [CrossRef]
- Le Roux, P.A.; Tregoning, S.; Zinn, P.; Van Der Spuy, Z. Inhibition of progesterone secretion with trilostane for mid-trimester termination of pregnancy: Randomized controlled trials. Hum. Reprod. 2002, 17, 1483–1489. [Google Scholar] [CrossRef]
- Le Roux, P.A.; Van Der Spuy, Z.M. Labor induction abortion utiling trilostane, a 3beta-hydroxysteroid dehydrogenase inhibitor. Contraception 2005, 71, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Behrend, E.N.; Civco, T.D.; Merton Boothe, D. Drug therapy for endocrinopathies. In Small Animal Clinical Pharmacology & Therapeutics; Merton Boothe, D., Ed.; Elsevier: St. Louis, MO, USA, 2012; p. 832. [Google Scholar]
- Braddock, J.A.; Church, D.B.; Robertson, I.D.; Watson, A.D.J. Trilostane treatment in dogs with pituitary-dependent hyperadrenocorticism. Aust. Vet. J. 2003, 81, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Zinn, P.M. Trilostane as Antiprogestin Therapy in Pregnancy Termination. Master’s Thesis, University of Cape Town, Cape Town, South Africa, November 2000. [Google Scholar]
- Bruyette, D. Pituitary-dependent hyuperadrenocorticism in dogs and cats. In Clinical Small Animal Internal Medicine; Bruyette, D., Ed.; Willey: Hoboken, NJ, USA, 2020; pp. 49–65. [Google Scholar]
- Webster, M.; Phipps, S.; Gillmer, M. Interruption of first trimester human pregnancy following epostane therapy. Effect of prostaglandin E2 pessaries. BJOG Int. J. Obstet. Gynaecol. 1985, 92, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Crooij, M.J.; De Nooyer, C.C.; Rao, B.R.; Berends, G.T.; Gooren, L.J.; Janssens, J. Termination of early pregnancy by the 3β–hydroxysteroid dehydrogenase inhibitor epostane. N. Engl. J. Med. 1988, 319, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.D.; Kang, J.H.; Chang, D.; Na, K.J.; Yang, M.P. Efficacy of low- and high-dose trilostane treatment in dogs (<5 kg) with pituitary-dependent hyperadrenocorticism. J. Vet. Intern. Med. 2013, 27, 91–98. [Google Scholar] [CrossRef]
- Galac, S.; Kooistra, H.; Butinar, J.; Bevers, M.; Dieleman, S.; Voorhout, G.; Okkens, A. Termination of mid-gestation pregnancy in bitches with aglepristone, a progesterone receptor antagonist. Theriogenology 2000, 53, 941–950. [Google Scholar] [CrossRef]
- Fieni, F.; Tainturier, D.; Bruyas, J.; Badinand, F.; Berthelot, X.; Ronsin, P.; Rachail, M.; Lefay, M.M. Etude clinique d’une anti-hormone pour provoquer l’avortement chez la chienne: L’aglépristone. Rec. Med. Vet. 1996, 172, 359–367. [Google Scholar]
- Haluska, G.J.; Cooka, M.J.; Novy, M.J. Inhibition and augmentation of progesterone production during pregnancy: Effects on parturition in rhesus monkeys. Am. J. Obstet. Gynecol. 1997, 176, 682–691. [Google Scholar] [CrossRef]
- England, G. Vaginal cytology and cervicovaginal mucus arborisation in the breeding management of bitches. J. Small Anim. Pract. 1992, 33, 577–582. [Google Scholar] [CrossRef]
- Silva, L.D.M.; Onclin, K.; Verstegen, J.P. Cervical opening in relation to progesterone and oestradiol during heat in beagle bitches. J. Reprod. Fertil. 1995, 104, 85–90. [Google Scholar] [CrossRef]
- Fusi, J.; Veronesi, M.C. Canine parturition: What is known about thw homonal setting? Domest. Anim. Endocrinol. 2022, 78, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gendler, A.; Brourman, J.D.; Graf, K.E. Canine dystocia: Medical and surgical management. Compend. Contin. Educ. Pract. Vet. North Am. Ed. 2007, 29, 551. [Google Scholar]
- Concannon, P. Canine pregnancy: Predicting parturition and timing events of gestation. In Recent Advances in Small Animal Reproduction; Concannon, P.W., England, E., Verstegen, J., Eds.; International Veterinary Information Service: Ithaca, NY, USA, 2000; pp. 1–7. [Google Scholar]
- Kimura, T.; Kotani, K. Perinatal veterinary medicine–related evaluation in hematological and serum biochemical profiles of experimental beagles throughout pregnancy and parturition. Anim. Model Exp. Med. 2018, 1, 282–294. [Google Scholar] [CrossRef] [PubMed]
- England, G. Ultrasonographic assessment of abnormal pregnancy. Vet. Clin. North Am. Small Anim. Pract. 1998, 28, 849–868. [Google Scholar] [CrossRef]
- Breukelman, S.P.; Szenci, O.; Beckers, J.-F.; Kindahl, H.; Mulder, E.J.; Jonker, F.H.; Van Der Weijden, B.; Revy, D.; Pogany, K.; Sulon, J. Ultrasonographic appearance of the conceptus, fetal heart rate and profiles of pregnancy-associated glycoproteins (PAG) and prostaglandin F2α–metabolite (PGF2α–metabolite) after induction of fetal death with aglepristone during early gestation in cattle. Theriogenology 2005, 64, 917–933. [Google Scholar] [CrossRef]
- Gil, E.; Garcia, D.; Giannico, A.; Froes, T. Canine fetal heart rate: Do accelerations or decelerations predict the parturition day in bitches? Theriogenology 2014, 82, 933–941. [Google Scholar] [CrossRef]
- Smith, F. Challenges in small animal parturition—Timing elective and emergency cesarian sections. Theriogenology 2007, 68, 348–353. [Google Scholar] [CrossRef]
- Johnson, C. Pregnancy management in the bitch. Theriogenology 2008, 70, 1412–1417. [Google Scholar] [CrossRef]
- Soothill, P.W.; Ajayi, R.A.; Champbell, S.; Nicolaides, K.H. Prediction of morbidity in small and normally grown fetuses by fetal heart rate variability, biophysical profile score and umbical artery doppler studies. BJOG Int. J. Obstet. Gynaecol. 1993, 100, 742–745. [Google Scholar] [CrossRef]
- Keister, D.; Kaiser, L.; Gensburg, L.; D’Ver, A.; Ehrhart, W. The use of epostane, a 3β-hydroxysteroid dehydrogenase delta 4-5 isomerase enzyme inhibitor, in oil suspension as a mismating agent in the dog. Theriogenology 1988, 30, 497–506. [Google Scholar] [CrossRef]
- Schane, H.P.; Potts, G.O.; Creange, J.E. Inhibition of ovarian, placental, and adrenal steroidogenesis in the rhesus monkey by trilostane. Fertil. Steril. 1979, 32, 464–467. [Google Scholar] [CrossRef]
- Fowden, A.; Silver, M. Effects of inhibiting 3 beta-hydroxysteroid dehydrogenase on plasma progesterone and other steroids in the pregnant mare near term. J. Reprod. Fertil. Suppl. 1987, 35, 539–545. [Google Scholar]
- Jenkin, G.; Thorburn, G.D. Inhibition of progesterone secretion by a 3β–hydroxysteroid dehydrogenase inhibitor in late pregnant sheep. Can. J. Physiol. Pharmacol. 1985, 63, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Alenza, D.P.; Arenas, C.; Lopez, M.L.; Melian, C. Long-term efficacy of trilostane administered twice daily in dogs with pituitary-dependent hyperadrenocorticism. J. Am. Anim. Hosp. Assoc. 2006, 42, 269–276. [Google Scholar] [CrossRef]
- Galac, S.; Buijtels, J.J.; Mol, J.A.; Kooistra, H.S. Effects of trilostane on the pituitary-adrenocortical and renin–aldosterone axis in dogs with pituitary-dependent hypercortisolism. Vet. J. 2010, 183, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Kowalewski, M.; Mason, J.; Howie, A.; Morley, S.; Schuler, G.; Hoffmann, B. Characterization of the canine 3β- hydroxysteroid dehydrogenase and its expression in the corpus luteum during diestrus. J. Steroid Biochem. Mol. Biol. 2006, 101, 254–262. [Google Scholar] [CrossRef]
- Ouschan, C.; Lepschy, M.; Zeugswetter, F.; Möstl, E. The influence of trilostane on steroid hormone metabolism in canine adrenal glands and corpora lutea—An in vitro study. Vet. Res. Commun. 2012, 36, 35–40. [Google Scholar] [CrossRef]
- Papacleovoulou, G.; Edmondson, R.J.; Critchley, H.O.; Hillier, S.G.; Mason, J.I. 3β-hydroxysteroid dehydrogenases and pre–receptor steroid metabolism in the human ovarian surface epithelium. Mol. Cell. Endocrinol. 2009, 301, 65–73. [Google Scholar] [CrossRef]
- Thomas, J.L.; Mack, V.L.; Glow, J.A.; Moshkelani, D.; Terrell, J.R.; Bucholtz, K.M. Structure/function of the inhibition of human 3β-hydroxysteroid dehydrogenase type 1 and type 2 by trilostane. J. Steroid Biochem. Mol. Biol. 2008, 111, 66–73. [Google Scholar] [CrossRef]
- Ayoub, N.M. Editorial: Novel Combination Therapies for the Treatment of Solid Cancers. Front. Oncol. 2021, 11, 2377. [Google Scholar] [CrossRef]
- Ağaoğlu, A.R.; Schafer-Somi, S.; Kaya, D.; Küçükaslan, İ.; Emre, B.; Gültiken, N.; Mülazımoğlu, B.S.; Çolak, A.; Aslan, S. The intravaginal application of misoprostol improves induction of abortion with aglepristone. Theriogenology 2011, 76, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, B.; Schuler, G. Receptor blockers-general aspects with respect to their use in domestic animal reproduction. Anim. Reprod. Sci. 2000, 60, 295–312. [Google Scholar] [CrossRef]
- Schafer-Somi, S.; Aksoy, O.; Beceriklisoy, H.; Einspanier, A.; Hoppen, H.; Aslan, S. Repeated induction of abortion in bitches and the effect on plasma concentrations of relaxin, progesterone and estradiol-17β. Theriogenology 2007, 68, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.; Höftmann, T.; Politt, E.; Hoppen, H.O.; Sohr, M.; Günzel-Apel, A.R.; Einspanier, A. Morphological examination of the corpora lutea from pregnant bitches treated with different abortifacient regimes. Reprod. Domest. Anim. 2009, 44, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Baan, M.; Taverne, M.A.M.; Kooistra, H.S.; De Gier, J.; Dieleman, S.J.; Okkens, A.C. Induction of parturition in the bitch with the progesterone-receptor blocker aglepristine. Theriogenology 2005, 63, 1958–1972. [Google Scholar] [CrossRef]
- Hoffmann, B.; Busges, F.; Engel, E.; Kowalewski, M.; Papa, P. Regulation of corpus luteum-function in the bitch. Reprod. Domest. Anim. 2004, 39, 232–240. [Google Scholar] [CrossRef]
- Kowalewski, M.P.; Beceriklisoy, H.B.; Pfarrer, C.; Aslan, S.; Kindahl, H.; Kücükaslan, İ.; Hoffmann, B. Canine placenta: A source of prepartal prostaglandins during normal and antiprogestin-induced parturition. Reproduction 2010, 139, 655–664. [Google Scholar] [CrossRef]
- Hoffmann, B.; Riesenbeck, A.; Klein, R. Reproductive endocrinology of the bitch. Anim. Reprod. Sci. 1996, 42, 275–288. [Google Scholar] [CrossRef]
- McLean, M.P.; Billheimer, J.T.; Warden, K.J.; Irby, R.B. Prostaglandin F2 alpha mediates ovarian sterol carrier protein–2 expression during luteolysis. Endocrinology 1995, 136, 4963–4972. [Google Scholar] [CrossRef]
- Rothchild, I. The regulation of mammalian corpus luteum. Recent Prog. Horm. Res. 1981, 37, 183–298. [Google Scholar] [CrossRef]
- Simard, J.; Ricketts, M.L.; Gingras, S.; Soucy, P.; Feltus, F.A.; Melner, M.H. Molecular biology of the 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase gene family. Endocr. Rev. 2005, 26, 525–582. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D. The Metabolism of Trilostane and Epostane. Ph.D. Thesis, University of Surrey, Northumberland, UK, February 1989. [Google Scholar]
- Benyo, D.F.; Little-Ihrig, L.; Zeleznik, A.J. Noncoordinated expression of luteal cell messenger ribonucleic acids during human chorionic gonadotropin stimulation of the primate corpus luteum. Endocrinology 1993, 133, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Martel, C.; Labrie, C.; Couet, J.; Dupont, E.; Trudel, C.; Luu–Tea, V.; Takahashi, M.; Pelletier, G.; Labrie, F. Effects of human chorionic gonadotropin (hCG) and prolactin (PRL) on 3β-hydroxy-5-ene-steroid dehydrogenase/Δ5-Δ4 isomerase (3β-HSD) expression and activity in the rat ovary. Mol. Cell. Endocrinol. 1990, 72, R7–R13. [Google Scholar] [CrossRef]
- McAlister, J.M.; Byrd, W.; Simpson, E.R. The effects of growth factors and phorbol esters on steroid biosynthesis in isolated human theca interna and granulosa-lutein cells in long term culture. J. Clin. Endocrinol. Metab. 1994, 79, 106–112. [Google Scholar] [CrossRef]





| Days Hours | d1 0 | 6 | 12 | 18 | d2 24 | 30 | 36 | 42 | d3 48 | 54 | 60 | 66 | d4 72 | 78 | 84 | 90 | d5 96 | 102 | 108 | 114 | d6 120 | 126 | 132 | 138 | d7 144 | 150 | 156 | 162 | d8 OHE | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | TRL | • | • | • | • | • | • | • | ||||||||||||||||||||||
| AGL | • | • | ||||||||||||||||||||||||||||
| Clinical assessments | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | |||||||||||||||
| CBC & Biochemistry | • | • | • | • | • | • | • | • | ||||||||||||||||||||||
| P4 assay | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | |
| Group | GA (d; X ± SE) | Age (m; X ± SE) | Body Weight (kg; X ± SE) | PT | |
|---|---|---|---|---|---|
| Rate (%) | Duration (h; X ± SE) | ||||
| TRL (n = 7) | 36.1 ± 0.9 | 22.8 ± 14.9 | 18.5 ± 6.1 | 0 (0/7) | - |
| (range) | (32–38) | (12–48) | (15.2–27.8) | ||
| AGL (n = 7) | 33.9 ± 1.2 | 20.6 ± 8.4 | 16.1 ± 5.1 | 100 (7/7) | 36.7 ± 23.8 |
| (range) | (30–38) | (12–36) | (10–24) | ||
| CON (n = 7) | 35.4 ± 1.1 | 25.2 ± 10.7 | 17.1 ± 6.4 | 0 (0/7) | - |
| (range) | (32–38) | (12–48) | (9.7–27) | ||
| p value | p > 0.05 | p > 0.05 | p > 0.05 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Binli, F.; İnan, İ.; Büyükbudak, F.; Gram, A.; Kaya, D.; Liman, N.; Aslan, S.; Fındık, M.; Ay, S.S. The Efficacy of a 3β-Hydroxysteroid Dehydrogenase Inhibitor for the Termination of Mid-Term Pregnancies in Dogs. Animals 2022, 12, 2475. https://doi.org/10.3390/ani12182475
Binli F, İnan İ, Büyükbudak F, Gram A, Kaya D, Liman N, Aslan S, Fındık M, Ay SS. The Efficacy of a 3β-Hydroxysteroid Dehydrogenase Inhibitor for the Termination of Mid-Term Pregnancies in Dogs. Animals. 2022; 12(18):2475. https://doi.org/10.3390/ani12182475
Chicago/Turabian StyleBinli, Firdevs, İpek İnan, Fatih Büyükbudak, Aykut Gram, Duygu Kaya, Narin Liman, Selim Aslan, Murat Fındık, and Serhan Serhat Ay. 2022. "The Efficacy of a 3β-Hydroxysteroid Dehydrogenase Inhibitor for the Termination of Mid-Term Pregnancies in Dogs" Animals 12, no. 18: 2475. https://doi.org/10.3390/ani12182475





