Salmonella Enteritidis Fatal Septicemia with Meningoencephalitis in a Tiger (Panthera tigris) Cub
Abstract
:Simple Summary
Abstract
1. Introduction
2. History and Case Presentation
2.1. Clinical Report
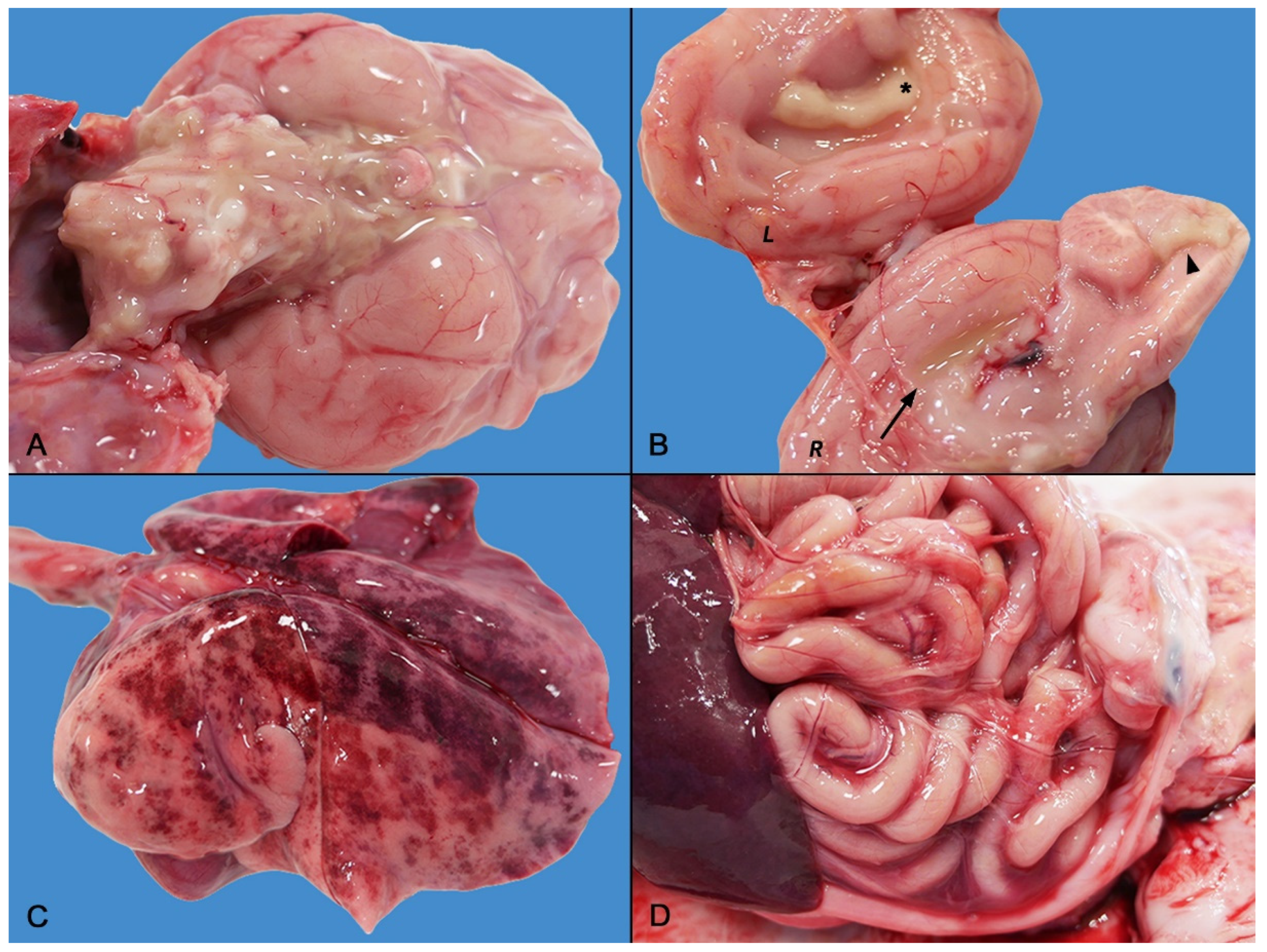
2.2. Gross Pathology
2.3. Histopathology
2.4. Microbiological Findings
3. Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grimont, P.A.D.; Weil, F. WHO Collaborating Centre for Reference and Research on Salmonella. Antigenic Formulae of the Salmonella Serovars, 9th ed.; Institute Pasteur: Paris, France, 2007; 167p. [Google Scholar]
- Issenhuth-Jeanjean, S.; Roggentin, P.; Mikoleit, M.; Guibourdenche, M.; de Pinna, E.; Nair, S.; Fields, P.I.; Weill, F.-X. Supplement 2008–2010 (no. 48) to the White–Kauffmann–Le Minor scheme. Res. Microbiol. 2014, 165, 526–530. [Google Scholar] [CrossRef]
- Uzal, F.A.; Plattner, B.L.; Hostetter, J.M. Alimentary System. In Jubb, Kennedy & Palmer’s Pathology of Domestic Animals; Maxie, M.G., Ed.; Elsevier: St. Louis, MO, USA, 2016; Volume 2, 259p. [Google Scholar]
- Gelberg, H.B. Alimentary System and the Peritoneum, Omentum, Mesentery, and Peritoneal Cavity. In Pathologic Basis of Veterinary Disease; Zachary, J.F., McGavin, M.D., Eds.; Mosby: St. Louis, MO, USA, 2011; pp. 322–404. [Google Scholar]
- Lewis, C.E.; Bemis, D.A.; Ramsay, E.C. Positive effects of diet change on shedding of Salmonella spp. in the feces of captive felids. J. Zoo Wildl. Med. 2002, 33, 83–84. [Google Scholar] [CrossRef]
- Venter, E.H.; van Vuuren, M.; Carstens, J.; van der Walt, M.L.; Nieuwoudt, B.; Steyn, H.; Kriek, N.P.J. A Molecular Epidemiologic Investigation of Salmonella from a Meat Source to the Feces of Captive Cheetah (Acinonyx Jubatus). J. Zoo Wildl. Med. 2003, 34, 76–81. [Google Scholar] [CrossRef]
- Rosario, I.; Calcines, M.I.; Rodríguez-Ponce, E.; Déniz, S.; Real, F.; Vega, S.; Marin, C.; Padilla, D.; Martín, J.L.; Acosta-Hernández, B. Salmonella enterica subsp. enterica serotypes isolated for the first time in feral cats: The impact on public health. Comp. Immunol. Microbiol. Infect. Dis. 2022, 84, 101792. [Google Scholar] [CrossRef]
- Wei, L.; Yang, C.; Shao, W.; Sun, T.; Wang, J.; Zhou, Z.; Chen, C.; Zhu, A.; Pan, Z. Prevalence and drug resistance of Salmonella in dogs and cats in Xuzhou, China. J. Veter. Res. 2020, 64, 263–268. [Google Scholar] [CrossRef]
- Srisanga, S.; Angkititrakul, S.; Sringam, P.; Le Ho, P.T.; Vo, A.T.T.; Chuanchuen, R. Phenotypic and genotypic antimicrobial resistance and virulence genes of Salmonella enterica isolated from pet dogs and cats. J. Veter. Sci. 2017, 18, 273–281. [Google Scholar] [CrossRef]
- Clyde, V.L.; Ramsay, E.C.; Bemis, D.A. Fecal Shedding of Salmonella in Exotic Felids. J. Zoo Wildl. Med. 1997, 28, 148–152. [Google Scholar]
- Iske, C.J.; Morris, C.L.; Kappen, K.L. Evaluation of raw pork as a commercially manufactured diet option for zoo-managed African wildcats (Felis silvestris lybica). Transl. Anim. Sci. 2017, 1, 397–405. [Google Scholar] [CrossRef]
- Stiver, S.L.; Frazier, K.S.; Mauel, M.J.; Styer, E.L. Septicemic Salmonellosis in Two Cats Fed a Raw-Meat Diet. J. Am. Anim. Hosp. Assoc. 2003, 39, 538–542. [Google Scholar] [CrossRef]
- Silva, C.; Calva, E.; Maloy, S. One Health and Food-Borne Disease: Salmonella Transmission between Humans, Animals, and Plants. Microbiol. Spectr. 2014, 2, 137–148. [Google Scholar] [CrossRef]
- Smith, A.M.; Ismail, H.; Henton, M.M.; Keddy, K.H. Similarities between Salmonella Enteritidis isolated from humans and captive wild animals in South Africa. J. Infect. Dev. Ctries. 2014, 8, 1615–1619. [Google Scholar] [CrossRef] [Green Version]
- Proverbio, D.; Perego, R.; Baggiani, L.; Ravasio, G.; Giambellini, D.; Spada, E. Hematological and Biochemical Reference Values in Healthy Captive Tigers (Panthera tigris). Animals 2021, 11, 3440. [Google Scholar] [CrossRef]
- Womble, M.; Georoff, T.A.; Helmick, K.; Carpenter, N.A.; Joslin, J.; Tupa, L.; Tetzloff, J.; McAloose, D. Mortality review for the North American snow leopard (Panthera uncia) zoo population from January 1999 to December 2019. J. Zoo Wildl. Med. 2021, 52, 145–156. [Google Scholar] [CrossRef]
- Cigler, P.; Kvapil, P.; Kastelic, M.; Gombač, M.; Švara, T.; Vobr, J.; Račnik, J.; Bartova, E. Retrospective study of causes of animal mortality in Ljubljana Zoo 2005–2015. J. Zoo Wildl. Med. 2020, 51, 571–577. [Google Scholar] [CrossRef]
- Junginger, J.; Hansmann, F.; Herder, V.; Lehmbecker, A.; Peters, M.; Beyerbach, M.; Wohlsein, P.; Baumgärtner, W. Pathology in Captive Wild Felids at German Zoological Gardens. PLoS ONE 2015, 10, e0130573. [Google Scholar] [CrossRef]
- Hussein, I.T.M.; Field, H.J. Development of a quantitative real-time TaqMan PCR assay for testing the susceptibility of feline herpesvirus-1 to antiviral compounds. J. Virol. Methods 2008, 152, 85–90. [Google Scholar] [CrossRef]
- Elia, G.; Decaro, N.; Martella, V.; Cirone, F.; Lucente, M.S.; Lorusso, E.; Di Trani, L.; Buonavoglia, C. Detection of canine distemper virus in dogs by real-time RT-PCR. J. Virol. Methods 2006, 136, 171–176. [Google Scholar] [CrossRef]
- Magnino, S.; Vigo, P.G.; Fabbi, M.; Colombo, M.; Colombo, N.; Genchi, C. Diagnostica Della Neosporosi Bovina Nel Nord Italia. Large Anim. Rev. 2000, 3, 25. [Google Scholar]
- Hofmann-Lehmann, R.; Huder, J.B.; Gruber, S.; Boretti, F.; Sigrist, B.; Lutz, H. Feline leukaemia provirus load during the course of experimental infection and in naturally infected cats. J. Gen. Virol. 2001, 82, 1589–1596. [Google Scholar] [CrossRef]
- Meli, M.L.; Berger, A.; Willi, B.; Spiri, A.M.; Riond, B.; Hofmann-Lehmann, R. Molecular detection of feline calicivirus in clinical samples: A study comparing its detection by RT-qPCR directly from swabs and after virus isolation. J. Virol. Methods 2018, 251, 54–60. [Google Scholar] [CrossRef]
- Decaro, N.; Elia, G.; Martella, V.; Desario, C.; Campolo, M.; Di Trani, L.; Tarsitano, E.; Tempesta, M.; Buonavoglia, C. A real-time PCR assay for rapid detection and quantitation of canine parvovirus type 2 in the feces of dogs. Veter. Microbiol. 2005, 105, 19–28. [Google Scholar] [CrossRef]
- De Laforcade, A. Diseases Associated with Thrombosis. Top. Companion Anim. Med. 2012, 27, 59–64. [Google Scholar] [CrossRef]
- Bauler, T.J.; Starr, T.; Nagy, T.A.; Sridhar, S.; Scott, D.; Winkler, C.W.; Steele-Mortimer, O.; Detweiler, C.S.; Peterson, K.E. Salmonella Meningitis Associated with Monocyte Infiltration in Mice. Am. J. Pathol. 2016, 187, 187–199. [Google Scholar] [CrossRef]
- Collins, F.M. Salmonellosis in orally infected specific pathogen-free C57B1 mice. Infect Immun. 1972, 5, 191–198. [Google Scholar] [CrossRef]
- Harrell, J.E.; Hahn, M.M.; D, S.J.; Vasicek, E.M.; Sandala, J.L.; Gunn, J.S.; McLachlan, J.B.; Edward Swords, W.; Subashchandrabose, S.; White, A.; et al. Salmonella Biofilm Formation, Chronic Infection, and Immunity Within the Intestine and Hepatobiliary Tract. Front. Cell. Infect. Microbiol 2021, 10, 624622. [Google Scholar] [CrossRef]
- Bernal-Bayard, J.; Ramos-Morales, F. Molecular Mechanisms Used by Salmonella to Evade the Immune System. Curr. Issues Mol. Biol. 2018, 25, 133–168. [Google Scholar] [CrossRef]
- Saunders, S.P.; Harris, T.; Traylor-Holzer, K.; Beck, K.G. Factors influencing breeding success, ovarian cyclicity, and cub survival in zoo-managed tigers (Panthera tigris). Anim. Reprod. Sci. 2014, 144, 38–47. [Google Scholar] [CrossRef]
- Hampson, M.C.; Schwitzer, C. Effects of Hand-Rearing on Reproductive Success in Captive Large Cats Panthera tigris altaica, Uncia uncia, Acinonyx jubatus and Neofelis nebulosa. PLoS ONE 2016, 11, e0155992. [Google Scholar] [CrossRef]
- Carvallo, F.R.; DebRoy, C.; Baeza, E.; Hinckley, L.; Gilbert, K.; Choi, S.J.; Risatti, G.; Smyth, J.A. Necrotizing Pneumonia and Pleuritis Associated with Extraintestinal Pathogenic Escherichia Coli in a Tiger (Panthera tigris) Cub. J. Veter. Diagn. Investig. 2010, 22, 136–140. [Google Scholar] [CrossRef]
- Duarte, M.D.; Barros, S.C.; Henriques, M.; Fernandes, T.L.; Bernardino, R.; Monteiro, M.; Fevereiro, M. Fatal Infection with Feline Panleukopenia Virus in Two Captive Wild Carnivores (Panthera tigris and Panthera leo). J. Zoo Wildl. Med. 2009, 40, 354–359. [Google Scholar] [CrossRef]
- Steinel, A.; Parrish, C.R.; Bloom, M.E.; Truyen, U. Parvovirus Infections in Wild Carnivores. J. Wildl. Dis. 2001, 37, 594–607. [Google Scholar] [CrossRef] [PubMed]
- Oladapo, O.O.; Kwaga, J.K.P.; Asabe, D.A.; Junaid, K. Prevalence of Salmonella Spp. in Captive Wildlife at the National Zoological Garden Jos, Nigeria. Curr. Res. Microbiol. Biotechnol. 2013, 1, 285–288. [Google Scholar]
- Davies, R.H.; Lawes, J.R.; Wales, A.D. Raw diets for dogs and cats: A review, with particular reference to microbiological hazards. J. Small Anim. Pract. 2019, 60, 329–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fauth, E.; Freeman, L.M.; Cornjeo, L.; Markovich, J.E.; Janecko, N.; Weese, J.S. Salmonella bacteriuria in a cat fed a Salmonella-contaminated diet. J. Am. Veter. Med. Assoc. 2015, 247, 525–530. [Google Scholar] [CrossRef]
- Andruzzi, M.N.; Krath, M.L.; Lawhon, S.D.; Boudreau, B. Salmonella enterica subspecies houtenae as an opportunistic pathogen in a case of meningoencephalomyelitis and bacteriuria in a dog. BMC Veter. Res. 2020, 16, 437. [Google Scholar] [CrossRef]
- Hoelzer, K.; Switt, A.I.M.; Wiedmann, M. Animal contact as a source of human non-typhoidal salmonellosis. Veter. Res. 2011, 42, 34. [Google Scholar] [CrossRef]
- Dróżdż, M.; Małaszczuk, M.; Paluch, E.; Pawlak, A. Zoonotic potential and prevalence of Salmonella serovars isolated from pets. Infect. Ecol. Epidemiol. 2021, 11, 1975530. [Google Scholar] [CrossRef]
- Mustafa, G.R.; Zhao, K.; He, X.; Chen, S.; Liu, S.; Mustafa, A.; He, L.; Yang, Y.; Yu, X.; Penttinen, P.; et al. Heavy Metal Resistance in Salmonella Typhimurium and Its Association with Disinfectant and Antibiotic Resistance. Front. Microbiol. 2021, 12, 702725. [Google Scholar] [CrossRef]
- Hosey, G.; Melfi, V. Human-Animal Bonds Between Zoo Professionals and the Animals in Their Care. Zoo Biol. 2012, 31, 13–26. [Google Scholar] [CrossRef]
- Rahman, M.M.; Alam Tumpa, M.A.; Zehravi, M.; Sarker, T.; Yamin; Islam, R.; Rashid, H.O.; Ahmed, M.; Ramproshad, S.; Mondal, B.; et al. An Overview of Antimicrobial Stewardship Optimization: The Use of Antibiotics in Humans and Animals to Prevent Resistance. Antibiotics 2022, 11, 667. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzotta, E.; Foiani, G.; De Benedictis, G.M.; Fiore, E.; Natale, A.; Spagnolo, E.; Vascellari, M.; Cento, G.; Corrò, M. Salmonella Enteritidis Fatal Septicemia with Meningoencephalitis in a Tiger (Panthera tigris) Cub. Animals 2022, 12, 2490. https://doi.org/10.3390/ani12192490
Mazzotta E, Foiani G, De Benedictis GM, Fiore E, Natale A, Spagnolo E, Vascellari M, Cento G, Corrò M. Salmonella Enteritidis Fatal Septicemia with Meningoencephalitis in a Tiger (Panthera tigris) Cub. Animals. 2022; 12(19):2490. https://doi.org/10.3390/ani12192490
Chicago/Turabian StyleMazzotta, Elisa, Greta Foiani, Giulia Maria De Benedictis, Enrico Fiore, Alda Natale, Elena Spagnolo, Marta Vascellari, Giulia Cento, and Michela Corrò. 2022. "Salmonella Enteritidis Fatal Septicemia with Meningoencephalitis in a Tiger (Panthera tigris) Cub" Animals 12, no. 19: 2490. https://doi.org/10.3390/ani12192490
APA StyleMazzotta, E., Foiani, G., De Benedictis, G. M., Fiore, E., Natale, A., Spagnolo, E., Vascellari, M., Cento, G., & Corrò, M. (2022). Salmonella Enteritidis Fatal Septicemia with Meningoencephalitis in a Tiger (Panthera tigris) Cub. Animals, 12(19), 2490. https://doi.org/10.3390/ani12192490








