Effect of Sheep Grazing, Stocking Rates and Dolomitic Limestone Application on the Floristic Composition of a Permanent Dryland Pasture, in the Montado Agroforestry System of Southern Portugal
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Overview of the Montado Ecosystem
1.2. Effects and Relationship of Different Grazing Systems on Pasture Floristic Composition
2. Materials and Methods
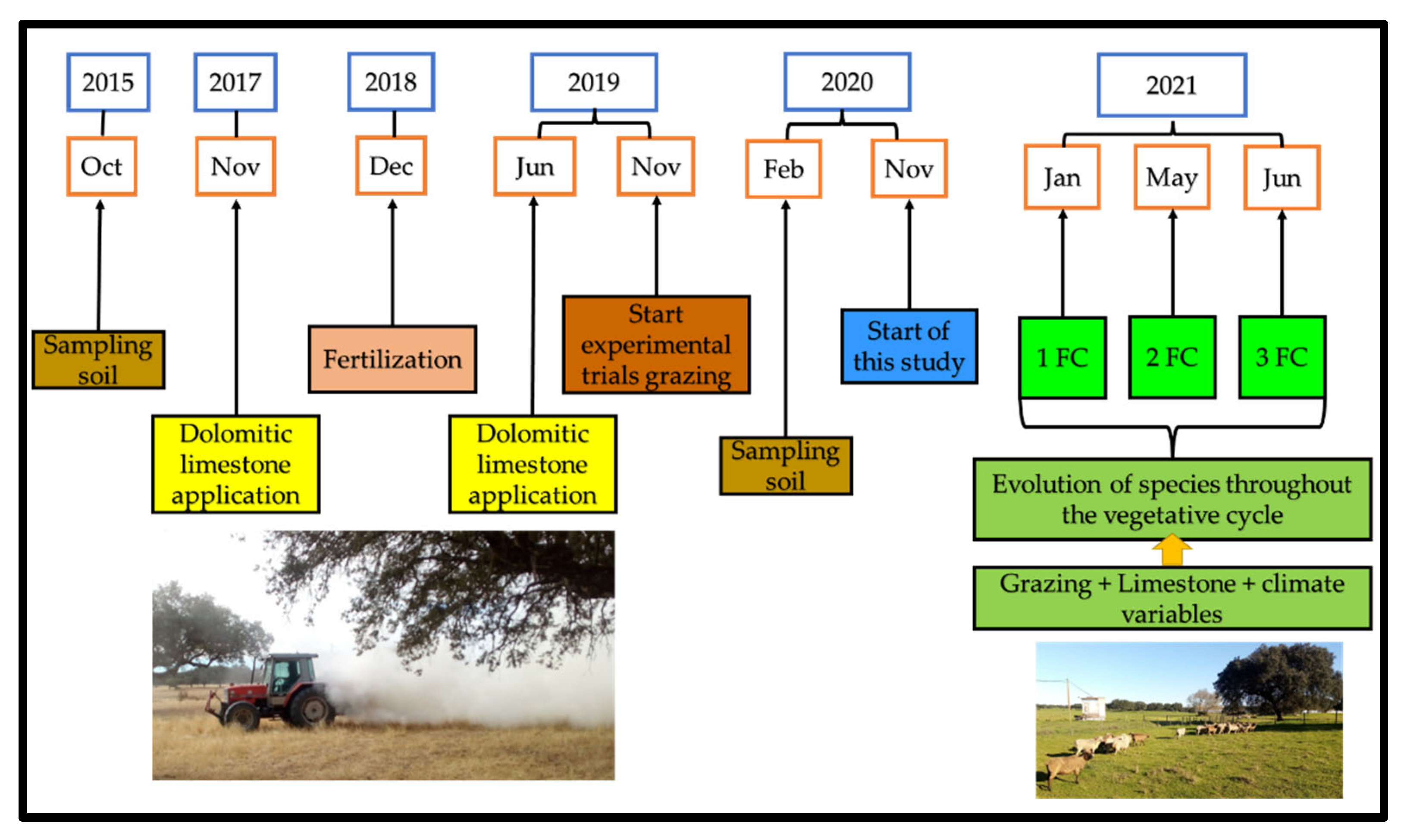
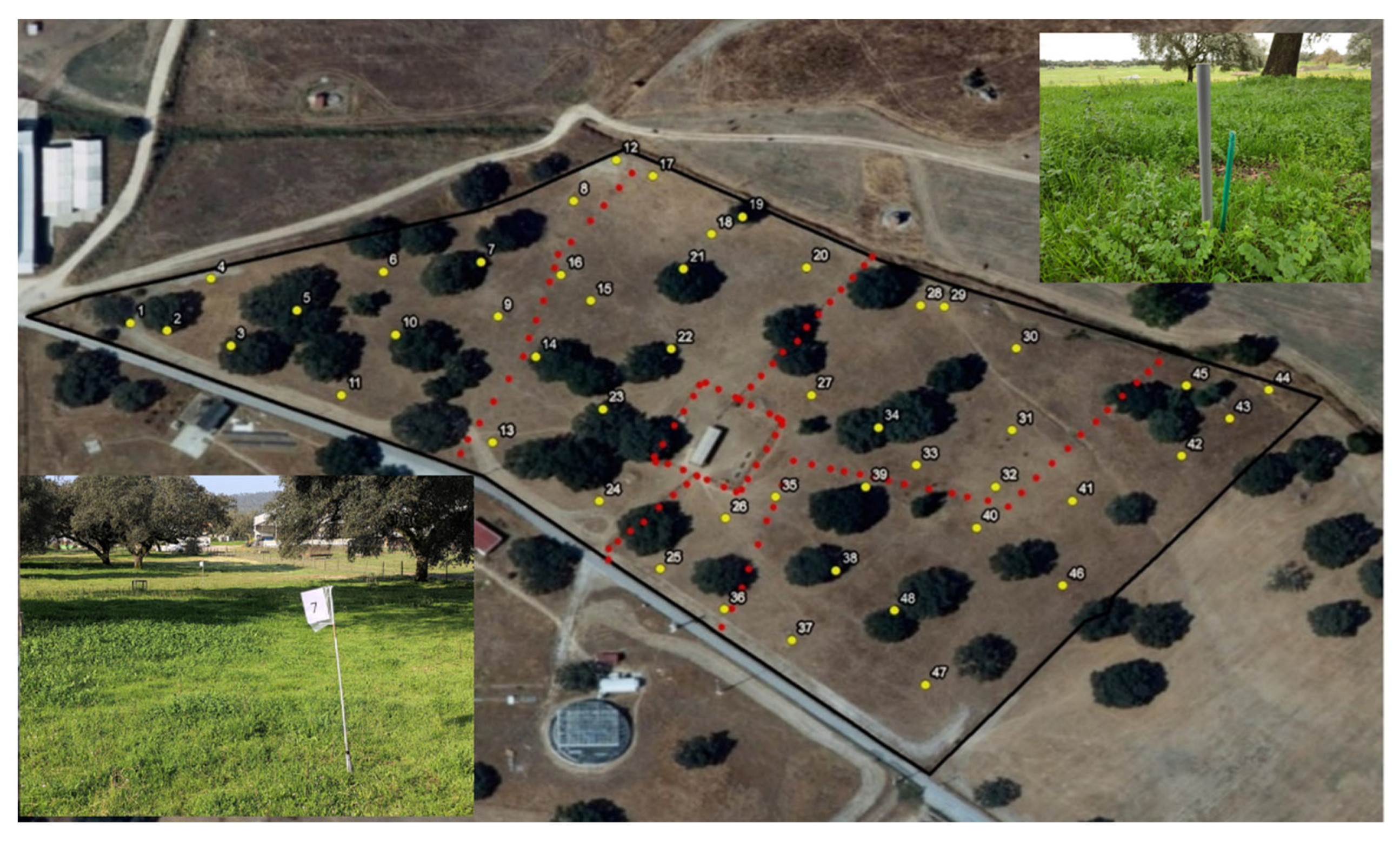
2.1. Study Area Framework
2.2. Study Design Description
2.3. Grazing Management
2.4. Characterization of the Floristic Composition
2.5. Statistics Analyses
3. Results
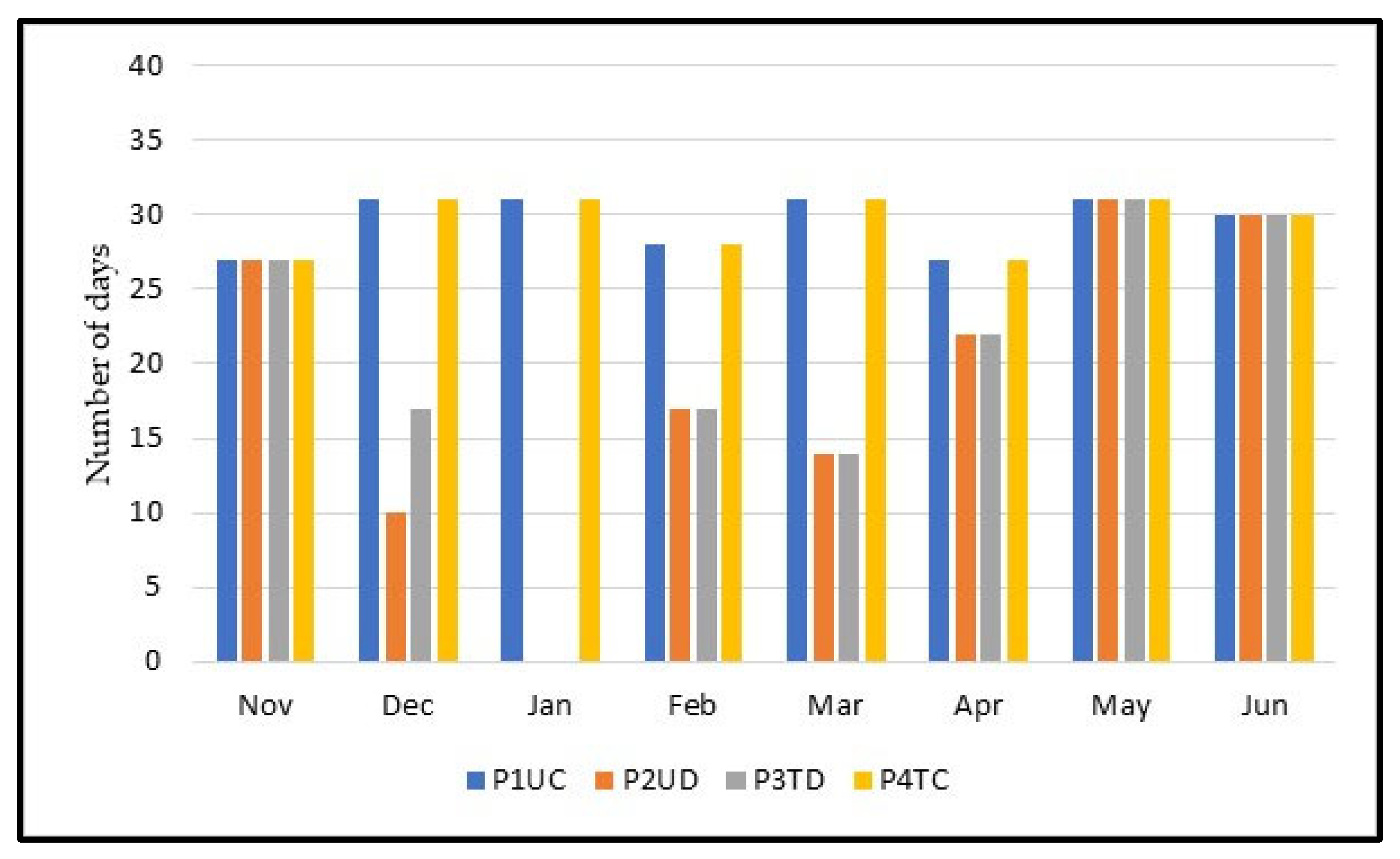
3.1. Meteorological Conditions
3.2. Grazing Days
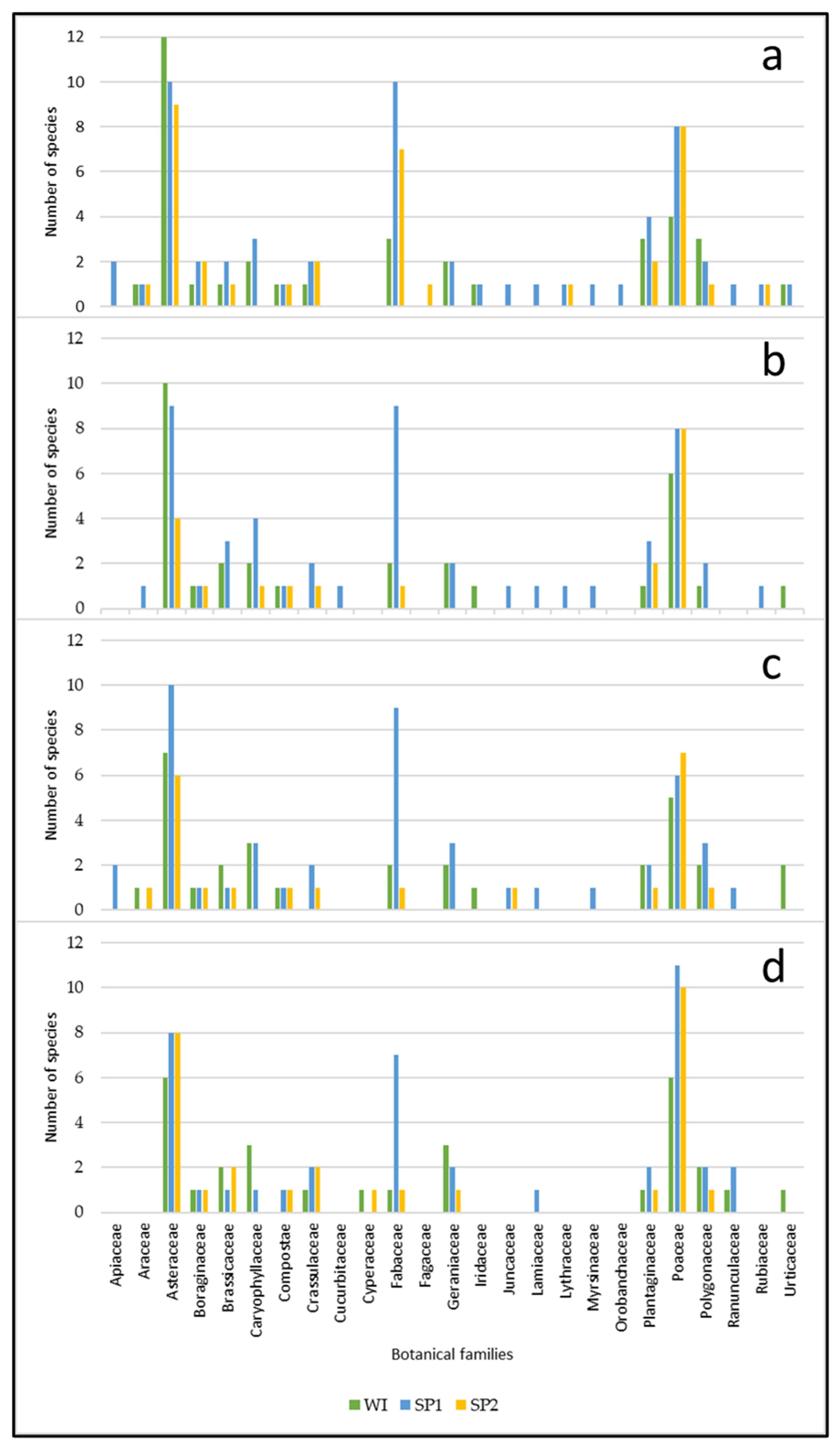
3.3. Characterization of the Floristic Composition
3.3.1. Descriptive Analysis
3.3.2. Seasonal Bioindicators
4. Discussion
4.1. Relationship between Climatic Variables, Pasture Development and Grazing Days
4.2. Evolution of the Floristic Composition: Field Observations
4.3. Evolution of the Floristic Composition: Field Observations vs. Indicator Species Analysis
4.4. Evolution of the Floristic Composition: Effects of Different Gazing Management
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Plant Taxa | Family | P1UC | P2UD | P3TD | P4TC |
|---|---|---|---|---|---|
| Agrostis castellana Boiss. & Reut. | Poaceae | N | L | L | L |
| Agrostis pourretii Willd. | Poaceae | L | L | L | L |
| Arum italicum subsp. italicum | Araceae | L | N | L | N |
| Bromus diandrus Roth | Poaceae | M | M | M | H |
| Calendula arvensis L. | Asteraceae | M | N | N | L |
| Carduus tenuiflorus Curtis | Compostae | L | L | L | N |
| Cerastium glomeratum Thuill. | Caryophyllaceae | N | N | L | L |
| Chamaemelum fuscatum (Brot.) Vasc. | Asteraceae | L | L | L | L |
| Chamaemelum mixtum L. | Asteraceae | L | L | N | N |
| Crassula tillaea Lest.-Garl. | Crassulaceae | N | N | N | L |
| Crepis vesicaria subsp. taraxacifolia (Thuill.) Thell. | Crassulaceae | L | N | N | N |
| Cynodon dactylon (L.) Pers. | Poaceae | L | L | L | L |
| Diplotaxis catholica (L.) DC. | Brassicaceae | M | H | H | H |
| Echium plantagineum L. | Boraginaceae | L | M | M | H |
| Erodium cicutarium subsp. bipinnatum (Cav.) Tourlet | Geraniaceae | H | M | H | H |
| Geranium molle L. | Geraniaceae | M | M | L | L |
| Geranium rotundifolium L. | Geraniaceae | N | N | N | L |
| Hypochaeris glabra L. | Asteraceae | L | L | L | L |
| Hypochaeris radicata L. | Asteraceae | L | L | N | N |
| Iris xiphium L. | Iridaceae | L | L | L | N |
| Leontodon taraxacoides (Vill.) Mérat | Asteraceae | L | M | L | M |
| Leontodon tuberosus L. | Asteraceae | N | L | L | N |
| Logfia gallica (L.) Coss. & Germ. | Asteraceae | N | L | N | N |
| Medicago polymorpha L. | Fabaceae | L | N | N | N |
| Ornithopus compressus L. | Fabaceae | L | L | L | L |
| Plantago coronopus L. | Plantaginaceae | 1 | N | N | N |
| Plantago lagopus L. | Plantaginaceae | N | N | L | N |
| Plantago lanceolata L. | Plantaginaceae | L | M | L | L |
| Plantago sp. | Plantaginaceae | L | N | N | N |
| Poa annua L. | Poaceae | N | L | N | N |
| Poa bulbosa L. | Poaceae | N | N | N | L |
| Pulicaria odora (L.) Rchb. | Asteraceae | L | L | L | N |
| Ranunculus ollissiponensis subsp. Ollissiponensis Pers. | Ranunculaceae | N | N | N | L |
| Raphanus raphanistrum L. | Brassicaceae | N | L | L | L |
| Rumex acetosella subsp. angiocarpus (Murb.) Murb. | Polygonaceae | N | N | L | N |
| Rumex bucephalophorus L. | Polygonaceae | N | N | N | L |
| Rumex crispus L. | Polygonaceae | L | N | N | N |
| Rumex pulcher subsp. woodsii (De Not.) Arcang. | Polygonaceae | L | L | L | L |
| Rumex sp. | Polygonaceae | L | N | N | N |
| Scirpoides holoschoenus (L.) Soják | Cyperaceae | N | N | N | L |
| Scolymus hispanicus L. | Asteraceae | L | N | N | N |
| Senecio gallicus Vill. | Asteraceae | L | N | N | N |
| Senecio jacobaea L. | Asteraceae | L | L | L | L |
| Senecio vulgaris L. | Asteraceae | M | H | H | M |
| Sonchus oleraceus L. | Asteraceae | L | N | N | N |
| Spergula arvensis L. | Caryophyllaceae | L | L | L | L |
| Stellaria media (L.) Vill. | Caryophyllaceae | L | L | L | L |
| Trifolium repens L. | Fabaceae | L | L | L | N |
| Urtica membranacea Poir. | Urticaceae | N | N | L | N |
| Urtica urens L. | Urticaceae | L | L | L | L |
| Vulpia geniculata L. | Poaceae | L | H | M | H |
| Plant Taxa | Family | P1UC | P2UD | P3TD | P4TC |
|---|---|---|---|---|---|
| Agrostis castellana Boiss. & Reut. | Poaceae | N | N | N | M |
| Agrostis pourretii Willd. | Poaceae | L | N | N | N |
| Anagallis arvensis L. | Myrsinaceae | L | L | L | N |
| Andryala integrifolia L. | Asteraceae | L | N | N | N |
| Anthriscus caucalis M.Bieb. | Apiaceae | L | N | L | N |
| Arum italicum subsp. italicum | Araceae | L | L | N | N |
| Avena barbata subsp. lusitanica (Tab.Morais) Romero Zarco | Poaceae | L | N | N | L |
| Biserrula pelecinus L. | Fabaceae | L | L | L | L |
| Bromus hordeaceus L. | Poaceae | M | L | H | H |
| Bromus sterilis L. | Poaceae | L | L | L | L |
| Bryonia dioica Jacq. | Cucurbitaceae | N | L | N | N |
| Callitriche stagnalis | Plantaginaceae | L | L | N | N |
| Carduus tenuiflorus Curtis | Compostae | L | L | L | L |
| Cerastium glomeratum Thuill. | Caryophyllaceae | L | L | L | L |
| Chamaemelum fuscatum (Brot.) Vasc. | Asteraceae | L | L | L | L |
| Chamaemelum mixtum | Asteraceae | M | H | M | M |
| Crepis capillaris (L.) Wallr. | Crassulaceae | L | M | M | M |
| Crepis vesicaria subsp. taraxacifolia (Thuill.) Thell. | Crassulaceae | M | L | L | L |
| Cynodon dactylon (L.) Pers. | Poaceae | L | L | L | L |
| Daucus carota subsp maximus L. | Apiaceae | L | N | L | N |
| Diplotaxis catholica (L.) DC. | Brassicaceae | M | M | H | M |
| Echium plantagineum L. | Boraginaceae | M | M | H | M |
| Erodium cicutarium subsp. bipinnatum (Cav.) Tourlet | Geraniaceae | M | M | M | M |
| Galactites tomentosus Moench | Asteraceae | N | N | N | L |
| Geranium molle L. | Geraniaceae | M | L | M | L |
| Geranium purpureum Vill. | Geraniaceae | N | N | L | N |
| Hedypnois cretica (L.) Dum.-Courset | Asteraceae | M | M | L | L |
| Heliotropium europaeum L. | Boraginaceae | L | N | N | N |
| Hordeum murinum subsp. leporinum (Link) Arcang. | Poaceae | M | L | M | 1++ |
| Hypochaeris glabra L. | Asteraceae | L | L | L | N |
| Hypochaeris radicata L. | Asteraceae | L | L | L | L |
| Iris xiphium L. | Iridaceae | L | N | N | N |
| Juncus bufonius L. | Juncaceae | L | L | M | N |
| Lamarckia aurea (L.) Moench | Poaceae | N | L | N | N |
| Lathyrus angulatus L. | Fabaceae | L | N | L | N |
| Leontodon taraxacoides (Vill.) Mérat | Asteraceae | M | L | M | L |
| Logfia gallica (L.) Coss. & Germ. | Asteraceae | N | L | N | N |
| Lolium rigidum subsp. Rigidum Gaudin | Poaceae | N | N | N | L |
| Lotus parviflorus Desf. | Fabaceae | N | L | N | N |
| Lythrum borysthenicum (Schrank) Litv. | Lythraceae | L | L | N | N |
| Medicago polymorpha L. | Fabaceae | L | L | L | L |
| Mentha pulegium L. | Lamiaceae | L | N | L | L |
| Ornithopus compressus L. | Fabaceae | L | L | L | L |
| Orobanche sp. | Orobanchaceae | L | N | N | N |
| Plantago coronopus L. | Plantaginaceae | L | M | L | L |
| Plantago lagopus L. | Plantaginaceae | M | M | H | M |
| Poa annua L. | Poaceae | L | L | N | N |
| Poa bulbosa L. | Poaceae | N | N | N | L |
| Pulicaria odora (L.) Rchb. | Asteraceae | N | N | L | N |
| Poa trivialis L. | Poaceae | N | N | N | L |
| Polycarpon tetraphyllum (L.) L. | Caryophyllaceae | N | L | L | N |
| Ranunculus ophioglossifolius Vill. | Ranunculaceae | N | N | N | L |
| Ranunculus parviflorus L. | Ranunculaceae | L | N | L | L |
| Raphanus raphanistrum L. | Brassicaceae | N | L | N | N |
| Rumex bucephalophorus L. | Polygonaceae | M | M | L | L |
| Rumex crispus L. | Polygonaceae | N | N | L | N |
| Rumex pulcher subsp. woodsii (De Not.) Arcang. | Polygonaceae | L | L | L | L |
| Senecio jacobaea L. | Asteraceae | L | L | L | L |
| Sherardia arvensis L. | Rubiaceae | L | L | N | N |
| Silene gallica L. | Caryophyllaceae | M | L | L | N |
| Sisymbrium officinale (L.) Scop. | Brassicaceae | L | L | N | N |
| Sonchus oleraceus L. | Asteraceae | L | N | L | N |
| Spergula arvensis L. | Caryophyllaceae | N | L | N | N |
| Stachys arvensis (L.) L. | Lamiaceae | L | L | M | N |
| Stellaria media (L.) Vill. | Caryophyllaceae | L | N | N | N |
| Tolpis umbellata Bertol. | Asteraceae | L | M | M | L |
| Trifolium campestre Schreb. | Fabaceae | N | M | L | L |
| Trifolium glomeratum L. | Fabaceae | L | H | M | L |
| Trifolium medium subsp. médium L. | Fabaceae | N | N | L | N |
| Trifolium repens L. | Fabaceae | L | H | H | L |
| Trifolium resupinatum L. | Fabaceae | L | L | L | L |
| Trifolium scabrum L. | Fabaceae | L | N | N | N |
| Trifolium subterraneum L. | Fabaceae | L | M | N | N |
| Urtica urens L. | Urticaceae | L | N | N | N |
| veronica sp. | Plantaginaceae | L | N | N | N |
| Vicia disperma DC. | Fabaceae | L | N | N | N |
| Vulpia bromoides (L.) S.F.Gray | Poaceae | N | L | L | L |
| Vulpia geniculata L. | Poaceae | H | H | H | H |
| Plant Taxa | Family | P1UC | P2UD | P3TD | P4TC |
|---|---|---|---|---|---|
| Agrostis castellana Boiss. & Reut. | Poaceae | N | N | N | L |
| Agrostis pourretii Willd. | Poaceae | H | H | M | M |
| Andryala integrifolia L. | Asteraceae | L | N | L | N |
| Arum italicum subsp. italicum | Araceae | L | N | L | N |
| Avena barbata subsp. lusitanica (Tab.Morais) Romero Zarco | Poaceae | M | L | N | M |
| Biserrula pelecinus L. | Fabaceae | L | N | L | N |
| Bromus diandrus L. | Poaceae | N | N | L | H |
| Bromus hordeaceus L. | Poaceae | N | L | N | N |
| Bromus sterilis L. | Poaceae | N | N | N | L |
| Bromus tectorum L. | Poaceae | L | L | N | L |
| Carduus tenuiflorus Curtis | Compostae | L | L | L | L |
| Chamaemelum fuscatum (Brot.) Vasc. | Asteraceae | L | N | N | L |
| Chamaemelum mixtum L. | Asteraceae | M | L | M | M |
| Crepis capillaris (L.) Wallr. | Crassulaceae | M | M | H | L |
| Crepis vesicaria subsp. taraxacifolia (Thuill.) Thell. | Crassulaceae | L | N | N | L |
| Cynodon dactylon (L.) Pers. | Poaceae | L | L | L | L |
| Cynosurus echinatus L. | Poaceae | L | N | L | N |
| Cyperus longus L. | Cyperaceae | N | N | N | L |
| Diplotaxis catholica (L.) DC. | Brassicaceae | L | N | L | L |
| Echium plantagineum L. | Boraginaceae | M | L | L | M |
| Erodium cicutarium subsp. bipinnatum (Cav.) Tourlet | Geraniaceae | N | N | N | L |
| Galactites tomentosus Moench | Asteraceae | N | N | N | L |
| Hedypnois cretica (L.) Dum.-Courset | Asteraceae | L | N | N | L |
| Heliotropium europaeum L. | Boraginaceae | L | N | N | N |
| Holcus lanatus L. | Poaceae | N | L | N | N |
| Hordeum murinum subsp. leporinum (Link) Arcang. | Poaceae | M | L | M | M |
| Hypochaeris glabra L. | Asteraceae | N | N | N | L |
| Hypochaeris radicata L. | Asteraceae | M | L | L | L |
| Juncus bufonius L. | Juncaceae | N | N | L | N |
| Leontodon taraxacoides (Vill.) Mérat | Asteraceae | L | L | L | N |
| Lolium perenne L. | Poaceae | L | N | N | N |
| Lythrum borysthenicum (Schrank) Litv. | Lythraceae | L | N | N | N |
| Medicago polymorpha L. | Fabaceae | L | N | N | N |
| Ornithopus compressus L. | Fabaceae | L | N | N | L |
| Phalaris arundinacea subsp. arundinacea | Poaceae | N | N | N | L |
| Plantago coronopus L. | Plantaginaceae | L | L | N | N |
| Plantago lagopus L. | Plantaginaceae | M | M | H | L |
| Pulicaria odora (L.) Rchb. | Asteraceae | L | N | L | N |
| Polycarpon tetraphyllum (L.) L. | Caryophyllaceae | N | L | N | N |
| Polypogon maritimus Willd. | Poaceae | N | N | L | N |
| Quercus rotundifolia Lam. | Fagaceae | L | N | N | N |
| Raphanus raphanistrum L. | Brassicaceae | N | N | N | L |
| Rumex bucephalophorus L. | Polygonaceae | N | N | N | L |
| Rumex crispus L. | Polygonaceae | N | N | L | N |
| Rumex pulcher subsp. woodsii (De Not.) Arcang. | Polygonaceae | L | N | N | N |
| Sherardia arvensis L. | Rubiaceae | L | N | N | N |
| Tolpis barbata (L.) Gaertn | Asteraceae | L | N | N | L |
| Tolpis umbellata Bertol. | Asteraceae | M | L | M | L |
| Trifolium campestre Schreb. | Fabaceae | L | L | N | N |
| Trifolium glomeratum L. | Fabaceae | L | N | N | N |
| Trifolium scabrum L. | Fabaceae | L | N | N | N |
| Trifolium subterraneum L. | Fabaceae | L | N | N | N |
| Vulpia geniculata L. | Poaceae | H | H | H | H |
References
- Pinto-Correia, T.; Ribeiro, N.; Potes, J. Livro verde dos Montados. In Edição: ICAAM—Instituto de Ciências Agrárias e Ambientais Mediterrânicas; Universidade de Évora: Évora, Portugal, 2013. [Google Scholar]
- Serrano, J.M. Utilização eficiente dos recursos no Montado: Sensores próximos e detecção remota no apoio à tomada de decisão. Bol. CCDR Alentejo Perspetiva 2020, 32, 18–25. [Google Scholar]
- David, T.S.; Pinto, C.A.; Nadezhdina, N.; Kurz-Besson, C.; Henriques, M.O.; Quilhó, T.; Cermak, J.; Chaves, M.M.; Pereira, J.S.; David, J.S. Root functioning, tree water use and hydraulic redistribution in Quercus suber trees: A modeling approach based on root sap flow. For. Ecol. Manag. 2013, 307, 136–146. [Google Scholar] [CrossRef]
- Serrano, J.; Shahidian, S.; Machado, E.; Paniagua, L.L.; Carreira, E.; Moral, F.; Pereira, A.; de Carvalho, M. Floristic Composition: Dynamic Biodiversity Indicator of Tree Canopy Effect on Dryland and Improved Mediterranean Pastures. Agriculture 2021, 11, 1128. [Google Scholar] [CrossRef]
- Feio, M. Clima e Agricultura: Exigências Climáticas das Principais Culturas e Potencialidade Agrícolas do Nosso Clima; Ministério da Agricultura, Pescas e Alimentação: Lisboa, Portugal, 1991; ISBN 972-9175-25-X. [Google Scholar]
- Carreira, E.; Serrano, J.; Shahidian, S.; Nogales-Bueno, J.; Rato, A.E. Real-Time Quantification of Crude Protein and Neutral Detergent Fibre in Pastures under Montado Ecosystem Using the Portable NIR Spectrometer. Appl. Sci. 2021, 11, 10638. [Google Scholar] [CrossRef]
- Belo, C.C.; Coelho, I.; Rolo, J.; Reis, P. Sistemas agroflorestais em Portugal continental. Parte II: Montados, condições de uso do solo e evolução. Rev. Ciências Agrárias 2014, 37, 122–130. [Google Scholar]
- Daniels, M.B.; Delaune, P.; Moore, P.A.; Mauromoustakos, A.; Chapman, S.L.; Langston, J.M. Soil Phosphorus Variability in Pastures: Implications for Sampling and Environmental Management Strategies. J. Environ. Qual. 2001, 30, 2157–2165. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.; Goss, M.J.; Teixeira, D. Manganese toxicity in Portuguese Cambisols derived from granite: Causes, limitations of soil analyses and possible solutions. Rev. Ciências Agrárias 2015, 38, 518–527. [Google Scholar] [CrossRef]
- Serrano, J.; Roma, L.; Shahidian, S.; Belo, A.D.F.; Carreira, E.; Paniagua, L.L.; Moral, F.; Paixão, L.; da Silva, J.M. A Technological Approach to Support Extensive Livestock Management in the Portuguese Montado Ecosystem. Agronomy 2022, 12, 1212. [Google Scholar] [CrossRef]
- Brougham, R.W. The effects of frequent hard grazings at different times of the year on the productivity and species yields of a grass-clover pasture. N. Z. J. Agric. Res. 1960, 3, 125–136. [Google Scholar] [CrossRef]
- Voisin, A.; Lecomte, A. La Vaca y la Hierba: Cómo Obtener Buenos Rendimientos del Ganado Vacuno; Tecnos: Madrid, Spain, 1968. [Google Scholar]
- Xiao, X.; Zhang, T.; Angerer, J.P.; Hou, F. Grazing Seasons and Stocking Rates Affects the Relationship between Herbage Traits of Alpine Meadow and Grazing Behaviors of Tibetan Sheep in the Qinghai–Tibetan Plateau. Animals 2020, 10, 488. [Google Scholar] [CrossRef]
- Nie, Z.N.; Barker, D.J.; Mackay, A.D.; Valentine, I.; Hodgson, J. Influence of the timing and duration of pastoral fallowing and nitrogen fertiliser on pasture and white clover (Trifolium repens) growth in hill country. N. Z. J. Agric. Res. 1998, 41, 19–29. [Google Scholar] [CrossRef]
- Heady, H.F. Continuous vs. Specialized Grazing Systems: A Review and Application to the California Annual Type. Rangel. Ecol. Manag. 1961, 14, 182. [Google Scholar] [CrossRef]
- Zhou, Y.; Gowda, P.H.; Wagle, P.; Ma, S.; Neel, J.P.S.; Kakani, V.G.; Steiner, J.L. Climate Effects on Tallgrass Prairie Responses to Continuous and Rotational Grazing. Agronomy 2019, 9, 219. [Google Scholar] [CrossRef]
- Holechek, J.L. Considerations concerning grazing systems. Rangelands 1983, 5, 208–211. [Google Scholar]
- Tozer, K.N.; Müller, K.; Craven, T.; Tarbotton, I.; Coster, A.; Burke, R.; Sherlock, J.; Cameron, C. Effect of deferred grazing during late spring and summer on pasture productivity in Waikato and Bay of Plenty hill country. J. N. Z. Grasslands 2020, 82, 111–119. [Google Scholar] [CrossRef]
- Lambert, M.; Clark, D.; Litherland, A. Advances in pasture management for animal productivity and health. N. Z. Vet. J. 2004, 52, 311–319. [Google Scholar] [CrossRef]
- McCallum, D.; Thomson, N.; Judd, T. Experiences with deferred grazing at the Taranaki Agricultural Research Station. Proc. N. Z. Grassl. Assoc. 1991, 53, 79–83. [Google Scholar] [CrossRef]
- Lambert, M.; Litherland, A. A practitioner’s guide to pasture quality. Proc. N. Z. Grassl. Assoc. 2000, 115, 111–115. [Google Scholar] [CrossRef]
- Watkinson, A.R.; Ormerod, S.J. Grasslands, grazing and biodiversity: Editors’ introduction. J. Appl. Ecol. 2001, 38, 233–237. [Google Scholar] [CrossRef]
- Lin, L.; Dickhoefer, U.; Müller, K.; Wang, C.; Glindemann, T.; Hao, J.; Wan, H.; Schönbach, P.; Gierus, M.; Taube, F.; et al. Growth of sheep as affected by grazing system and grazing intensity in the steppe of Inner Mongolia, China. Livest. Sci. 2011, 144, 140–147. [Google Scholar] [CrossRef]
- Stewart, G.B.; Pullin, A.S. The relative importance of grazing stock type and grazing intensity for conservation of mesotrophic ‘old meadow’ pasture. J. Nat. Conserv. 2008, 16, 175–185. [Google Scholar] [CrossRef]
- Edwards, G.R.; Hay, M.J.M.; Brock, J.L. Seedling recruitment dynamics of forage and weed species under continuous and rotational sheep grazing in a temperate New Zealand pasture. Grass Forage Sci. 2005, 60, 186–199. [Google Scholar] [CrossRef]
- Buttolph, L.P.; Coppock, D.L. Influence of deferred grazing on vegetation dynamics and livestock productivity in an Andean pastoral system. J. Appl. Ecol. 2004, 41, 664–674. [Google Scholar] [CrossRef]
- Beetz, A.E.; Rinehart, L. rotational grazing [n]. In Encyclopedic Dictionary of Landscape and Urban Planning; Springer: Berlin/Heidelberg, Germany, 2010; p. 857. [Google Scholar] [CrossRef]
- Nie, Z.N.; Zollinger, R.P. Impact of deferred grazing and fertilizer on plant population density, ground cover and soil moisture of native pastures in steep hill country of southern Australia. Grass Forage Sci. 2012, 67, 231–242. [Google Scholar] [CrossRef]
- Di Grigoli, A.; Todaro, M.; Di Miceli, G.; Genna, V.; Tornambé, G.; Alicata, M.L.; Giambalvo, D.; Bonanno, A. Effects of continuous and rotational grazing of different forage species on ewe milk production. Small Rumin. Res. 2012, 106, S29–S36. [Google Scholar] [CrossRef]
- Serrano, J.; Shahidian, S.; Da Silva, J.M.; Sales-Baptista, E.; De Oliveira, I.F.; De Castro, J.L.; Pereira, A.; De Abreu, M.C.; Machado, E.; de Carvalho, M. Tree influence on soil and pasture: Contribution of proximal sensing to pasture productivity and quality estimation in montado ecosystems. Int. J. Remote Sens. 2018, 39, 4801–4829. [Google Scholar] [CrossRef]
- Serrano, J.; Shahidian, S.; da Silva, J.M.; Paixão, L.; Carreira, E.; Pereira, A.; Carvalho, M. Climate Changes Challenges to the Management of Mediterranean Montado Ecosystem: Perspectives for Use of Precision Agriculture Technologies. Agronomy 2020, 10, 218. [Google Scholar] [CrossRef]
- Serrano, J.; Shahidian, S.; Da Silva, J.M.; Moral, F.; Carvajal-Ramirez, F.; Carreira, E.; Pereira, A.; De Carvalho, M. Evaluation of the Effect of Dolomitic Lime Application on Pastures—Case Study in the Montado Mediterranean Ecosystem. Sustainability 2020, 12, 3758. [Google Scholar] [CrossRef]
- Serrano, J.; Shahidian, S.; Costa, F.; Carreira, E.; Pereira, A.; Carvalho, M. Can Soil pH Correction Reduce the Animal Supplementation Needs in the Critical Autumn Period in Mediterranean Montado Ecosystem? Agronomy 2021, 11, 514. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; Update 2015; World Soil Resources Report 106; Food and Agriculture Organization (FAO): Rome, Italy, 2015; 188p. [Google Scholar]
- Serrano, J.; Shahidian, S.; da Silva, J.M. Monitoring Seasonal Pasture Quality Degradation in the Mediterranean Montado Ecosystem: Proximal versus Remote Sensing. Water 2018, 10, 1422. [Google Scholar] [CrossRef]
- Russel, A. Body condition scoring of sheep. Practice 1984, 6, 91–93. [Google Scholar] [CrossRef]
- Marley, C.L.; Fraser, M.D.; Fisher, W.J.; Forbes, A.B.; Jones, R.; Moorby, J.M.; Macrae, J.C.; Theodorou, M.K. Effects of continuous or rotational grazing of two perennial ryegrass varieties on the chemical composition of the herbage and the performance of finishing lambs. Grass Forage Sci. 2007, 62, 255–264. [Google Scholar] [CrossRef]
- Walton, P.D.; Martinez, R.; Bailey, A.W. A Comparison of Continuous and Rotational Grazing. Rangel. Ecol. Manag. 1981, 34, 19. [Google Scholar] [CrossRef]
- Santos, M.; Afonso, L.; Carvalho, B.; Rêgo, A.; Queiroz, G.; Medica, J.; Moraes, L.; Carmo, L. Apparent selectivity of sheep in deferred marandu palisadegrass pastures with variable initial heights. Arq. Bras. Med. Vet. Zootec. 2019, 71, 1727–1734. [Google Scholar] [CrossRef]
- Houlbrooke, D.J.; Drewry, J.J.; Monaghan, R.M.; Paton, R.J.; Smith, L.C.; Littlejohn, R.P. Grazing strategies to protect soil physical properties and maximise pasture yield on a Southland dairy farm. N. Z. J. Agric. Res. 2009, 52, 323–336. [Google Scholar] [CrossRef]
- Cannas, A.; Boe, F. Prediction of the relationship between body weight and body condition score in sheep. Ital. J. Anim. Sci. 2003, 2 (Suppl. 1), 527–529. [Google Scholar] [CrossRef]
- Shore, A. DESeq and Indicator Species Analysis R Script, Figshare Software: Cambridge, MA, USA, 2020.
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Wagner, H. Vegan: Community Ecology Package; R Package Version 2.5-3; The Comprehensive R Archive Network: Wien, Austria, 2018. [Google Scholar]
- Beals, E.W. Bray-Curtis Ordination: An Effective Strategy for Analysis of Multivariate Ecological Data. Adv. Ecol. Res. 1984, 14, 1–55. [Google Scholar] [CrossRef]
- De Cáceres, M.; Legendre, P. Beals smoothing revisited. Oecologia 2008, 156, 657–669. [Google Scholar] [CrossRef]
- Carvalho, M. A Seca E a Intensificação Sustentável Dos Sistemas Agro-Pecuários: Desafios E Soluções. Dossier Secas e Cheias—Como Gerir. 2018, pp. 24–27. Available online: http://dspace.uevora.pt/rdpc/bitstream/10174/23541/1/ (accessed on 7 July 2022).
- Moreira, N. Agronomia das Forragens e Pastagens; Sector Editorial; Universidade de Trás-os-Montes e Alto Douro: Vila Real, Portugal, 2002; 183p. [Google Scholar]
- Paço, A.; Da-Silva, J.R.; Torres, D.P.; Glick, B.R.; Brígido, C. Exogenous ACC Deaminase Is Key to Improving the Performance of Pasture Legume-Rhizobial Symbioses in the Presence of a High Manganese Concentration. Plants 2020, 9, 1630. [Google Scholar] [CrossRef]
- Mendes, P.; Meireles, C.; Vila-Viçosa, C.; Musarella, C.; Pinto-Gomes, C. Best management practices to face degraded territories occupied by Cistus ladanifer shrublands—Portugal case study. Plant Biosyst. 2015, 149, 494–502. [Google Scholar] [CrossRef]
- Ferreira, P.M.A.; Andrade, B.O.; Podgaiski, L.; Dias, A.C.; Pillar, V.D.; Overbeck, G.E.; Milton, D.S.M.; Boldrini, I.I. Long-term ecological research in southern Brazil grasslands: Effects of grazing exclusion and deferred grazing on plant and arthropod communities. PLoS ONE 2020, 15, e0227706. [Google Scholar] [CrossRef]
- Nie, Z.; Valentine, I.; Mackay, A.; Barker, D.; Hodgson, J. Long-term effects of pastoral fallowing on the distribution and performance of white clover (Trifolium repens L.) in a hill country pasture. NZGA: Res. Pract. Ser. 1996, 6, 75–78. [Google Scholar] [CrossRef]
- Avdiu, B.; Aliu, S.; Fetahu, S.; Zeka, D.; Rusinovci, I. THE FLORISTIC COMPOSITION OF THE NATURAL PASTURES IN MASSIVE OF NOVOBERDA. J. Agric. For. 2018, 64, 235–241. [Google Scholar] [CrossRef]
- Köycü, E.; Sezenler, T.; Özder, M.; Karadağ, O. The Relationship Between Body Weight and Body Condition Score in Karacabey Merino Ewes. J. Tekirdag Agric. Fac. 2008, 5, 61–65. [Google Scholar]




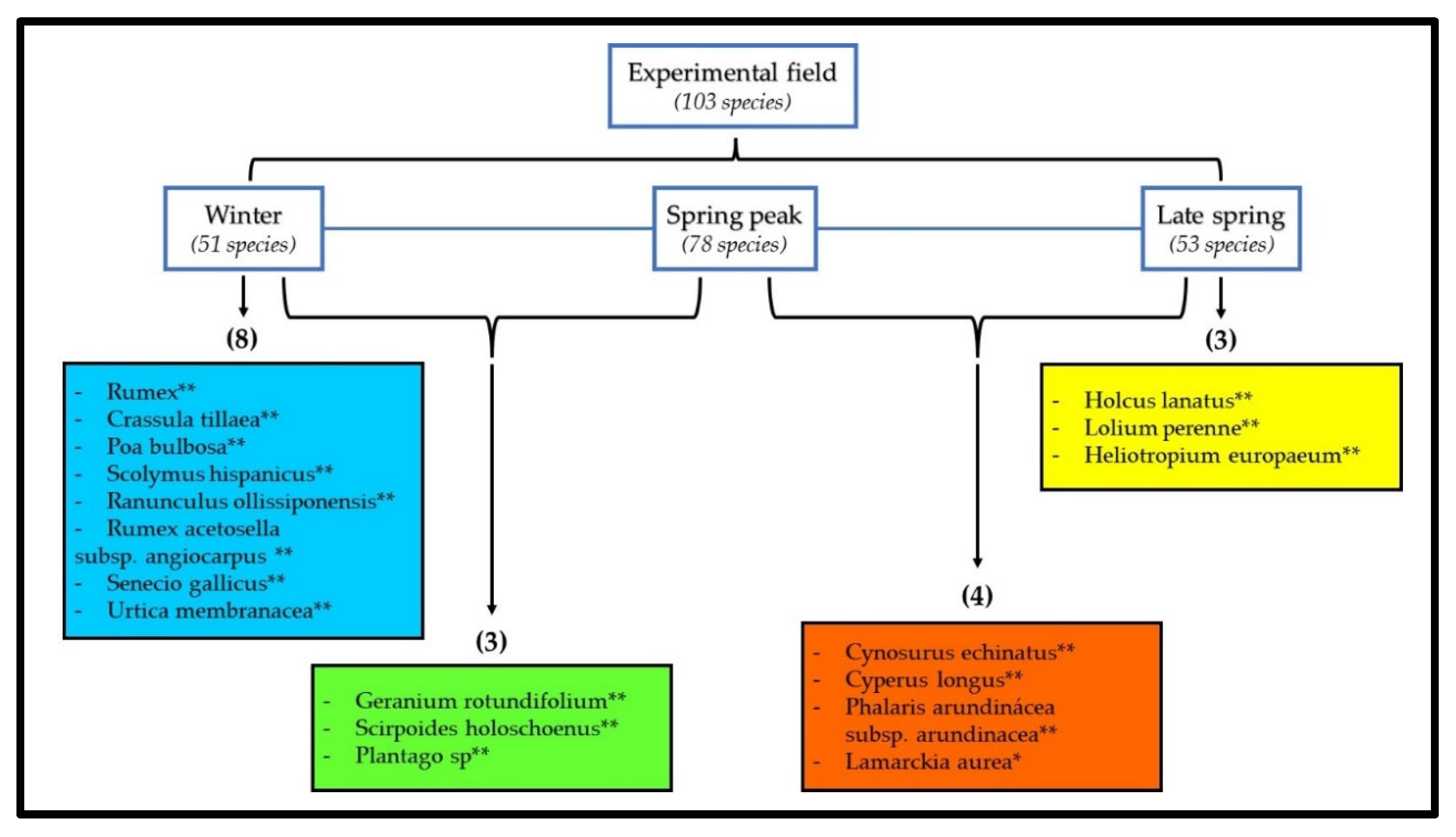

| Combinations: Seasons and Plots | Species | p-Value |
|---|---|---|
| WI_P1UC; WI_P2UD; WI_P3TD | Urtica membranacea | 0.005 ** |
| WI_P1UC; WI_P2UD; WI_P3TD; WI_P4TC | Rumex sp. | 0.005 ** |
| Crassula tillaea | 0.005 ** | |
| Poa bulbosa | 0.005 ** | |
| Ranunculus ollissiponensis | 0.005 ** | |
| Silene gallica | 0.005 ** | |
| WI_P1UC; WI_P2UD; WI_P3TD; WI_P4TC; SP1_P3TD | Plantago sp. | 0.005 ** |
| WI_P1UC; WI_P2UD; WI_P3TD; WI_P4TC; SP1_P1UC; SP1_P3TD | Scolymus hispanicus | 0.005 ** |
| WI_P1UC; WI_P2UD; WI_P3TD; WI_P4TC; SP1_P2UD; SP1_P3TD; SP1_P4TC | Rumex acetosella subsp. angiocarpus | 0.005 ** |
| SP2_P1UC; SP2_P2UD; SP2_P3TD; SP2_P4TC | Holcus lanatus | 0.005 ** |
| SP2_P1UC; SP2_P2UD; SP2_P3TD; SP2_P4TC; SP1_P4TC | Cyperus longus | 0.005 ** |
| Phalaris arundinacea subsp. arundinacea | 0.005 ** | |
| Lolium perenne | 0.005 ** | |
| SP1_P1UC; SP1_P2UD; SP1_P4TC; SP2_P1UC; SP2_P2UD; SP2_P3TD; SP2_P4TC | Cynosurus echinatus | 0.005 ** |
| WI_P1UC; WI_P2UD; WI_P3TD; WI_P4TC; SP1_P1UC; SP1_P2UD; SP1_P3TD; SP1_P4TC | Geranium rotundifolium | 0.005 ** |
| Scirpoides holoschoenus | 0.005 ** | |
| Leontodon tuberosus | 0.005 ** | |
| WI_P1UC; WI_P2UD; SP1_P1UC; SP1_P4TC; SP2_P1UC; SP2_P2UD; SP2_P3TD; SP2_P4TC | Heliotropium europaeum | 0.035 * |
| WI_P4TC; SP1_P1UC; SP1_P2UD; SP1_P3TD; SP1_P4TC; SP2_P1UC; SP2_P2UD; SP2_P3TD; SP2_P4TC | Tolpis barbata | 0.005 ** |
| WI_P2UD; WI_P4TC; SP1_P1UC; SP1_P2UD; SP1_P3TD; SP1_P4TC; SP2_P1UC; SP2_P2UD; SP2_P3TD; SP2_P4TC | Orobanche sp. | 0.01 ** |
| WI_P1UC; WI_P2UD; WI_P3TD; WI_P4TC; SP1_P1UC; SP1_P2UD; SP1_P3TD; SP1_P4TC; SP2_P1UC; SP2_P3TD; SP2_P4TC | Stellaria media | 0.005 ** |
| Sonchus oleraceus | 0.02 * | |
| WI_P1UC; WI_P3TD; WI_P4TC; SP1_P1UC; SP1_P2UD; SP1_P3TD; SP1_P4TC; SP2_P1UC; SP2_P2UD; SP2_P3TD; SP2_P4TC | Quercus rotundifolia | 0.005 ** |
| WI_P2UD; WI_P3TD; WI_P4TC; SP1_P1UC; SP1_P2UD; SP1_P3TD; SP1_P4TC; SP2_P1UC; SP2_P2UD; SP2_P3TD; SP2_P4TC | Bromus tectorum | 0.005 ** |
| Lolium rigidum subsp. rigidum | 0.005 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carreira, E.; Serrano, J.; Gomes, C.J.P.; Shahidian, S.; Paniagua, L.L.; Pilirito, A.; Lopes Castro, J.; Carvalho, M.; Pereira, A.F. Effect of Sheep Grazing, Stocking Rates and Dolomitic Limestone Application on the Floristic Composition of a Permanent Dryland Pasture, in the Montado Agroforestry System of Southern Portugal. Animals 2022, 12, 2506. https://doi.org/10.3390/ani12192506
Carreira E, Serrano J, Gomes CJP, Shahidian S, Paniagua LL, Pilirito A, Lopes Castro J, Carvalho M, Pereira AF. Effect of Sheep Grazing, Stocking Rates and Dolomitic Limestone Application on the Floristic Composition of a Permanent Dryland Pasture, in the Montado Agroforestry System of Southern Portugal. Animals. 2022; 12(19):2506. https://doi.org/10.3390/ani12192506
Chicago/Turabian StyleCarreira, Emanuel, João Serrano, Carlos J. Pinto Gomes, Shakib Shahidian, Luís L. Paniagua, Alexandre Pilirito, José Lopes Castro, Mário Carvalho, and Alfredo F. Pereira. 2022. "Effect of Sheep Grazing, Stocking Rates and Dolomitic Limestone Application on the Floristic Composition of a Permanent Dryland Pasture, in the Montado Agroforestry System of Southern Portugal" Animals 12, no. 19: 2506. https://doi.org/10.3390/ani12192506







