RNA Sequencing Reveals the Regulation of Betaine on Chicken Myogenesis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Isolation and Culture of Chicken Primary Myoblast Cells
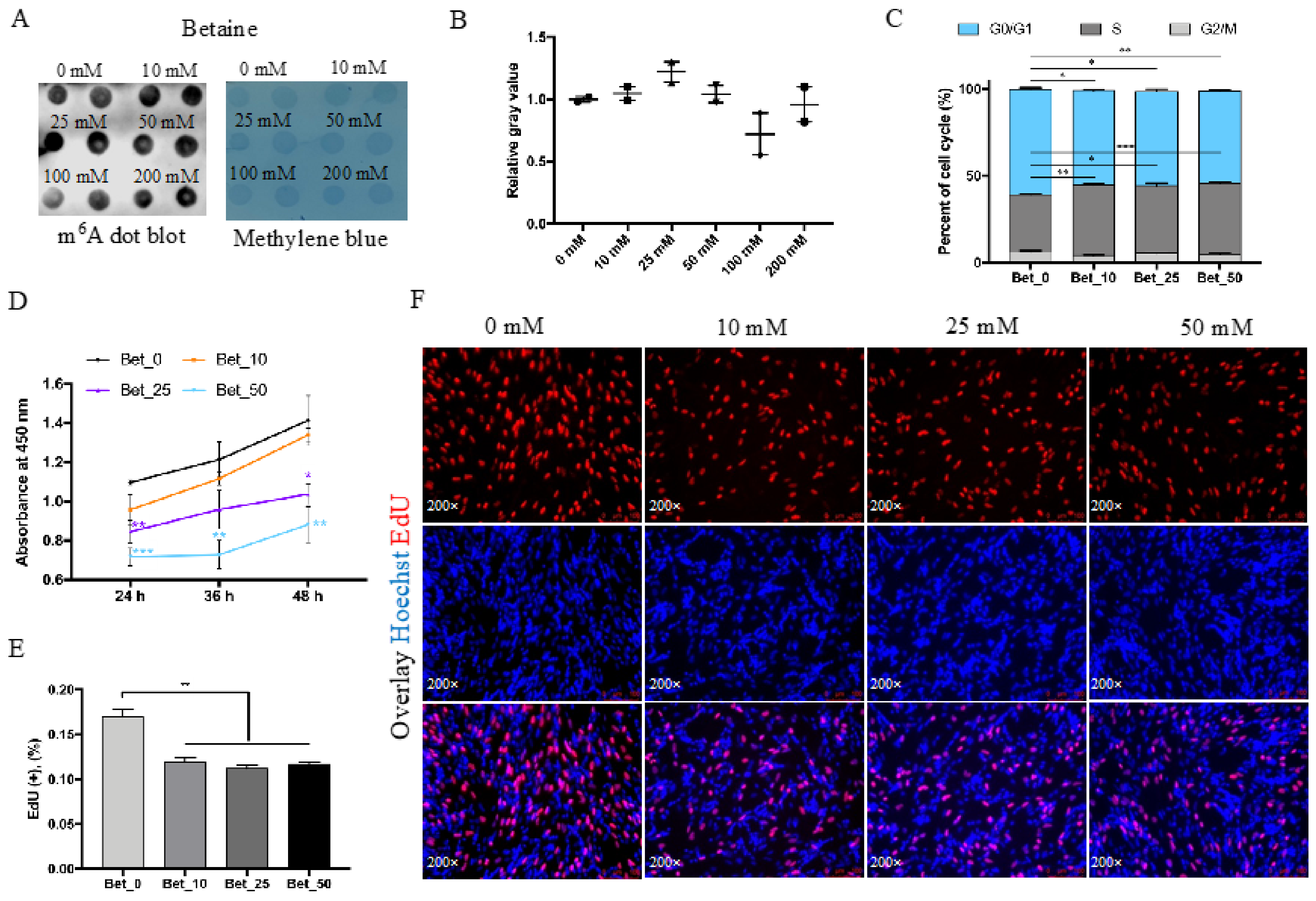
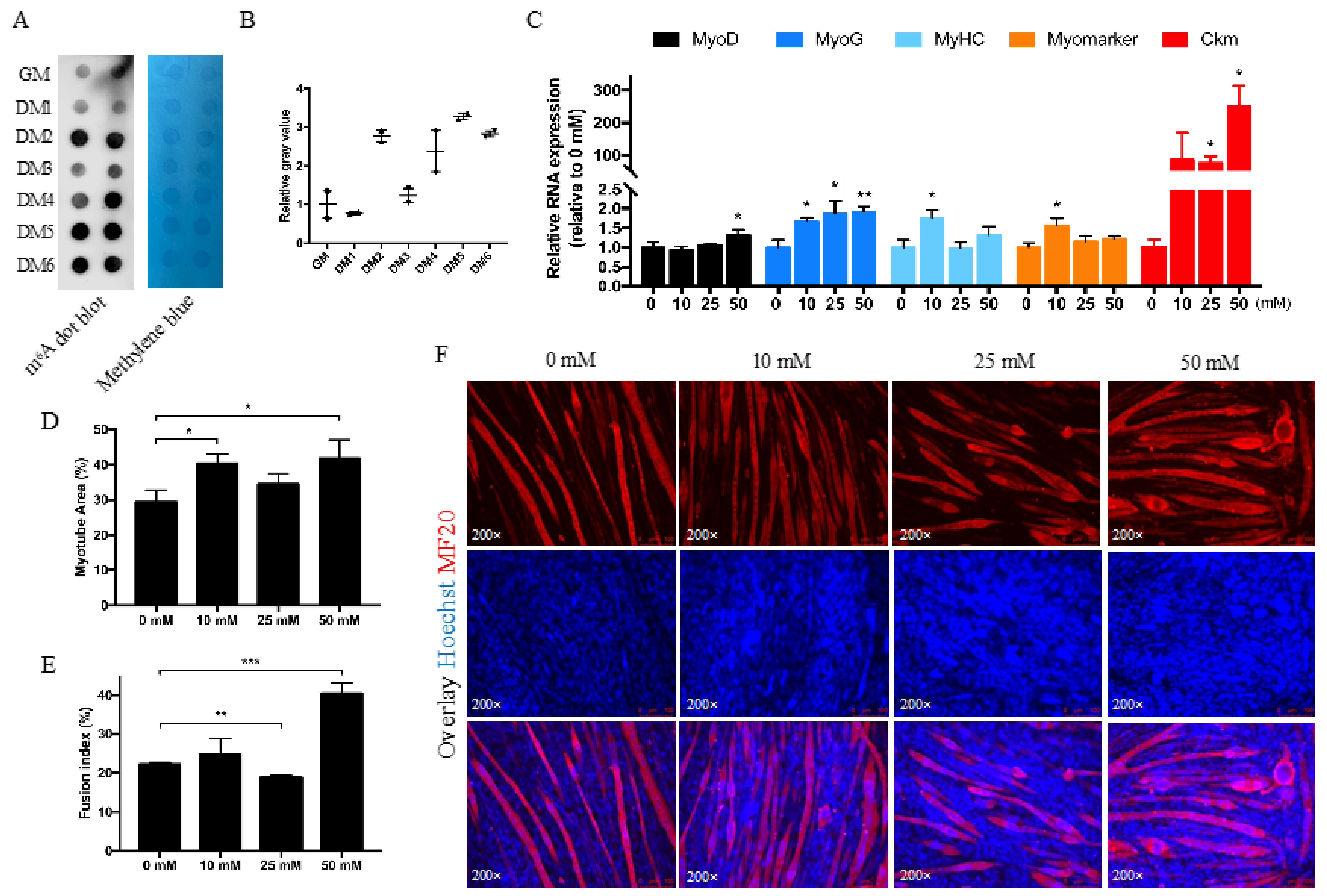
2.3. Betaine Treatment and RNA Dot Blot
2.4. RNA Isolation, cDNA Synthesis and Quantitative Real-Time PCR (qRT-RCR)
2.5. Proliferation Assay
2.6. Differentiation Assay
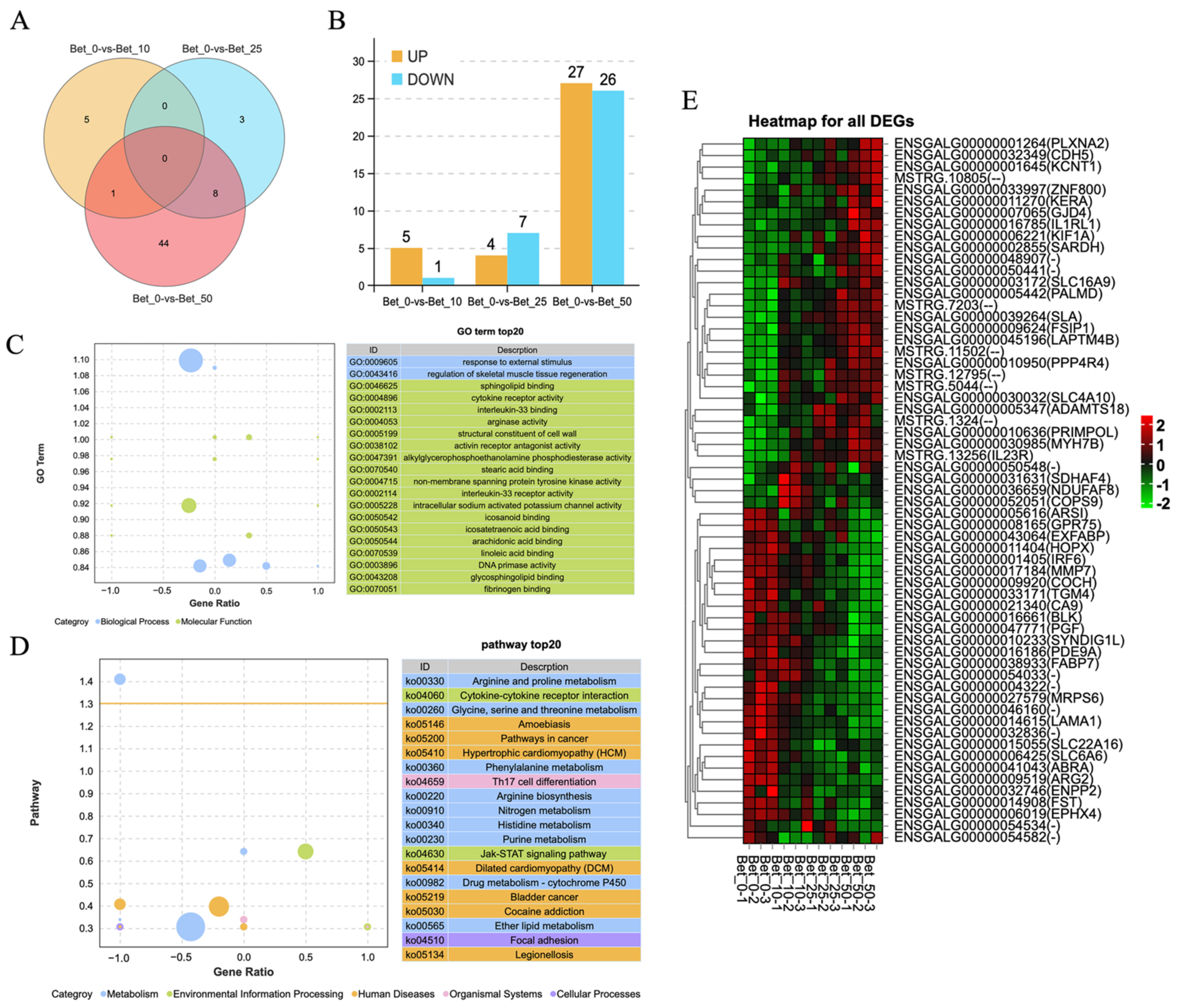
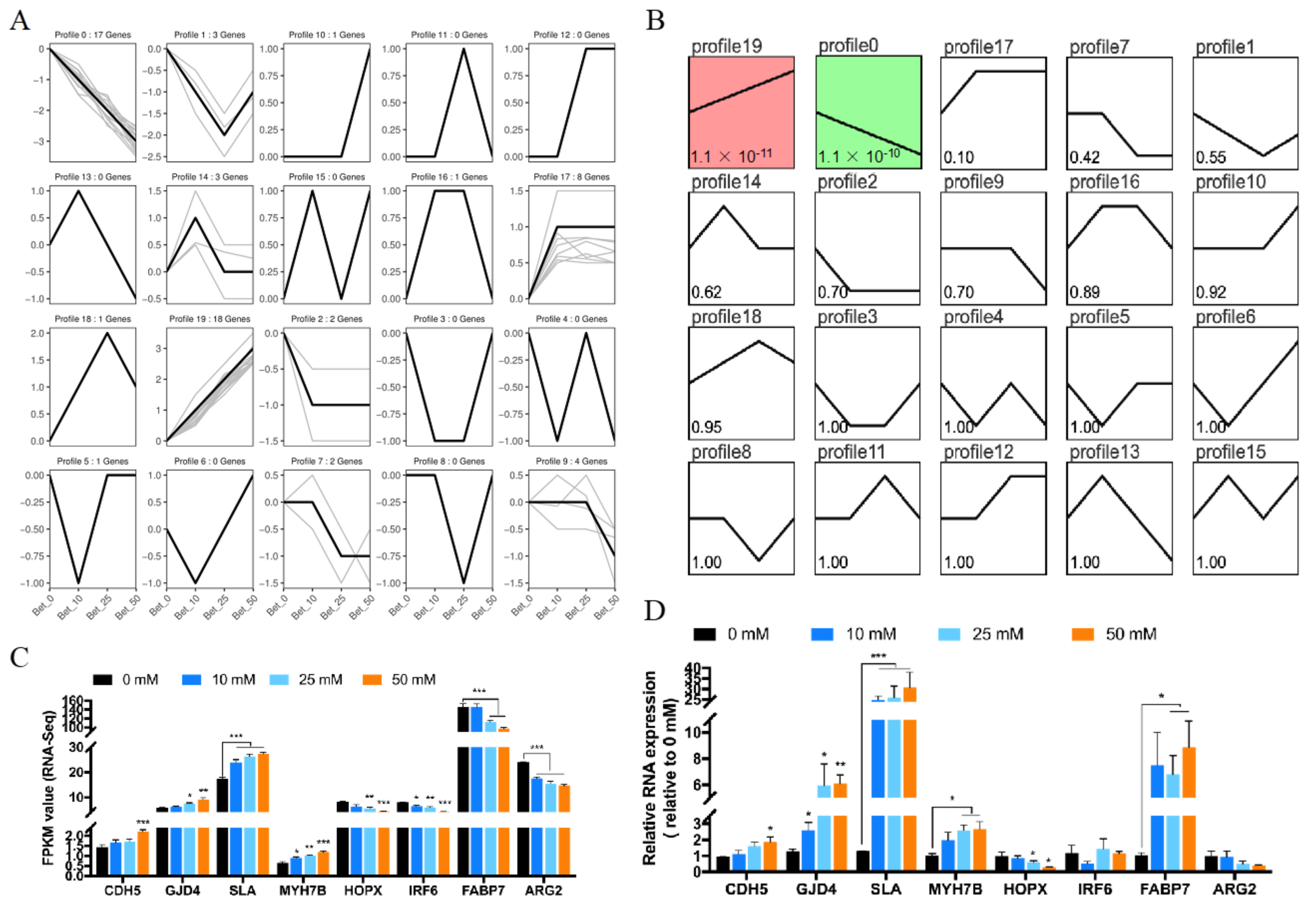
2.7. RNA Sequencing
2.8. Statistical Analysis
3. Results
3.1. Betaine Inhibits Myoblast Cell Proliferation In Vitro
3.2. Betaine Promotes Myotube Formation In Vitro
3.3. RNA-Sequencing Analysis for DEGs in Betaine-Treated Myoblast Cells
3.4. Trend Analysis of Gene Expression for Betaine Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mitchell, A.D.; Chappell, A.; Knox, K.L. Metabolism of betaine in the ruminant. J. Anim. Sci. 1979, 49, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, B.V.; Schinckel, A.P.; Adeola, O.; Cera, K. Impact of betaine on pig finishing performance and carcass composition. J. Anim. Sci. 2002, 80, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; He, F.; Wu, C.; Li, P.; Li, N.; Deng, J.; Zhu, G.; Ren, W.; Peng, Y. Betaine in Inflammation: Mechanistic Aspects and Applications. Front. Immunol. 2018, 9, 1070. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Cheng, X.; Klimasauskas, S.; Mi, S.; Posfai, J.; Roberts, R.J.; Wilson, G.G. The DNA (cytosine-5) methyltransferases. Nucleic Acids Res. 1994, 22, 1–10. [Google Scholar] [CrossRef]
- Hori, H. Methylated nucleosides in tRNA and tRNA methyltransferases. Front. Genet. 2014, 5, 144. [Google Scholar] [CrossRef]
- Traube, F.R.; Carell, T. The chemistries and consequences of DNA and RNA methylation and demethylation. RNA Biol. 2017, 14, 1099–1107. [Google Scholar] [CrossRef]
- Nishimaki-Mogami, T.; Yao, Z.; Fujimori, K. Inhibition of phosphatidylcholine synthesis via the phosphatidylethanolamine methylation pathway impairs incorporation of bulk lipids into VLDL in cultured rat hepatocytes. J. Lipid Res. 2002, 43, 1035–1045. [Google Scholar] [CrossRef]
- Boisvert, F.M.; Côté, J.; Boulanger, M.C.; Richard, S. A proteomic analysis of arginine-methylated protein complexes. Mol. Cell Proteom. 2003, 2, 1319–1330. [Google Scholar] [CrossRef]
- Jia, G.; Fu, Y.; Zhao, X.; Dai, Q.; Zheng, G.; Yang, Y.; Yi, C.; Lindahl, T.; Pan, T.; Yang, Y.G.; et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 2011, 7, 885–887. [Google Scholar] [CrossRef]
- Zheng, G.; Dahl, J.A.; Niu, Y.; Fedorcsak, P.; Huang, C.M.; Li, C.J.; Vågbø, C.B.; Shi, Y.; Wang, W.L.; Song, S.H.; et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell 2013, 49, 18–29. [Google Scholar] [CrossRef]
- Lee, M.; Kim, B.; Kim, V.N. Emerging roles of RNA modification: m6A and U-tail. Cell 2014, 158, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Dominissini, D.; Rechavi, G.; He, C. Gene expression regulation mediated through reversible m⁶A RNA methylation. Nat. Rev. Genet. 2014, 15, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Toth, J.I.; Petroski, M.D.; Zhang, Z.; Zhao, J.C. N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat. Cell Biol. 2014, 16, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.; Agarwala, S.D.; Mumbach, M.R.; Jovanovic, M.; Mertins, P.; Shishkin, A.; Tabach, Y.; Mikkelsen, T.S.; Satija, R.; Ruvkun, G. High-resolution mapping reveals a conserved, widespread, dynamic mRNA methylation program in yeast meiosis. Cell 2013, 155, 1409–1421. [Google Scholar] [CrossRef]
- Wang, T.; Kong, S.; Tao, M.; Ju, S. The potential role of RNA N6-methyladenosine in Cancer progression. Mol. Cancer 2020, 19, 88. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, C.; Zhu, G. Profiling of RNA N6-methyladenosine methylation during follicle selection in chicken ovary. Poult. Sci. 2019, 98, 6117–6124. [Google Scholar] [CrossRef]
- Cheng, B.; Leng, L.; Li, Z.; Wang, W.; Jing, Y.; Li, Y.; Wang, N.; Li, H.; Wang, S. Profiling of RNA N6 -Methyladenosine Methylation Reveals the Critical Role of m6A in Chicken Adipose Deposition. Front. Cell Dev. Biol. 2021, 9, 590468. [Google Scholar] [CrossRef]
- Zuo, Q.; Jing, J.; Zhou, J.; Zhang, Y.; Wei, W.; Chen, G.; Li, B. Dual regulatory actions of LncBMP4 on BMP4 promote chicken primordial germ cell formation. EMBO Rep. 2022, 23, e52491. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Wang, X.; Sun, C.; Zheng, J.; Li, J.; Yi, G.; Yang, N. The m6A methylation regulates gonadal sex differentiation in chicken embryo. J. Anim. Sci. Biotechnol. 2022, 13, 52. [Google Scholar] [CrossRef]
- Gheller, B.J.; Blum, J.E.; Fong, E.H.H.; Malysheva, O.V.; Cosgrove, B.D.; Thalacker-Mercer, A.E. A defined N6-methyladenosine (m6A) profile conferred by METTL3 regulates muscle stem cell/myoblast state transitions. Cell Death Discov. 2020, 6, 95. [Google Scholar] [CrossRef]
- Petrosino, J.M.; Hinger, S.A.; Golubeva, V.A.; Barajas, J.M.; Dorn, L.E.; Iyer, C.C.; Sun, H.L.; Arnold, W.D.; He, C.; Accornero, F. The m6A methyltransferase METTL3 regulates muscle maintenance and growth in mice. Nat. Commun. 2022, 13, 168. [Google Scholar] [CrossRef] [PubMed]
- Senesi, P.; Luzi, L.; Montesano, A.; Mazzocchi, N.; Terruzzi, I. Betaine supplement enhances skeletal muscle differentiation in murine myoblasts via IGF-1 signaling activation. J. Transl. Med. 2013, 11, 174. [Google Scholar] [CrossRef] [PubMed]
- Pesti, G.M.; Harper, A.E.; Sunde, M.L. Choline/methionine nutrition of starting broiler chicks. Three models for estimating the choline requirement with economic considerations. Poult. Sci. 1980, 59, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Saunderson, C.L.; Mackinlay, J. Changes in body-weight, composition and hepatic enzyme activities in response to dietary methionine, betaine and choline levels in growing chicks. Br. J. Nutr. 1990, 63, 339–349. [Google Scholar] [CrossRef]
- Campbell, R.G.; Morley, W.C.; Zabaras-Krick, B. The effects of betaine on protein and energy metabolism of growing pigs. In Manipulating Pig Production VI; Cranwell, P.D., Ed.; Australasian Pig Science Association: Werribee, Australia, 1997; p. 243. [Google Scholar]
- Wang, Z.; Zhang, M.; Li, K.; Chen, Y.; Cai, D.; Chen, B.; Nie, Q. CircMGA Depresses Myoblast Proliferation and Promotes Myotube Formation through miR-144-5p/FAP Signal. Animals 2022, 12, 873. [Google Scholar] [CrossRef]
- Ernst, J.; Bar-Joseph, Z. STEM: A tool for the analysis of short time series gene expression data. BMC Bioinform. 2006, 7, 191. [Google Scholar] [CrossRef]
- Glickman, M.E.; Rao, S.R.; Schultz, M.R. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J. Clin. Epidemiol. 2014, 67, 850–857. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Natalello, A.; Liu, J.; Ami, D.; Doglia, S.M.; Marco, A. The osmolyte betaine promotes protein misfolding and disruption of protein aggregates. Proteins 2009, 75, 509–517. [Google Scholar] [CrossRef]
- Lin, D.W.; Chung, B.P.; Kaiser, P. S-adenosylmethionine limitation induces p38 mitogen-activated protein kinase and triggers cell cycle arrest in G1. J. Cell Sci. 2014, 127 Pt 1, 50–59. [Google Scholar] [CrossRef]
- Chen, J.N.; Chen, Y.; Wei, Y.Y.; Raza, M.A.; Zou, Q.; Xi, X.Y.; Zhu, L.; Tang, G.Q.; Jiang, Y.Z.; Li, X.W. Regulation of m6A RNA Methylation and Its Effect on Myogenic Differentiation in Murine Myoblasts. Mol. Biol. 2019, 53, 436–445. [Google Scholar] [CrossRef]
- Jang, Y.N.; Baik, E.J. JAK-STAT pathway and myogenic differentiation. JAK-STAT 2013, 2, e23282. [Google Scholar] [CrossRef] [PubMed]
- Serrano, A.L.; Baeza-Raja, B.; Perdiguero, E.; Jardí, M.; Muñoz-Cánoves, P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab. 2008, 7, 33–44. [Google Scholar] [CrossRef]
- Sun, L.; Ma, K.; Wang, H.; Xiao, F.; Gao, Y.; Zhang, W.; Wang, K.; Gao, X.; Ip, N.; Wu, Z. JAK1–STAT1–STAT3, a key pathway promoting proliferation and preventing premature differentiation of myoblasts. J. Cell Biol. 2007, 179, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Tierney, M.T.; Aydogdu, T.; Sala, D.; Malecova, B.; Gatto, S.; Puri, P.L.; Latella, L.; Sacco, A. STAT3 signaling controls satellite cell expansion and skeletal muscle repair. Nat. Med. 2014, 20, 1182–1186. [Google Scholar] [CrossRef]
- Michalak, E.M.; Burr, M.L.; Bannister, A.J.; Dawson, M.A. The roles of DNA, RNA and histone methylation in ageing and cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 573–589. [Google Scholar] [CrossRef]
- Turner, D.C.; Gorski, P.P.; Maasar, M.F.; Seaborne, R.A.; Baumert, P.; Brown, A.D.; Kitchen, M.O.; Erskine, R.M.; Dos-Remedios, I.; Voisin, S.; et al. DNA methylation across the genome in aged human skeletal muscle tissue and muscle-derived cells: The role of HOX genes and physical activity. Sci. Rep. 2020, 10, 15360. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, H.; Li, Z.; Zheng, X.; Jia, X.; Nie, Q.; Zhang, X. Comparison of the genome-wide DNA methylation profiles between fast-growing and slow-growing broilers. PLoS ONE 2013, 8, e56411. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Cai, D.; Ju, X.; Li, K.; Liang, S.; Fang, M.; Nie, Q. RNA Sequencing Reveals the Regulation of Betaine on Chicken Myogenesis. Animals 2022, 12, 2508. https://doi.org/10.3390/ani12192508
Wang Z, Cai D, Ju X, Li K, Liang S, Fang M, Nie Q. RNA Sequencing Reveals the Regulation of Betaine on Chicken Myogenesis. Animals. 2022; 12(19):2508. https://doi.org/10.3390/ani12192508
Chicago/Turabian StyleWang, Zhijun, Danfeng Cai, Xing Ju, Kan Li, Sisi Liang, Meixia Fang, and Qinghua Nie. 2022. "RNA Sequencing Reveals the Regulation of Betaine on Chicken Myogenesis" Animals 12, no. 19: 2508. https://doi.org/10.3390/ani12192508
APA StyleWang, Z., Cai, D., Ju, X., Li, K., Liang, S., Fang, M., & Nie, Q. (2022). RNA Sequencing Reveals the Regulation of Betaine on Chicken Myogenesis. Animals, 12(19), 2508. https://doi.org/10.3390/ani12192508






