Impact of Dietary Supplementation with Sodium Butyrate Protected by Medium-Chain Fatty Acid Salts on Gut Health of Broiler Chickens
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
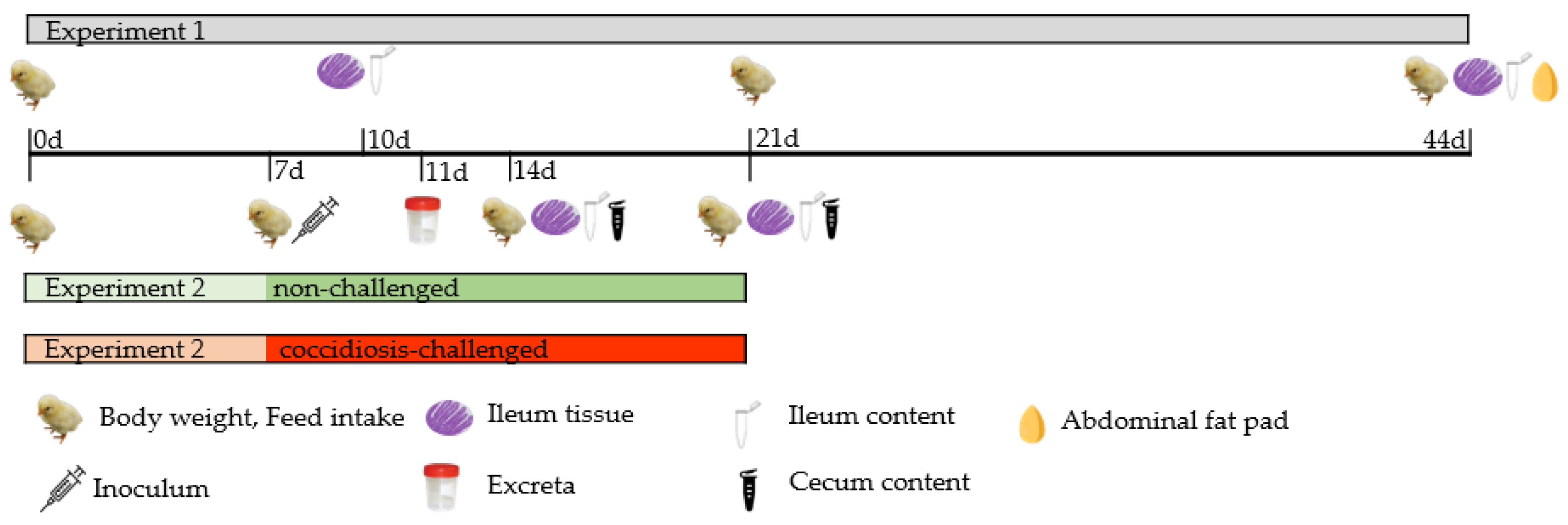
2.1. Animals, Housing, and Treatments
2.2. Diets
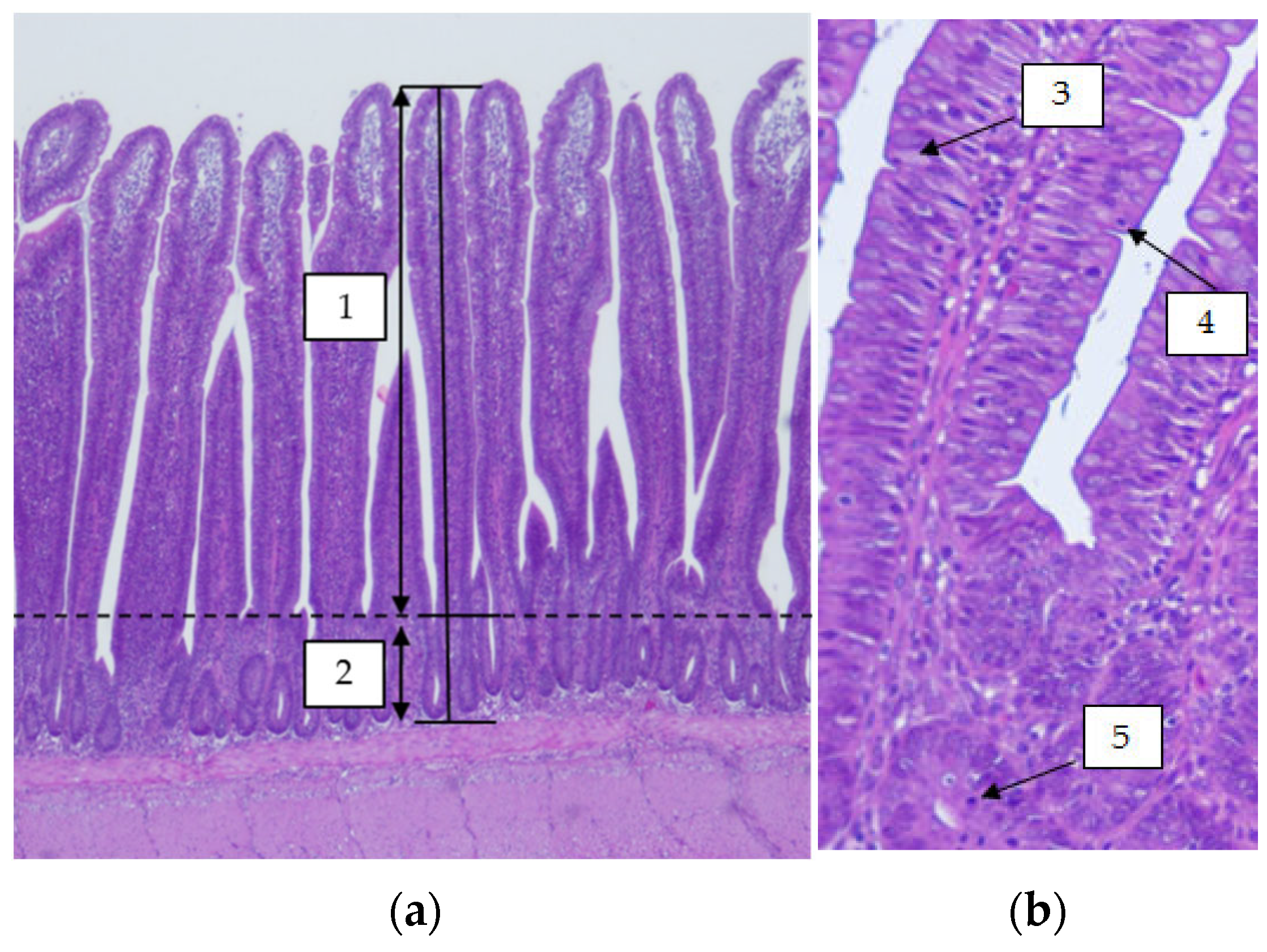
2.3. Controls, Sampling and Analytical Determinations
[N]d)}] × 100
2.4. Statistical Analysis
3. Results
3.1. Experiment 1
3.1.1. Experimental Diet Characterization
3.1.2. Performance Parameters
3.1.3. Ileal Histomorphometry
3.1.4. Microbiological Analysis
3.1.5. Abdominal Fat Pad Fatty Acid Composition
3.2. Experiment 2
3.2.1. Experimental Diet Characterization
3.2.2. Performance Parameters
3.2.3. Ileal Histomorphometry
3.2.4. Microbiological Analysis
3.2.5. Digestibility Balance
4. Discussion
4.1. Experimental Diet Characterization
4.2. Performance Parameters
4.3. Ileal Histomorphometry
4.4. Microbiological Analysis
4.5. Abdominal Fat Pad Fatty Acid Composition
4.6. Digestibility Balance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Parliament and European Council. Regulation (EC) No. 2019/6 of the European Parliament and of the Council of 11 December 2018 on Veterinary Medicinal Products and Repealing Directive 2001/83/EC Off. J. Eur. Union L:4–43; European Parliament and European Council: Brussels, Belgium, 2018. [Google Scholar]
- Oviedo-Rondón, E.O. Holistic view of intestinal health in poultry. Anim. Feed Sci. Technol. 2019, 250, 1–8. [Google Scholar] [CrossRef]
- Amer, S.A.; A.-Nasser, A.; Al-Khalaifah, H.S.; AlSadek, D.M.M.; Abdel fattah, D.M.; Roushdy, E.M.; Sherief, W.R.I.A.; Farag, M.F.M.; Altohamy, D.E.; Abdel-Wareth, A.A.A.; et al. Effect of dietary medium-chain -monoglycerides on the growth performance, intestinal histomorphology, amino acid digestibility, and broiler chickens’ blood biochemical parameters. Animals 2021, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.; Naeem, T.; Zulfiqar, A. Importance and promotion of gut health in broilers through dietary interventions. Appro. Poult. Dairy Vet. Sci. 2018, 3, 1–5. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Lee, K.Y.; Mohammadigheisar, M.; Kim, I.H. Evaluation of the blend of organic acids and medium-chain fatty acids in matrix coating as antibiotic growth promoter alternative on growth performance, nutrient digestibility, blood profiles, excreta microflora, and carcass quality in broilers. Poult. Sci. 2018, 97, 1–8. [Google Scholar] [CrossRef]
- Van Immerseel, F.; Fievez, V.; De Buck, J.; Pasmans, F.; Martel, A.; Haesebrouck, F.; Ducatelle, R. Microencapsulated short-chain fatty acids in feed modify colonization and invasion early after infection with Salmonella Enteritidis in young chickens. Poult. Sci. 2014, 83, 69–74. [Google Scholar] [CrossRef]
- Baltić, B.; Ćirić, J.; Šefer, D.; Radovanović, A.; Đorđević, J.; Glišić, M.; Bošković, M.; Baltić, M.Ž.; Đorđević, V.; Marković, R. Effect of dietary supplementation with medium chain fatty acids on growth performance, intestinal histomorphology, lipid profile and intestinal microflora of broiler chickens. South Afr. J. Anim. Sci. 2018, 48, 884–896. [Google Scholar] [CrossRef]
- Bortoluzzi, C.; Pedroso, A.A.; Mallo, J.J.; Puyalto, M.; Kim, W.K.; Applegate, T.J. Sodium butyrate improved performance while modulating the cecal microbiota and regulating the expression of intestinal immune-related genes of broiler chickens. Poult. Sci. 2017, 96, 3981–3993. [Google Scholar] [CrossRef]
- Leeson, S.; Namkung, H.; Antongiovanni, M.; Lee, E.H. Effect of butyric acid on the performance and carcass yield of broiler chickens. Poult. Sci. 2005, 84, 1418–1422. [Google Scholar] [CrossRef]
- Thompson, J.L.; Hinton, M. Antibacterial activity of formic and propionic acids in the diet of hens on salmonellas in the crop. Br. Poult. Sci. 1997, 38, 59–65. [Google Scholar] [CrossRef]
- Kaczmarek, S.A.; Barri, A.; Hejdysz, M.; Rutkowski, A. Effect of different doses of coated butyric acid on growth performance and energy utilization in broilers. Poult. Sci. 2016, 95, 851–859. [Google Scholar] [CrossRef]
- Smith, D.J.; Barri, A.; Herges, G.; Hahn, J.; Yersin, A.G.; Jourdan, A. In vitro dissolution and in vivo absorption of calcium [1-14C] butyrate in free or protected forms. J. Agric. Food Chem. 2012, 60, 3151–3157. [Google Scholar] [CrossRef]
- Boas, A.D.C.V.; Budiño, F.E.L.; Neto, M.A.T.; Scmidt, A.; Dadalt, J.C.; Monferdini, R.P.; Sitanaka, N.Y.; Moraes, J.E.; Pizzolante, C.C. Organic acid in diets of weaned piglets: Performance, digestibility and economical viability. Arg. Bras. Med. Vet. Zootec. 2016, 68, 1015–1022. [Google Scholar] [CrossRef]
- Yan, F.; Chen, J.; Kuttappan, V.; Vazquez Anon, M. Efficacy of protected benzoic acid in broilers subject to Eimeria challenge as affected by diet type. PSA Annual Meeting (abstract). Polutry Ind. 2018, 97, 180–181. [Google Scholar]
- Del Alamo, A.G.; De Los Mozos, J.; Van Dam, J.T.P.; De Ayala, P.P. The use of short and medium chain fatty acids as an alternative to antibiotic growth promoters in broilers infected with malabsorption syndrome. In Proceedings of the 16th European Symposium on Poultry Nutrition Strasbourg, Paris, France, 26–30 August 2007; pp. 317–320. [Google Scholar]
- Ali, A.M.; Seddiek, S.A.; Khater, H.F. Effect of butyrate, clopidol and their combination on the performance of broilers infected with Eimeria maxima. Br. Poult. Sci. 2014, 55, 474–482. [Google Scholar] [CrossRef]
- Cervantes, H.M.; McDonald, L.R.; Jenkins, M.C. Diseases of Poultry, 14th ed.; Swayne, D.E., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2020; pp. 1193–1217. [Google Scholar]
- European Parliament. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. L:276-33; European Parliament: Brussels, Belgium, 2010. [Google Scholar]
- Ferrini, G.; Baucells, M.D.; Esteve-García, E.; Barroeta, A.C. Dietary polyunsaturated fat reduces skin fat as well as abdominal fat in broiler chickens. Poult. Sci. 2008, 87, 528–535. [Google Scholar] [CrossRef]
- Viñado, A.; Castillejos, L.; Rodriguez-Sanchez, R.; Barroeta, A.C. Crude soybean lecithin as alternative energy source for broiler chicken diets. Poult. Sci. 2019, 98, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fundación Española para el Desarrollo de la Nutrición Animal. Necesidades Nutricionales para Avicultura: Pollos de Carne y Aves de Puesta; FEDNA: Madrid, Spain, 2008; ISBN 9788409065295. [Google Scholar]
- AOAC International. Official Methods of Analysis, 18th ed; AOAC: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Sukhija, P.S.; Palmquist, D.L. Rapid method for determination of total fatty acid content and composition of feedstuffs and feces. J. Agric. Food Chem. 1998, 36, 1202–1206. [Google Scholar] [CrossRef]
- Cortinas, L.; Villaverde, C.; Galobart, J.; Baucells, M.D.; Codony, R.; Barroeta, A.C. Fatty acid content in chicken thigh and breast as affected by dietary polyunsaturation level. Poult. Sci. 2004, 83, 1155–1164. [Google Scholar] [CrossRef]
- Nofrarías, M.; Manzanilla, E.G.; Pujols, J.; Gibert, X.; Majó, N.; Segalés, J.; Gasa, J. Effect of spray-dried porcine plasma and plant extracts on intestinal morphology and on leukocyte cell subsets of weaned pigs. J. Anim. Sci. 2006, 84, 2735–2742. [Google Scholar] [CrossRef]
- Carrapiso, A.I.; Timón, M.L.; Petrón, M.J.; Tejeda, J.F.; García, C. In situ transesterification of fatty acids from Iberian pig subcutaneous adipose tissue. Meat Sci. 2000, 56, 159–164. [Google Scholar] [CrossRef]
- Short, F.J.; Gorton, P.; Wiseman, J.; Boorman, K.N. Determination of titanium dioxide added as an inert marker in chicken digestibility studies. Anim. Feed Sci. Technol. 1996, 5, 215–221. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Li, J.; Chen, Y.; Yang, W.; Zhang, L. Effects of dietary coconut oil as a medium-chain fatty acid source on performance, carcass composition and serum lipids in male broilers. Asian Australas. J. Anim. Sci. 2015, 28, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Smulikowska, S.; Czerwiński, J.; Mieczkowska, A.; Jankowiak, J. The effect of fat-coated organic acid salt and a feed enzyme on growth performance, nutrient utilization, microflora activity, and morphology of the small intestine in broiler chickens. J. Anim. Feed Sci. 2009, 18, 478–489. [Google Scholar] [CrossRef]
- Pinchasov, Y.; Jensen, L. Effect of short-chain fatty acids on voluntary feed of broiler chicks. Poult. Sci. 1989, 68, 1612–1618. [Google Scholar] [CrossRef]
- Murakami, A.E.; Eyng, C.; Torrent, J. Effects of functional oils on coccidiosis and apparent metabolizable energy in broiler chickens. Asian Australas. J. Anim. Sci. 2014, 27, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Leung, H.; Yitbarek, A.; Snyder, R.; Patterson, R.; Barta, J.R.; Karrow, N.; Kiarie, E. Responses of broiler chickens to Eimeria challenge when fed a nucleotide-rich yeast extract. Poult. Sci. 2019, 98, 1622–1633. [Google Scholar] [CrossRef]
- Belote, B.L.; Soares, I.; Tujimoto-Silva, A.; Sanches, A.W.D.; Kraieski, A.L.; Santin, E. Applying I see inside histological methodology to evaluate gut health in broilers challenged with Eimeria. Vet. Parasitol. 2019, 276, 1000004. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Encinas, J.; Menoyo, D.; Blanch, M.; Pastor, J.J.; Rochell, S.J. Response of broiler chickens fed diets supplemented with a bioactive olive pomace extract from Olea europaea to an experimental coccidial vaccine challenge. Poult. Sci. 2020, 100, 575–584. [Google Scholar] [CrossRef]
- Song, B.; Li, H.; Wu, Y.; Zhen, W.; Wang, Z.; Xia, Z.; Guo, Y. Effect of microencapsulated sodium butyrate dietary supplementation on growth performance and intestinal barrier function of broiler chickens infected with necrotic enteritis. Anim. Feed Sci. Technol. 2017, 232, 6–15. [Google Scholar] [CrossRef]
- Liu, J.D.; Lumpkins, B.; Mathis, G.; Williams, S.M.; Fowler, J. Evaluation of encapsulated sodium butyrate with varying releasing times on growth performance and necrotic enteritis mitigation in broilers. Poult. Sci. 2019, 98, 3240–3245. [Google Scholar] [CrossRef]
- Liu, T.; Li, C.; Zhong, H.; Feng, F. Dietary medium-chain a-monoglycerides increase BW, feed intake, and carcass yield in broilers with muscle composition alteration. Poult. Sci. 2021, 100, 186–195. [Google Scholar] [CrossRef]
- Khatibjoo, A.; Mahmoodi, M.; Fattahnia, F.; Akbari-Gharaei, M.; Shokri, A.G.; Soltani, S. Effects of dietary short- and medium-chain fatty acids on performance, carcass traits, jejunum morphology, and serum parameters of broiler chickens. J. Appl. Anim. Res. 2018, 46, 492–498. [Google Scholar] [CrossRef]
- Czerwinski, J.; Hojberg, O.; Smulikowska, S.; Engberg, R.M.; Mieczkowska, A. Effects of sodium butyrate and salinomycin upon intestinal microbiota, mucosal morphology and performance of broiler chickens. Arch. Anim. Nutr. 2012, 66, 102–116. [Google Scholar] [CrossRef]
- Chamba, F.; Puyalto, M.; Ortiz, A.; Torrealba, H.; Mallo, J.J.; Riboty, R. Effect of partially protected sodium butyrate on performance, digestive organs, intestinal villi and E. coli development in broilers chickens. Int. J. Poult. Sci. 2014, 13, 390–396. [Google Scholar] [CrossRef]
- Wu, W.; Xiao, Z.; An, W.; Dong, Y.; Zhang, B. Dietary sodium butyrate improves intestinal development and function by modulating the microbial community in broilers. PLoS ONE 2018, 13, e0197762. [Google Scholar] [CrossRef]
- Teshfam, M.; Rahbari, S. Alteration in small intestinal structure induced by experimental subclinical coccidiosis in chicken. J. Appl. Anim. Res. 2003, 24, 33–39. [Google Scholar] [CrossRef]
- Choct, M. Managing gut health through nutrition. Br. Poult. Sci. 2009, 50, 9–15. [Google Scholar] [CrossRef]
- Barcelo, A.; Claustre, J.; Moro, F.; Chayvialle, J.; Cuber, J.; Plaisancie, P. Mucin secretion is modulated by luminal factors in the isolated vascularly perfused rat colon. Gut 2000, 46, 218–224. [Google Scholar] [CrossRef]
- Sikandar, A.; Zaneb, H.; Younus, M.; Masood, S.; Aslam, A.; Khattak, F.; Ashraf, S.; Yousaf, M.S.; Rehman, H. Effect of sodium butyrate on performance, immune status, microarchitercture of small intestinal mucosa and lymphoid organs in broiler chickens. Asian Australas. J. Anim. Sci. 2017, 30, 690–699. [Google Scholar] [CrossRef]
- Ferrara, F.; Tedin, L.; Pieper, R.; Meyer, W.; Zentek, J. Influence of medium-chain fatty acids and short-chain organic acids on jejunal morphology and intra-epithelial immune cells in weaned piglets. J. Anim. Physiol. Anim. Nutr. 2016, 101, 531–540. [Google Scholar] [CrossRef]
- Decuypere, J.A.; Dierick, N.A. The Combined use of triacylglycerols containing medium-chain fatty acids and exogenous lipolytic enzymes as an alternative to in-feed antibiotics in piglets: Concept, possibilities and limitations. An overview. Nutr. Res. Rev. 2003, 16, 193–209. [Google Scholar] [CrossRef]
- Bessay, M.; Le Vern, Y.; Kerboeuf, D.; Yvoré, P.; Quéré, P. Changes in intestinal intra-epithelial and systemic T-cell subpopulations after an Eimeria infection in chickens: Comparative study between E acervulina and E tenella. Vet. Res. 1996, 27, 503–514. [Google Scholar] [PubMed]
- Hong, Y.H.; Lillehoj, H.S.; Lillehoj, E.P.; Lee, S.H. Changes in immune-related gene expression and intestinal lymphocyte subpopulations following Eimeria maxima infection of chickens. Vet. Immunol. Immunopathol. 2006, 114, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Onrust, L.; Ducatelle, R.; Van Driessche, K.; De Maesschalck, C.; Vermeulen, K.; Haesebrouck, F.; Eeckhaut, V.; Van Immerseel, F. Steering endogenous butyrate production in the intestinal tract of broilers as a tool to improve gut health. Front. Vet. Sci. 2015, 75, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Van der Wielen, P.W.; Biesterveld, S.; Noterman, S.; Hofstra, H.; Urlings, B.A.; Van Knapen, F. Role of volatile fatty acids in development of the cecal microflora in broiler chickens during growth. Appl Environ. Microbiol. 2000, 66, 2536–2540. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.M.; Soratto, T.A.T.; Cardinal, K.M.; Wagner, G.; Hauptli, L.; Lima, A.L.F.; Dahlke, F.; Neto, D.P.; Moraes, P.O.; Ribeiro, A.M.L. Modulation of the intestinal microbiota of broilers supplemented with monensin or functional oils in response to challenge by Eimeria spp. PLoS ONE 2020, 15, e0237118. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.B. Anticoccidial vaccines for broiler chickens: Pathways to success. Avian Pathol. 2002, 31, 317–353. [Google Scholar] [CrossRef]
- Kien, C.L.; Blauwiekel, R.; Bunn, J.Y.; Jetton, T.L.; Frankel, W.L.; Holst, J.J. Cecal infusion of butyrate increases intestinal cell proliferation in piglets. J. Nutr. 2007, 37, 916–922. [Google Scholar] [CrossRef]
- Timbermont, L.; Lanckriet, A.; Dewulf, J.; Nollet, N.; Schwarzer, K.; Haesebrouk, F.; Ducatelle, R.; Van Immerseel, F. Control of Clostridium perfringens-induced necrotic enteritis in broilers by target-released butyric acid, fatty acids and essential oils. Avian Pathol. 2010, 39, 117–121. [Google Scholar] [CrossRef]
- Namkung, H.; Yu, H.; Gong, J.; Leeson, S. Antimicrobial activity of butyrate toward Salmonella Typhimurium and Clostridium perfringens. Poult. Sci. 2011, 90, 2217–2222. [Google Scholar] [CrossRef]
- Zhou, Z.; Nie, K.; Huang, Q.; Li, K.; Sun, Y.; Zhou, R.; Wang, Z.; Hu, S. Changes of cecal microflora in chickens following Eimeria tenella challenge and regulating effect of coated sodium butyrate. Exp. Parasitol. 2017, 177, 73–81. [Google Scholar] [CrossRef]
- Skřivanová, E.; Marounek, M.; Dlouhá, G.; Kaňka, J. Susceptibility of Clostridium perfringens to C2-C18 fatty acids. Lett. Appl. Microbiol. 2005, 41, 77–81. [Google Scholar] [CrossRef]
- Panda, A.K.; Rao, S.V.R.; Raju, M.V.L.N.; Sunder, C.S. Effect of butyric acid on performance, gastrointestinal tract health and carcass characteristics in broiler chickens. Asian Australas. J. Anim. Sci. 2009, 22, 1026–1031. [Google Scholar] [CrossRef]
- Shokrollahi, B.; Yavari, Z.; Kordestani, A.H. Effects of dietary medium-chain fatty acids on performance, carcass characteristics, and some serum parameters of broiler chickens. Br. Poult. Sci. 2014, 55, 662–667. [Google Scholar] [CrossRef]
- Bach, A.C.; Ingenbleek, Y.; Frey, A. The usefulness of dietary medium-chain triglycerides in body weight control: Fact or fancy? J. Lipid Res. 1996, 37, 708–726. [Google Scholar] [CrossRef]
- Fetterer, R.H.; Miska, K.B.; Jenkins, M.C.; Wong, E.A. Expression of nutrient transporters in duodenum, jejunum, and ileum of Eimeria maxima-infected broiler chickens. Parasitol. Res. 2014, 113, 3891–3894. [Google Scholar] [CrossRef]
- Su, S.; Miska, K.B.; Fetterer, R.H.; Jenkins, M.C.; Wong, E.A. Expression of digestive enzymes and nutrient transporters in Eimeria-challenged broilers. Exp. Parasitol. 2015, 150, 13–21. [Google Scholar] [CrossRef]
- Adedokun, S.A.; Adeola, O. The response in jejunal and ileal nutrient and energy digestibility and the expression of markers of intestinal inflammation in broiler chickens to coccidial vaccine challenge and phytase supplementation. Can. J. Anim. Sci. 2017, 97, 258–267. [Google Scholar] [CrossRef]
- Gautier, A.E.; Latorre, J.D.; Matsler, P.L.; Rochell, S.J. Longitudinal characterization of coccidiosis control methods on live performance and nutrient utilization in broilers. Front. Vet. Sci. 2020, 6, 468. [Google Scholar] [CrossRef]
- Teng, P.Y.; Yadav, S.; Castro, F.L.S.; Tompkins, Y.H.; Fuller, A.L.; Kim, W.K. Graded Eimeria challenge on broiler gastrointestinal health parameters. Poult. Sci. 2020, 99, 4203–4216. [Google Scholar] [CrossRef]
- Liu, J.D.; Bayir, H.O.; Cosby, D.E.; Cox, N.A.; Williams, S.M.; Fowler, J. Evaluation of encapsulated sodium butyrate on growth performance, energy digestibility, gut development, and Salmonella colonization in broilers. Poult. Sci. 2017, 96, 3638–3644. [Google Scholar] [CrossRef] [PubMed]
- Dibner, J.J.; Buttin, P. Use of organic acid as a model to study the impact of gut microflora on nutrition and metabolism. J. Appl. Poult. Res. 2002, 11, 453–463. [Google Scholar] [CrossRef]
| Experiment 1 | Experiment 2 | ||
|---|---|---|---|
| Starter Diet (From 0 to 21 days) | Grower-Finisher Diet (From 22 to 44 days) | Starter Diet (From 0 to 21 days) | |
| Ingredients, % | |||
| Corn | 39.99 | 39.97 | 39.63 |
| Wheat | 24.46 | 30.76 | 24.46 |
| Soybean meal 47% | 28.97 | 22.90 | 28.97 |
| Soybean oil | 2.96 | 3.00 | 2.96 |
| Bicalcium phosphate | 1.14 | 0.69 | 1.14 |
| Calcium carbonate | 0.88 | 1.16 | 0.88 |
| Salt | 0.35 | 0.29 | 0.35 |
| Premix 1 | 0.44 | 0.44 | 0.30 |
| L-Lysine | 0.34 | 0.28 | 0.34 |
| DL-Methionine | 0.31 | 0.23 | 0.31 |
| L-Threonine | 0.15 | 0.10 | 0.15 |
| L-Valine | 0.03 | 0.20 | 0.03 |
| Sodium bicarbonate | 0.05 | 0.04 | 0.05 |
| Choline chloride | 0.06 | 0.07 | 0.06 |
| Titanium dioxide | - | - | 0.50 |
| Analyzed nutrient and energy content | |||
| Dry matter, % | 90.97 | 89.30 | 88.93 |
| Crude protein, % | 20.22 | 17.68 | 19.58 |
| Ether extract, % | 5.12 | 4.89 | 4.87 |
| Crude fiber, % | 1.83 | 2.66 | 2.72 |
| Ash, % | 5.82 | 4.58 | 6.33 |
| Gross energy, kcal/kg | 4072 | 4096 | 3978 |
| Starter Diet (From 0 to 21 days) | Grower-Finisher Diet (From 22 to 44 days) | |||||||
|---|---|---|---|---|---|---|---|---|
| Fatty Acids, % | CTR | 0.5DIC | 1DIC | 2DIC | CTR | 0.5DIC | 1DIC | 2DIC |
| Saturated fatty acids | 18.3 | 18.3 | 18.8 | 20.1 | 18.6 | 19.6 | 21.1 | 19.7 |
| C12:0 | - | 0.19 | 0.42 | 0.87 | - | 0.21 | 0.40 | 0.63 |
| C14:0 | 0.15 | 0.37 | 0.47 | 0.73 | - | 0.25 | 0.37 | 0.45 |
| C16:0 | 14.1 | 13.8 | 13.9 | 14.4 | 14.6 | 15.1 | 16.0 | 14.6 |
| C18:0 | 3.72 | 3.63 | 3.63 | 3.74 | 3.66 | 3.78 | 3.92 | 3.69 |
| Monounsaturated fatty acids | 25.3 | 25.3 | 25.0 | 25.9 | 25.3 | 25.9 | 26.2 | 25.0 |
| C18:1n9c | 23.7 | 23.8 | 23.3 | 23.9 | 24.0 | 24.8 | 24.9 | 23.7 |
| Polyunsaturated fatty acids | 56.4 | 56.3 | 56.2 | 54.0 | 56.1 | 54.5 | 52.7 | 55.3 |
| C18:2n6c | 51.1 | 51.2 | 50.9 | 49.0 | 51.2 | 50.0 | 48.1 | 50.5 |
| C18:3n3 | 5.30 | 5.27 | 5.26 | 4.96 | 4.89 | 4.75 | 4.60 | 4.81 |
| Minor fatty acids | 2.02 | 2.02 | 2.13 | 2.41 | 1.66 | 1.67 | 1.65 | 1.65 |
| UFA:SFA | 4.46 | 4.46 | 4.32 | 3.97 | 4.37 | 4.09 | 3.74 | 4.07 |
| Experimental Treatments 1 | Statistics | |||||
|---|---|---|---|---|---|---|
| CTR | 0.5DIC | 1DIC | 2DIC | SEM | p-Value | |
| From 0 to 21 d | ||||||
| BW at 0 d, g | 38.5 | 38.5 | 38.4 | 38.5 | 0.042 | 0.988 |
| BW at 21 d, g | 820 | 812 | 796 | 822 | 19.94 | 0.491 |
| ADFI, g/d per bird | 53.5 | 52.7 | 52.0 | 52.9 | 1.061 | 0.696 |
| ADG, g/d per bird | 37.0 | 36.6 | 35.9 | 36.9 | 0.910 | 0.547 |
| FCR, g/g | 1.45 | 1.44 | 1.44 | 1.43 | 0.022 | 0.960 |
| From 22 to 44 d | ||||||
| BW at 44 d, g | 2663 | 2601 | 2588 | 2588 | 74.82 | 0.730 |
| ADFI, g/d per bird | 142 | 139 | 142 | 140 | 4.228 | 0.895 |
| ADG, g/d per bird | 80.5 | 77.6 | 77.2 | 76.2 | 2.343 | 0.508 |
| FCR, g/g | 1.82 | 1.79 | 1.85 | 1.84 | 0.053 | 0.818 |
| From 0 to 44 d | ||||||
| ADFI, g/d per bird | 102 | 98.4 | 99.9 | 101 | 2.563 | 0.684 |
| ADG, g/d per bird | 59.7 | 58.4 | 58.0 | 57.9 | 1.703 | 0.730 |
| FCR, g/g | 1.70 | 1.69 | 1.72 | 1.71 | 0.042 | 0.913 |
| Experimental Treatments 1 | Statistics | ||||||
|---|---|---|---|---|---|---|---|
| Ileum | Age | CTR | 0.5DIC | 1DIC | 2DIC | SEM | p-Value |
| Villus | |||||||
| Height, µm | 10 d | 352 | 356 | 371 | 368 | 20.70 | 0.778 |
| 39 d | 627 | 629 | 601 | 765 | 91.79 | 0.182 | |
| Goblet cells/villus | 10 d | 56.6 ab | 60.9 a | 60.2 a | 49.8 b | 3.861 | 0.023 |
| 39 d | 120 | 138 | 126 | 138 | 9.087 | 0.238 | |
| IEL/villus | 10 d | 4.72 | 5.82 | 5.55 | 5.53 | 0.812 | 0.558 |
| 39 d | 22.1 xy | 23.7 xy | 19.3 y | 28.5 x | 3.200 | 0.085 | |
| Crypt | |||||||
| Depth, µm | 10 d | 160 | 164 | 167 | 158 | 11.41 | 0.854 |
| 39 d | 183 | 179 | 181 | 179 | 7.748 | 0.956 | |
| Mitosis/crypt | 10 d | 2.00 | 2.87 | 3.63 | 2.88 | 0.524 | 0.103 |
| 39 d | 1.08 | 1.73 | 1.25 | 1.99 | 0.523 | 0.303 | |
| Villus:crypt ratio | |||||||
| 10 d | 2.28 | 2.21 | 2.32 | 2.37 | 0.198 | 0.914 | |
| 39 d | 3.48 | 3.57 | 3.39 | 4.37 | 0.598 | 0.187 | |
| Experimental Treatments 1 | Statistics | ||||||
|---|---|---|---|---|---|---|---|
| Ileum, Logcfu/g | Age | CTR | 0.5DIC | 1DIC | 2DIC | SEM | p-Value |
| Total lactic acid bacteria | 10 d | 8.72 | 8.57 | 8.66 | 8.60 | 0.110 | 0.677 |
| 39 d | 8.95 | 8.96 | 8.99 | 8.90 | 0.041 | 0.077 | |
| Enterobacteriaceae | 10 d | 6.37 | 5.58 | 4.97 | 5.37 | 0.815 | 0.672 |
| 39 d | 3.68 | 3.57 | 3.82 | 4.30 | 0.849 | 0.798 | |
| Total lactic acid bacteria: Enterobacteriaceae ratio | 10 d | 1.50 | 1.67 | 1.85 | 1.65 | 0.208 | 0.691 |
| 39 d | 2.94 | 2.98 | 2.71 | 2.55 | 0.494 | 0.642 | |
| Total coliform bacteria | 10 d | 5.64 | 4.79 | 4.26 | 5.11 | 1.008 | 0.820 |
| 39 d | 3.53 | 2.91 | 3.67 | 3.81 | 0.747 | 0.686 | |
| Experimental Treatments 1 | Statistics | |||||
|---|---|---|---|---|---|---|
| Abdominal Fat Pat | CTR | 0.5DIC | 1DIC | 2DIC | SEM | p-Value |
| g | 48.6 | 38.9 | 40.4 | 42.0 | 3.567 | 0.220 |
| % 2 | 1.83 | 1.48 | 1.55 | 1.62 | 0.128 | 0.190 |
| Fatty acid profile, % | ||||||
| Saturated fatty acids | 29.4 | 29.3 | 29.5 | 29.1 | 0.655 | 0.958 |
| C12:0 | 0.01 D | 0.06 C | 0.10 B | 0.16 A | 0.005 | <0.001 |
| C14:0 | 0.52 C | 0.54 BC | 0.58 AB | 0.62 A | 0.020 | <0.001 |
| C16:0 | 23.2 | 22.8 | 23.1 | 22.7 | 0.510 | 0.746 |
| C18:0 | 5.36 | 5.59 | 5.40 | 5.35 | 0.179 | 0.646 |
| C20:0 | 0.08 | 0.07 | 0.07 | 0.07 | 0.010 | 0.971 |
| Monounsaturated fatty acids | 44.7 | 44.6 | 45.5 | 45.4 | 0.737 | 0.499 |
| C18:1n9c | 36.5 | 36.7 | 37.5 | 37.3 | 0.518 | 0.250 |
| Polyunsaturated fatty acids | 25.9 | 26.1 | 25.0 | 25.5 | 0.654 | 0.553 |
| C18:2n6c | 23.0 | 23.2 | 22.2 | 22.7 | 0.570 | 0.544 |
| C18:3n3 | 2.25 | 2.27 | 2.13 | 2.20 | 0.078 | 0.470 |
| Minor fatty acids | 9.18 | 8.81 | 8.92 | 8.98 | 0.324 | 0.738 |
| UFA:SFA | 2.40 | 2.51 | 2.37 | 2.38 | 0.068 | 0.240 |
| Starter Diet (From 0 to 21 days) | |||
|---|---|---|---|
| Fatty Acids, % | NC | CC | DC |
| Saturated fatty acids | 18.1 | 18.5 | 18.6 |
| C12:0 | - | - | 0.29 |
| C14:0 | - | - | 0.31 |
| C16:0 | 16.0 | 16.3 | 15.9 |
| C18:0 | 1.23 | 1.26 | 1.17 |
| Monounsaturated fatty acids | 29.5 | 29.8 | 29.5 |
| C18:1n9c | 27.7 | 28.0 | 27.7 |
| Polyunsaturated fatty acids | 52.4 | 51.7 | 52.0 |
| C18:2n6c | 48.4 | 47.9 | 48.0 |
| C18:3n3 | 3.97 | 3.85 | 3.95 |
| Minor fatty acids | 2.69 | 2.71 | 2.68 |
| UFA:SFA | 4.53 | 4.40 | 4.38 |
| Experimental Treatments 1 | Statistics | ||||
|---|---|---|---|---|---|
| NC | CC | DC | SEM | p-Value | |
| From 0 to 7 d | |||||
| BW at 0 d, g | 40.6 | 40.6 | 40.6 | 0.021 | 0.524 |
| BW at 7 d, g | 145 | 147 | 148 | 2.872 | 0.794 |
| ADFI, g/d per bird | 18.0 | 18.4 | 18.3 | 0.551 | 0.764 |
| ADG, g/d per bird | 15.0 | 15.2 | 15.3 | 0.409 | 0.787 |
| FCR, g/g | 1.20 | 1.21 | 1.21 | 0.030 | 0.959 |
| From 8 to 14 d | |||||
| BW at 14 d, g | 378 A | 351 B | 355 B | 5.600 | <0.001 |
| ADFI, g/d per bird | 46.3 a | 41.5 b | 41.9 b | 1.206 | 0.002 |
| ADG, g/d per bird | 33.3 A | 28.5 B | 29.4 B | 0.523 | <0.001 |
| FCR, g/g | 1.39 | 1.29 | 1.43 | 0.132 | 0.402 |
| From 15 to 21 d | |||||
| BW at 21 d, g | 687 | 700 | 720 | 12.19 | 0.124 |
| ADFI, g/d per bird | 67.6 B | 76.2 A | 80.5 A | 1.951 | <0.001 |
| ADG, g/d per bird | 44.3 B | 50.2 A | 52.0 A | 1.592 | <0.001 |
| FCR, g/g | 1.56 | 1.53 | 1.55 | 0.033 | 0.733 |
| From 0 to 21 d | |||||
| ADFI, g/d per bird | 43.8 b | 44.9 ab | 46.3 a | 0.637 | 0.017 |
| ADG, g/d per bird | 30.7 y | 31.2 xy | 32.3 x | 0.553 | 0.060 |
| FCR, g/g | 1.43 | 1.44 | 1.45 | 0.016 | 0.567 |
| Experimental Treatments 1 | Statistics | |||||
|---|---|---|---|---|---|---|
| Age | NC | CC | DC | SEM | p-Value | |
| Villus | ||||||
| Height, µm | 14 d | 508 | 506 | 508 | 19.53 | 0.994 |
| 21 d | 637 | 582 | 619 | 22.34 | 0.103 | |
| Goblet cells/villus | 14 d | 80.7 | 85.7 | 86.5 | 4.621 | 0.498 |
| 21 d | 85.6 a | 71.0 b | 86.1 a | 3.159 | 0.001 | |
| IEL/villus | 14 d | 17.7 | 16.6 | 19.0 | 1.476 | 0.359 |
| 21 d | 16.6 | 14.5 | 17.5 | 1.209 | 0.129 | |
| Crypt | ||||||
| Depth, µm | 14 d | 174 b | 182 ab | 196 a | 6.066 | 0.019 |
| 21 d | 148 | 159 | 164 | 5.598 | 0.114 | |
| Mitotic cells/crypt | 14 d | 1.35 | 1.37 | 1.36 | 0.193 | 0.995 |
| 21 d | 0.91 | 1.25 | 1.26 | 0.160 | 0.182 | |
| Villus:crypt ratio | ||||||
| 14 d | 2.98 x | 2.87 xy | 2.63 y | 0.118 | 0.075 | |
| 21 d | 4.49 a | 3.79 b | 3.91 ab | 0.233 | 0.048 | |
| Experimental Treatments 1 | Statistics | |||||
|---|---|---|---|---|---|---|
| Logcfu/g | Age | NC | CC | DC | SEM | p-Value |
| Ileum | ||||||
| Total lactic acid bacteria | 14 d | 6.91 b | 7.19 a | 7.18 ab | 0.100 | 0.021 |
| 21 d | 7.47 b | 8.32 ab | 8.43 a | 0.237 | 0.024 | |
| Enterobacteriaceae | 14 d | 7.37 b | 7.38 ab | 7.60 a | 0.217 | 0.042 |
| 21 d | 5.86 a | 4.80 b | 5.17 ab | 0.320 | 0.048 | |
| Total lactic acid bacteria:Enterobacteriaceae ratio | 14 d | 0.95 | 0.98 | 0.95 | 0.045 | 0.119 |
| 21 d | 1.32 b | 1.80 a | 1.66 ab | 0.102 | 0.003 | |
| Escherichica coli | 14 d | 5.05 | 5.73 | 5.90 | 0.315 | 0.116 |
| 21 d | 5.80 a | 4.68 b | 4.86 ab | 0.326 | 0.042 | |
| Cecum | ||||||
| Clostridium perfringens | 14 d | 2.91 | 3.06 | 4.20 | 0.681 | 0.178 |
| 21 d | 2.73 | 2.74 | 1.85 | 0.685 | 0.620 | |
| Experimental Treatments 1 | Statistics | ||||
|---|---|---|---|---|---|
| NC | CC | DC | SEM | p-Value | |
| AME, kcal/kg | 3138 | 3082 | 3180 | 39.78 | 0.361 |
| Digestibility, % | |||||
| Dry matter | 90.65 | 90.35 | 90.42 | 0.384 | 0. 835 |
| Organic matter | 70.09 | 68.89 | 70.26 | 0.981 | 0.375 |
| Total FA | 73.97 | 72.54 | 75.74 | 2.305 | 0.517 |
| SFA | 60.87 | 58.14 | 62.73 | 3.523 | 0.531 |
| MUFA | 72.34 | 70.91 | 74.36 | 2.707 | 0.557 |
| PUFA | 77.74 | 76.64 | 79.46 | 1.958 | 0.494 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadurní, M.; Barroeta, A.C.; Sala, R.; Sol, C.; Puyalto, M.; Castillejos, L. Impact of Dietary Supplementation with Sodium Butyrate Protected by Medium-Chain Fatty Acid Salts on Gut Health of Broiler Chickens. Animals 2022, 12, 2496. https://doi.org/10.3390/ani12192496
Sadurní M, Barroeta AC, Sala R, Sol C, Puyalto M, Castillejos L. Impact of Dietary Supplementation with Sodium Butyrate Protected by Medium-Chain Fatty Acid Salts on Gut Health of Broiler Chickens. Animals. 2022; 12(19):2496. https://doi.org/10.3390/ani12192496
Chicago/Turabian StyleSadurní, Meritxell, Ana Cristina Barroeta, Roser Sala, Cinta Sol, Mónica Puyalto, and Lorena Castillejos. 2022. "Impact of Dietary Supplementation with Sodium Butyrate Protected by Medium-Chain Fatty Acid Salts on Gut Health of Broiler Chickens" Animals 12, no. 19: 2496. https://doi.org/10.3390/ani12192496
APA StyleSadurní, M., Barroeta, A. C., Sala, R., Sol, C., Puyalto, M., & Castillejos, L. (2022). Impact of Dietary Supplementation with Sodium Butyrate Protected by Medium-Chain Fatty Acid Salts on Gut Health of Broiler Chickens. Animals, 12(19), 2496. https://doi.org/10.3390/ani12192496






