Changes in the Expression of MIF and Other Key Enzymes of Energy Metabolism in the Myocardia of Broiler Chickens with Ascites Syndrome
Abstract
:Simple Summary
Abstract
1. Introduction
- (1)
- Genetic factors: Broilers are artificially bred strains whose most significant biological characteristics are their exceedingly rapid growth rate and efficient feed conversion. However, they also possess genetic defects; for instance, an imbalance between the development of their cardio-respiratory system and their fast-growing bodies occurs before four weeks of age [9,10], which leaves their organs and muscles extremely vulnerable to hypoxia. Therefore, broiler chickens are especially prone to developing ascites.
- (2)
2. Materials and Methods
2.1. Source and Screening of Ascitic Cases
2.2. Antibodies and Chemicals
2.3. Hematoxylin and Eosin (HE) Staining
2.4. Western Blotting
2.5. Statistical Analysis
3. Results
3.1. The Performance of Clinical Ascitic Broilers
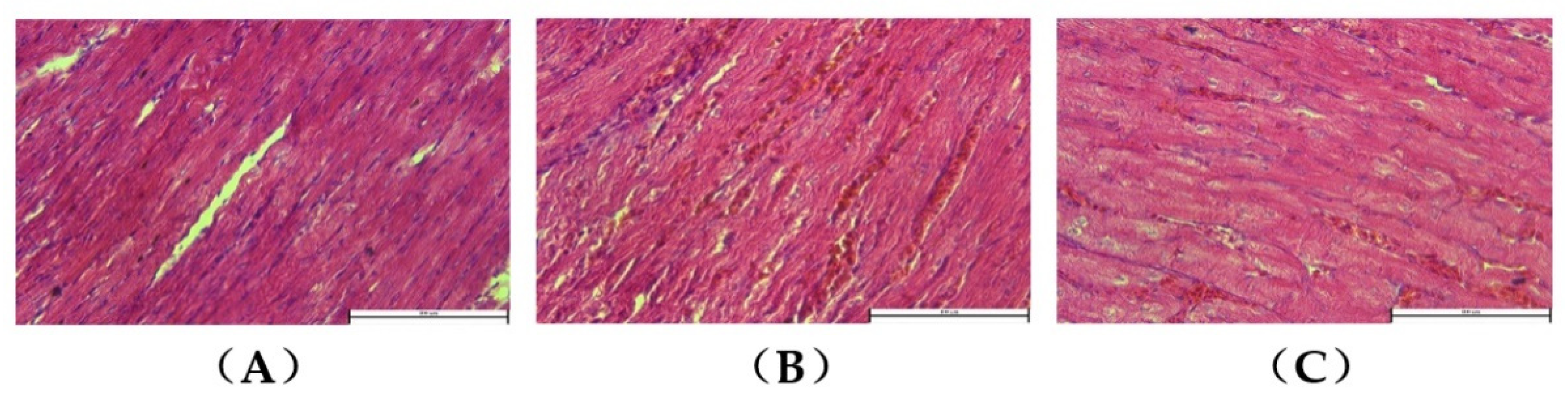
3.2. Myocardial Histopathology
3.3. Expressions of MIF and p-AMPK in Ascitic Broilers
3.4. Expressions of Key Enzymes of the Energy Metabolic Pathway of Myocardial Tissue in Ascitic Broilers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, W.A. Trial Diagnosis of Ascites Syndrome in Broiler Chickens. Pak. J. Biol. Sci. 2016, 19, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Cao, H.; Xiao, Q.; Guo, X.; Zhuang, Y.; Zhang, C.; Wang, T.; Lin, H.; Song, Y.; Hu, G.; et al. Transcriptome Analysis and Gene Identification in the Pulmonary Artery of Broilers with Ascites Syndrome. PLoS ONE 2016, 11, e0156045. [Google Scholar]
- Ahmadpanah, J.; Ghavi Hossein-Zadeh, N.; Shadparvar, A.A.; Pakdel, A. Genetic parameters of body weight and ascites in broilers: Effect of different incidence rates of ascites syndrome. Br. Poult. Sci. 2017, 58, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Druyan, S.; Cahaner, A. Segregation among test-cross progeny suggests that two complementary dominant genes explain the difference between ascites-resistant and ascites-susceptible broiler lines. Poult. Sci. 2007, 86, 2295–2300. [Google Scholar] [CrossRef]
- Parveen, A.; Jackson, C.D.; Dey, S.; Tarrant, K.; Anthony, N.; Rhoads, D.D. Identification and validation of quantitative trait loci for ascites syndrome in broiler chickens using whole genome resequencing. BMC Genet. 2020, 21, 54. [Google Scholar] [CrossRef]
- Akit, M.; Altan, O.; Karul, A.B.; Balkaya, M. Effects of cold temperature and vitamin E supplementation on oxidative stress, Troponin-T level, and other ascites-related traits in broilers. Arch. Geflugelkd. 2008, 72, 221–230. [Google Scholar]
- Mahjoor, A.A.; Hadipoor, M.M. Clinical, Gross and Histopathological Studies on Natural Ascites Syndrome in Broiler Chickens. J. Altern. Vet. Med. 2020, 3, 333–342. [Google Scholar]
- Julian, R.J. Physiological, management and environmental triggers of the ascites syndrome: A review. Avian Pathol. 2000, 29, 519–527. [Google Scholar] [CrossRef]
- Wideman, R.F.; Rhoads, D.D.; Erf, G.F.; Anthony, N.B. Pulmonary arterial hypertension (ascites syndrome) in broilers: A review. Poult. Sci. 2013, 92, 64–83. [Google Scholar] [CrossRef]
- Baghbanzadeh, A.; Decuypere, E. Ascites syndrome in broilers: Physiological and nutritional perspectives. Avian Pathol. 2008, 37, 117–126. [Google Scholar] [CrossRef]
- Buys, N.; Buyse, J.; Hassanzadeh-Ladmakhi, M.; Decuypere, E. Intermittent lighting reduces the incidence of ascites in broilers: An interaction with protein content of feed on performance and the endocrine system. Poult. Sci. 1998, 77, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Ekanayake, S.; Silva, S.S.; Priyankarage, N.; Asekara, M.J.; Horadagoda, N.; Abeynayake, P.; Gunaratne, S.P. The effect of increased sodium in feed on pulmonary hypertension-induced ascites and right ventricular failure in broiler chickens. Br. Poult. Sci. 2004, 45, S29–S30. [Google Scholar] [CrossRef] [PubMed]
- Parr, N.; Wilkes, M.; Hawkes, L.A. Natural climbers: Insights from avian physiology at high altitude. High Alt. Med. Biol. 2019, 20, 427–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khajali, F. Managing broiler production challenges at high altitude. Vet. Med. Sci. 2022, 8, 1519–1527. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.P.; Anthony, N.B.; Orlowski, S.K.; Rhoads, D.D. SNP-based breeding for broiler resistance to ascites and evaluation of correlated production traits. Hereditas 2022, 159, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Y.; Chen, L.; Han, L.; Li, L.; He, H.; Li, Y.; Huang, N.; Ren, H.; Pei, F.; et al. The role of MIF, cyclinD1 and ERK in the development of pulmonary hypertension in broilers. Avian Pathol. 2017, 46, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Yang, F.; Zhuang, Y.; Xiao, Q.; Cao, H.; Zhang, C.; Wang, T.; Lin, H.; Guo, X.; Hu, G. Dysregulated expression of microRNAs and mRNAs in pulmonary artery remodeling in ascites syndrome in broiler chickens. Oncotarget 2017, 8, 1993–2007. [Google Scholar] [CrossRef]
- Cheng, S.; Liu, X.; Liu, P.; Li, G. Dysregulated Expression of mRNAs and SNPs in Pulmonary Artery Remodeling in Ascites Syndrome in Broilers. Poult. Sci. 2020, 100, 100877. [Google Scholar] [CrossRef]
- Ozkan, S.; Takma, C.; Yahav, S.; Sögüt, B.; Türkmut, L.; Erturun, H.; Cahaner, A. The effects of feed restriction and ambient temperature on growth and ascites mortality of broilers reared at high altitude. Poult. Sci. 2010, 89, 974–985. [Google Scholar] [CrossRef]
- Acar, N.; Sizemore, F.G.; Leach, G.R.; Wideman, R.F.; Barbato, G.F. Growth of broiler chickens in response to feed restriction regimens to reduce ascites. Poult. Sci. 1995, 74, 833–843. [Google Scholar] [CrossRef]
- Moghaddam, A.Z.; Hamzekolaei, M.H.; Khajali, F.; Hassanpour, H. Role of Selenium from Different Sources in Prevention of Pulmonary Arterial Hypertension Syndrome in Broiler Chickens. Biol. Trace Elem. Res. 2017, 180, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Sutendra, G.; Michelakis, E.D. The metabolic basis of pulmonary arterial hypertension. Cell Metab. 2014, 19, 558–573. [Google Scholar] [CrossRef] [PubMed]
- Gurtu, V.; Michelakis, E.D. Emerging therapies and future directions in pulmonary arterial hypertension. Can. J. Cardiol. 2015, 31, 489–501. [Google Scholar] [CrossRef]
- Olkowski, A.A.; Classen, H.L. Progressive bradycardia, a possible factor in the pathogenesis of ascites in fast growing broiler chickens raised at low altitude. Br. Poult. Sci. 1998, 39, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.H.; Wu, J.; Yang, S.J.; Cheng, D.C.; Guo, D.Z. Intravenous endothelin-1 triggers pulmonary hypertension syndrome (ascites) in broilers. Vet. Med.-Czech. 2008, 53, 381–391. [Google Scholar] [CrossRef]
- De Smit, L.; Tona, K.; Bruggeman, V.; Onagbesan, O.; Hassanzadeh, M.; Arckens, L.; Decuypere, E. Comparison of three lines of broilers differing in ascites susceptibility or growth rate. 2. Egg weight loss, gas pressures, embryonic heat production, and physiological hormone levels. Poult. Sci. 2005, 84, 1446–1452. [Google Scholar] [CrossRef]
- Yang, S.; Guo, D.; Yao, B. Histopathology of the lymphatic system in ascitic broilers. Vet. Med.-Czech. 2002, 47, 264–269. [Google Scholar] [CrossRef]
- Swire, P.W. Ascites in broilers. Vet. Rec. 1980, 107, 541. [Google Scholar] [PubMed]
- Julian, R.J. Rapid growth problems: Ascites and skeletal deformities in broilers. Poult. Sci. 1998, 77, 1773–1780. [Google Scholar] [CrossRef]
- Bahadoran, S.; Hassanpour, H.; Arab, S.; Abbasnia, S.; Kiani, A. Changes in the expression of cardiac genes responsive to thyroid hormones in the chickens with cold-induced pulmonary hypertension. Poult. Sci. 2021, 100, 101263. [Google Scholar] [CrossRef] [PubMed]
- Zaha, V.G.; Young, L.H. AMP-activated protein kinase regulation and biological actions in the heart. Circ. Res. 2012, 111, 800–814. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Carling, D. The AMP-activated protein kinase--fuel gauge of the mammalian cell? Eur. J. Biochem. 1997, 246, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.M.; Lai, K.W.; Chen, Y.X.; Huang, X.R.; Lan, H.Y. Expression of macrophage migration inhibitory factor in acute ischemic myocardial injury. J. Histochem. Cytochem. 2003, 51, 625–631. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, M.; Nishihira, J.; Shimpo, M.; Mizue, Y.; Ueno, S.; Mano, H.; Kobayashi, E.; Ikeda, U.; Shimada, K. Macrophage migration inhibitory factor as a redox-sensitive cytokine in cardiac myocytes. Cardiovasc. Res. 2001, 52, 438–445. [Google Scholar] [CrossRef]
- Miller, E.J.; Li, J.; Leng, L.; McDonald, C.; Atsumi, T.; Bucala, R.; Young, L.H. Macrophage migration inhibitory factor stimulates AMP-activated protein kinase in the ischaemic heart. Nature 2008, 451, 578–582. [Google Scholar] [CrossRef]
- Ma, H.; Wang, J.; Thomas, D.P.; Tong, C.; Leng, L.; Wang, W.; Merk, M.; Zierow, S.; Bernhagen, J.; Ren, J.; et al. Impaired macrophage migration inhibitory factor-AMP-activated protein kinase activation and ischemic recovery in the senescent heart. Circulation 2010, 122, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Marsin, A.S.; Bertrand, L.; Rider, M.H.; Deprez, J.; Beauloye, C.; Vincent, M.F.; Carling, D.; Hue, L. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr. Biol. 2000, 10, 1247–1255. [Google Scholar] [CrossRef]
- Benigni, F.; Atsumi, T.; Calandra, T.; Metz, C.; Echtenacher, B.; Peng, T.; Bucala, R. The proinflammatory mediator macrophage migration inhibitory factor induces glucose catabolism in muscle. J. Clin. Investig. 2000, 106, 1291–1300. [Google Scholar] [CrossRef]
- Kudo, N.; Barr, A.J.; Barr, R.L.; Desai, S.; Lopaschuk, G.D. High rates of fatty acid oxidation during reperfusion of ischemic hearts are associated with a decrease in malonyl-CoA levels due to an increase in 5’-AMP-activated protein kinase inhibition of acetyl-CoA carboxylase. J. Biol. Chem. 1995, 270, 17513–17520. [Google Scholar] [CrossRef]
- Dyck, J.R.; Kudo, N.; Barr, A.J.; Davies, S.P.; Hardie, D.G.; Lopaschuk, G.D. Phosphorylation control of cardiac acetyl-CoA carboxylase by cAMP-dependent protein kinase and 5’-AMP activated protein kinase. Eur. J. Biochem. 1999, 262, 184–190. [Google Scholar] [CrossRef]
- Stanley, W.C.; Lopaschuk, G.D.; Hall, J.L.; McCormack, J.G. Regulation of myocardial carbohydrate metabolism under normal and ischaemic conditions. Potential for pharmacological interventions. Cardiovasc. Res. 1997, 33, 243–257. [Google Scholar] [CrossRef]
- Eyben, F.; Parraga-Alava, J.; Tu, S.M. Testicular germ cell tumors type 2 have high RNA expression of LDHB, the gene for lactate dehydrogenase subunit B. Asian J. Androl. 2021, 23, 357–362. [Google Scholar] [CrossRef]
- Modak, J.; Deckwer, W.D.; Zeng, A.P. Metabolic control analysis of eucaryotic pyruvate dehydrogenase multienzyme complex. Biotechnol. Prog. 2002, 18, 1157–1169. [Google Scholar] [CrossRef]
- Harris, R.A.; Bowker-kinley, M.M.; Huang, B.L.; Wu, P.F. Regulation of the Activity of the Pyruvate Dehydrogenase Complex. Adv. Enzym. Regul. 2015, 42, 49–59. [Google Scholar] [CrossRef]
- Stacpoole, P.W. Therapeutic Targeting of the Pyruvate Dehydrogenase Complex/Pyruvate Dehydrogenase Kinase (PDC/PDK) Axis in Cancer. J. Natl. Cancer Inst. 2017, 109, djx071. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Jia, Q.; Chen, L.; Wang, W. Changes in the Expression of MIF and Other Key Enzymes of Energy Metabolism in the Myocardia of Broiler Chickens with Ascites Syndrome. Animals 2022, 12, 2488. https://doi.org/10.3390/ani12192488
Li L, Jia Q, Chen L, Wang W. Changes in the Expression of MIF and Other Key Enzymes of Energy Metabolism in the Myocardia of Broiler Chickens with Ascites Syndrome. Animals. 2022; 12(19):2488. https://doi.org/10.3390/ani12192488
Chicago/Turabian StyleLi, Lifang, Qiufeng Jia, Lingli Chen, and Wenkui Wang. 2022. "Changes in the Expression of MIF and Other Key Enzymes of Energy Metabolism in the Myocardia of Broiler Chickens with Ascites Syndrome" Animals 12, no. 19: 2488. https://doi.org/10.3390/ani12192488





