Novel Proteoliposome-Based Vaccine against E. coli: A Potential New Tool for the Control of Bovine Mastitis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Bacterial Proteoliposomes
2.2. Characterization of Bacterial Proteoliposomes
2.3. Animals and Vaccination
2.4. Intramammary Infection
2.5. Measurement of Systemic and Local Humoral Immune Responses
2.6. Vaccine Effectiveness Evaluation
2.6.1. Clinical Evaluation
2.6.2. Histopathological Evaluation
2.6.3. Bacterial Count in the Mammary Gland
2.7. Statistical Analysis
3. Results
3.1. Physicochemical Characterization of Proteoliposomes
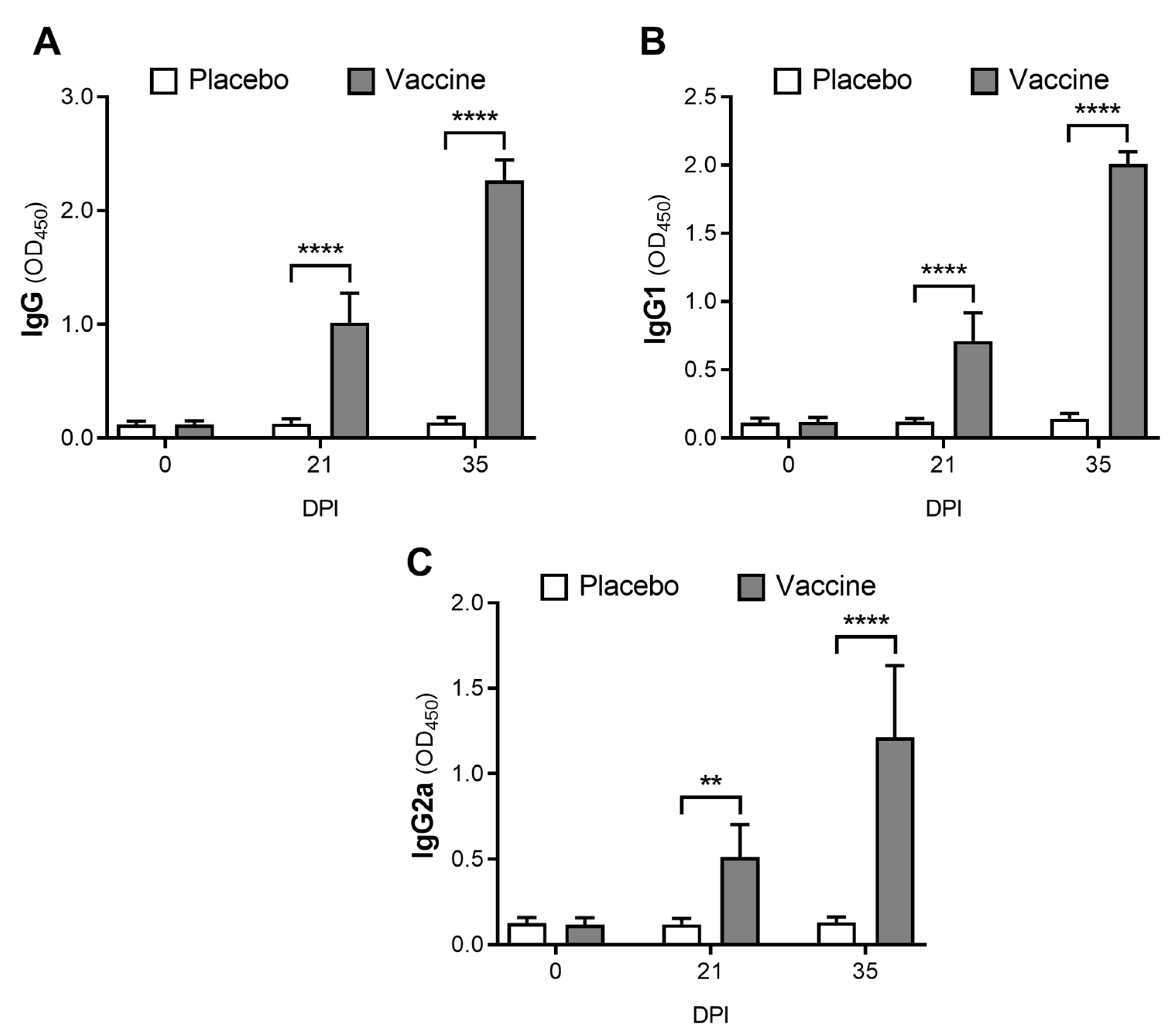
3.2. The Novel Vaccine Induces a Systemic Immune Response
3.3. The Proteoliposome Vaccine Induces a Local Immune Response
3.4. Vaccination Protects Mice from Induced E. coli Clinical Mastitis and Reduces the Bacterial Load in Mammary Glands
3.5. Vaccination Decreases Mammary Inflammation and Tissue Damage
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Klaas, I.C.; Zadoks, R.N. An update on environmental mastitis: Challenging perceptions. Transbound. Emerg. Dis. 2018, 65, 166–185. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.; Henriques, M. Control of Bovine Mastitis: Old and Recent therapeutic approaches. Curr. Microbiol. 2016, 72, 377–382. [Google Scholar] [CrossRef]
- Kaniyamattam, K.; De Vries, A.; Tauer, L.W.; Gröhn, Y.T. Economics of reducing antibiotic usage for clinical mastitis and metritis through genomic selection. J. Dairy Sci. 2020, 103, 473–491. [Google Scholar] [CrossRef] [PubMed]
- Jamali, H.; Barkema, H.W.; Jacques, M.; Lavallée-Bourget, E.M.; Malouin, F.; Saini, V.; Stryhn, H.; Dufour, S. Invited review: Incidence, risk factors, and effects of clinical mastitis recurrence in dairy cows. J. Dairy Sci. 2018, 101, 4729–4746. [Google Scholar] [CrossRef] [PubMed]
- Petersson-Wolfe, C.S.; Leslie, K.E.; Swartz, T.H. An update on the effect of clinical mastitis on the welfare of dairy cows and potential therapies. Vet. Clin. North Am. Food Anim. Pract. 2018, 34, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Sharun, K.; Dhama, K.; Tiwari, R.; Gugjoo, M.B.; Iqbal Yatoo, M.; Patel, S.K.; Pathak, M.; Karthik, K.; Khurana, S.K.; Singh, R.; et al. Advances in therapeutic and managemental approaches of bovine mastitis: A comprehensive review. Vet. Q. 2021, 41, 107–136. [Google Scholar] [CrossRef]
- Abdi, R.D.; Gillespie, B.E.; Ivey, S.; Pighetti, G.M.; Almeida, R.A.; Kerro Dego, O. Antimicrobial resistance of major bacterial pathogens from dairy cows with high somatic cell count and clinical mastitis. Animals 2021, 11, 131. [Google Scholar] [CrossRef]
- Nagasawa, Y.; Kiku, Y.; Sugawara, K.; Yabusaki, T.; Oono, K.; Fujii, K.; Suzuki, T.; Maehana, K.; Hayashi, T. The bacterial load in milk is associated with clinical severity in cases of bovine coliform mastitis. J. Vet. Med. Sci. 2019, 81, 107–112. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, G.; Liu, W.; Liu, Y.; Ali, T.; Chen, W.; Yin, J.; Han, B. Phylogenetic group, virulence factors and antimicrobial resistance of Escherichia coli associated with bovine mastitis. Res. Microbiol. 2014, 165, 273–277. [Google Scholar] [CrossRef]
- Blum, S.E.; Heller, E.D.; Sela, S.; Elad, D.; Edery, N.; Leitner, G. Genomic and phenomic study of mammary pathogenic Escherichia coli. PLoS ONE 2015, 10, e0136387. [Google Scholar] [CrossRef] [Green Version]
- Hoeben, D.; Burvenich, C.; Trevisi, E.; Bertoni, G.; Hamann, J.; Bruckmaier, R.M.; Blum, J.W. Role of endotoxin and TNF-alpha in the pathogenesis of experimentally induced coliform mastitis in periparturient cows. J. Dairy Res. 2000, 67, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, L.M. Mammary gland immunobiology and resistance to mastitis. Vet. Clin. North Am. Food Anim. Pract. 2018, 34, 507–523. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Xu, Y.; Lu, J.; Liu, M.; Bin, D.; Miao, J.; Yin, Y. Variant innate immune responses of mammary epithelial cells to challenge by Staphylococcus aureus, Escherichia coli and the regulating effect of taurine on these bioprocesses. Free Radic. Biol. Med. 2016, 96, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Dosogne, H.; Vangroenweghe, F.; Burvenich, C. Potential mechanism of action of J5 vaccine in protection against severe bovine coliform mastitis. Vet. Res. 2002, 33, 1–12. [Google Scholar] [CrossRef]
- Shpigel, N.Y.; Elazar, S.; Rosenshine, I. Mammary pathogenic Escherichia coli. Curr. Opin. Microbiol. 2008, 11, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Leimbach, A.; Poehlein, A.; Vollmers, J.; Görlich, D.; Daniel, R.; Dobrindt, U. No evidence for a bovine mastitis Escherichia coli pathotype. BMC Genom. 2017, 18, 359. [Google Scholar] [CrossRef]
- Burvenich, C.; Van Merris, V.; Mehrzad, J.; Diez-Fraile, A.; Duchateu, L. Severity of E. coli mastitis is mainly determined by cow factors. Vet. Res. 2003, 34, 521–564. [Google Scholar] [CrossRef]
- Jiang, L.; Sørensen, P.; Røntved, C.; Vels, L.; Ingvartsen, K.L. Gene expression profiling of liver from dairy cows treated intra-mammary with lipopolysaccharide. BMC Genom. 2008, 9, 443. [Google Scholar] [CrossRef]
- Zhao, X.; Lacasse, P. Mammary tissue damage during bovine mastitis: Causes and control. J. Anim. Sci. 2008, 86, 57–65. [Google Scholar] [CrossRef]
- Blum, S.E.; Heller, E.D.; Jacoby, S.; Krifucks, O.; Leitner, G. Comparison of the immune responses associated with experimental bovine mastitis caused by different strains of Escherichia Coli. J. Dairy Res. 2017, 84, 190–197. [Google Scholar] [CrossRef]
- Hogan, J.; Smith, K.L. Coliform mastitis. Vet. Res. 2003, 34, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Furuya, Y.; Regner, M.; Lobigs, M.; Koskinen, A.; Müllbacher, A.; Alsharifi, M. Effect of inactivation method on the cross-protective immunity induced by whole “killed” influenza A viruses and commercial vaccine preparations. J. Gen. Virol. 2010, 91, 1450–1460. [Google Scholar] [CrossRef] [PubMed]
- Kon, T.C.; Onu, A.; Berbecila, L.; Lupulescu, E.; Ghiorgisor, A.; Kersten, G.F.; Cui, Y.-Q.; Amorij, J.-P.; Van der Pol, L. Influenza vaccine manufacturing: Effect of inactivation, splitting and site of manufacturing. comparison of influenza vaccine production processes. PLoS ONE 2016, 11, e0150700. [Google Scholar] [CrossRef] [PubMed]
- Di Tommaso, A.; De Magistris, M.T.; Bugnoli, M.; Marsili, I.; Rappuoli, R.; Abrignani, S. Formaldehyde treatment of proteins can constrain presentation to T cells by limiting antigen processing. Infect. Immun. 1994, 62, 1830–1834. [Google Scholar] [CrossRef] [PubMed]
- Steele, N.M.; Swartz, T.H.; Enger, K.M.; Schramm, H.; Cockrum, R.R.; Lacy-Hulbert, S.J.; White, R.R.; Hogan, J.; Petersson-Wolfe, C.S. The effect of J5 bacterins on clinical, behavioral, and antibody response following an Escherichia coli intramammary challenge in dairy cows at peak lactation. J. Dairy Sci. 2019, 102, 11233–11249. [Google Scholar] [CrossRef]
- Kawai, K.; Kondo, Y.; Shinozuka, Y.; Kawata, R.; Kaneko, S.; Iwano, H.; Enokidani, M.; Watanabe, A.; Yuliza-Purba, F.; Isobe, N.; et al. Immune response during the onset of coliform mastitis in dairy cows vaccinated with STARTVAC®. Anim. Sci. J. 2021, 92, e13502. [Google Scholar] [CrossRef]
- Vangroenweghe, F.; Duchateau, L.; Burvenich, C. Short communication: J-5 Escherichia coli vaccination does not influence severity of an Escherichia coli intramammary challenge in primiparous cows. J. Dairy Sci. 2020, 103, 6692–6697. [Google Scholar] [CrossRef]
- Jorge, S.; Dellagostin, O.A. The development of veterinary vaccines: A review of traditional methods and modern biotechnology approaches. Biotechnol. Res. Innov. 2017, 1, 6–13. [Google Scholar] [CrossRef]
- Day, M.J. Vaccine side effects: Fact and fiction. Vet. Microbiol. 2006, 117, 51–58. [Google Scholar] [CrossRef]
- Kallerup, R.S.; Foged, C. Chapter 2. Classification of vaccines. In Subunit Vaccine Delivery; Foged, C., Rades, T., Perrie, Y., Hook, S., Eds.; Springer: New York, NY, USA, 2015; pp. 15–30. ISBN 978-1-4939-1416-6. [Google Scholar]
- Caruana, J.C.; Walper, S.A. Bacterial membrane vesicles and their applications as vaccines and in biotechnology. In Bacterial Membrane Vesicles; Kaparakis-Liaskos, M., Kufer, T.A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 219–251. ISBN 978-3-030-36331-4. [Google Scholar]
- Nishikawa, H.; Sasaki, M.; Nishiyama, K. In vitro assay for bacterial membrane protein integration into proteoliposomes. Bio-Protocol 2020, 10, e3626. [Google Scholar] [CrossRef]
- Parmar, M.M.; Edwards, K.; Madden, T.D. Incorporation of bacterial membrane proteins into liposomes: Factors influencing protein reconstitution. Biochim. Biophys. Acta Biomembr. 1999, 1421, 77–90. [Google Scholar] [CrossRef]
- Vartak, A.; Sucheck, S. Recent advances in subunit vaccine carriers. Vaccines 2016, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Ellis, T.N.; Kuehn, M.J. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol. Mol. Biol. Rev. 2010, 74, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Zhang, Y.; Wang, H.; Jin, J.; Piao, J.; Piao, J.; Liu, Q.; Li, W. Reduction of salmonella enteritidis number after infections by immunization of liposome-associated recombinant SefA. Avian Dis. 2013, 57, 627–633. [Google Scholar] [CrossRef]
- Roberts, R.; Moreno, G.; Bottero, D.; Gaillard, M.E.; Fingermann, M.; Graieb, A.; Rumbo, M.; Hozbor, D. Outer membrane vesicles as acellular vaccine against pertussis. Vaccine 2008, 26, 4639–4646. [Google Scholar] [CrossRef]
- Caruffo, M.; Vidal, S.; Santis, L.; Siel, D.; Pérez, O.; Huenchullan, P.R.; Sáenz, L. Effectiveness of a proteoliposome-based vaccine against salmonid rickettsial septicaemia in Oncorhynchus mykiss. Vet. Res. 2021, 52, 111. [Google Scholar] [CrossRef]
- Lee, C.H.; Frasch, C.E. Quantification of bacterial polysaccharides by the purpald assay: Measurement of periodate-generated formaldehyde from glycol in the repeating unit. Anal. Biochem. 2001, 296, 73–82. [Google Scholar] [CrossRef]
- Cheng, H.R.; Jiang, N. Extremely rapid extraction of DNA from bacteria and yeasts. Biotechnol. Lett. 2006, 28, 55–59. [Google Scholar] [CrossRef]
- Siel, D.; Loaiza, A.; Vidal, S.; Caruffo, M.; Paredes, R.; Ramirez, G.; Lapierre, L.; Briceño, C.; Pérez, O.; Sáenz, L. The immune profile induced is crucial to determine the effects of immunocastration over gonadal function, fertility, and GnRH-I expression. Am. J. Reprod. Immunol. 2018, 79, e12772. [Google Scholar] [CrossRef]
- Chandler, R.L. Experimental bacterial mastitis in the mouse. J. Med. Microbiol. 1970, 3, 273–282. [Google Scholar] [CrossRef]
- Morton, D.; Griffiths, P. Guidelines on the recognition of pain, distress and discomfort in experimental animals and an hypothesis for assessment. Vet. Rec. 1985, 116, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Pusey, P.N.; Zaccarelli, E.; Valeriani, C.; Sanz, E.; Poon, W.C.K.; Cates, M.E. Hard spheres: Crystallization and glass formation. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2009, 367, 4993–5011. [Google Scholar] [CrossRef] [PubMed]
- Kirby, B.J.; Hasselbrink, E.F. Zeta potential of microfluidic substrates: 1. Theory, experimental techniques, and effects on separations. Electrophoresis 2004, 25, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Ghaffar, K.; Giddam, A.; Zaman, M.; Skwarczynski, M.; Toth, I. Liposomes as nanovaccine delivery systems. Curr. Top. Med. Chem. 2014, 14, 1194–1208. [Google Scholar] [CrossRef]
- Schwendener, R.A. Liposomes as vaccine delivery systems: A review of the recent advances. Ther. Adv. Vaccines 2014, 2, 159–182. [Google Scholar] [CrossRef]
- Kastner, E.; Schmidt, S.T.; Wilkinson, A.; Christensen, D.; Perrie, Y. Chapter 5: The application of liposomes as vaccine adjuvants. In Subunit Vaccine Delivery, Advances in Delivery Science and Technology; Foged, C., Ed.; Springer Science & Business Media: New York, NY, USA, 2015; pp. 77–94. ISBN 9781493914173. [Google Scholar]
- Wojnicz, D.; Cisowska, A. Composition of the outer membrane proteins of Escherichia coli strains in relation to serum susceptibility after exposure to subinhibitory concentrations of amikacin and ciprofloxacin. Int. J. Antimicrob. Agents 2009, 33, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Agafitei, O.; Kim, E.J.; Maguire, T.; Sheridan, J. The role of Escherichia coli porins OmpC and OmpF in antibiotic cross resistance induced by sub-inhibitory concentrations of kanamycin. J. Exp. Microbiol. Immunol. 2010, 14, 34–39. [Google Scholar]
- Tie, Z.; Chun-guang, W.; Xing-hua, Z. Clinical isolating outer membrane protein pattern from Avian Escherichia coli of China. J. Microbiol. 2010, 4, 4–7. [Google Scholar]
- Liu, C.; Chen, Z.; Tan, C.; Liu, W.; Xu, Z.; Zhou, R.; Chen, H. Immunogenic characterization of outer membrane porins OmpC and OmpF of porcine extraintestinal pathogenic Escherichia coli. FEMS Microbiol. Lett. 2012, 337, 104–111. [Google Scholar] [CrossRef]
- Urban-Chmiel, R.; Dec, M.; Puchalski, A.; Wernicki, A. Characterization of heat-shock proteins in Escherichia coli strains under thermal stress in vitro. J. Med. Microbiol. 2013, 62, 1897–1901. [Google Scholar] [CrossRef]
- Dehghani, B.; Mottamedifar, M.; Khoshkharam-Roodmajani, H.; Hassanzadeh, A.; Zomorrodian, K.; Rahimi, A. SDS-PAGE analysis of the outer membrane proteins of uropathogenic Escherichia coli isolated from patients in different wards of Nemazee Hospital, Shiraz, Iran. Iran. J. Med. Sci. 2016, 41, 399–405. [Google Scholar]
- Wang, X.; Teng, D.; Guan, Q.; Mao, R.; Hao, Y.; Wang, X.; Yao, J.; Wang, J. Escherichia coli outer membrane protein F (OmpF): An immunogenic protein induces cross-reactive antibodies against Escherichia coli and Shigella. AMB Express 2017, 7, 155. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, N.; Rong, N.; Kang, C.; Chen, C.; Wu, S.; Liu, X.; Zhang, X. Immunoprotective evaluation of Escherichia coli outer membrane protein A against the main pathogens of animal mastitis. Trop. J. Pharm. Res. 2020, 19, 155–162. [Google Scholar] [CrossRef]
- Pen, G.; Yang, N.; Teng, D.; Hao, Y.; Mao, R.; Wang, J. The outer membrane proteins and their synergy triggered the protective effects against pathogenic Escherichia coli. Microorganisms 2022, 10, 982. [Google Scholar] [CrossRef]
- Liu, X.; Sun, W.; Wu, N.; Rong, N.; Kang, C.; Jian, S.; Chen, C.; Chen, C.; Zhang, X. Synthesis of Escherichia coli OmpA oral nanoparticles and evaluation of immune functions against the major etiologic agent of cow mastitis. Vaccines 2021, 9, 304. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Campos, C.; Burguete-García, A.I.; Madrid-Marina, V. Role of TLR9 in oncogenic virus-produced cancer. Viral Immunol. 2017, 30, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Deng, G.M. The role of bacterial DNA containing CpG motifs in diseases. J. Leukoc. Biol. 2021, 109, 991–998. [Google Scholar] [CrossRef]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef]
- Manolova, V.; Flace, A.; Bauer, M.; Schwarz, K.; Saudan, P.; Bachmann, M.F. Nanoparticles target distinct dendritic cell populations according to their size. Eur. J. Immunol. 2008, 38, 1404–1413. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M. Biomimetic nanoparticles: Preparation, characterization and biomedical applications. Int. J. Nanomed. 2010, 5, 249–259. [Google Scholar] [CrossRef]
- Shah, R.R.; O’hagan, D.T.; Amiji, M.M.; Brito, L.A. The impact of size on particulate vaccine adjuvants. Nanomedicine 2014, 9, 2671–2681. [Google Scholar] [CrossRef]
- Mann, J.F.S.; Shakir, E.; Carter, K.C.; Mullen, A.B.; Alexander, J.; Ferro, V.A. Lipid vesicle size of an oral influenza vaccine delivery vehicle influences the Th1/Th2 bias in the immune response and protection against infection. Vaccine 2009, 27, 3643–3649. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, A.S. Biophysical aspects of using liposomes as delivery vehicles. Biosci. Rep. 2002, 22, 129–150. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gong, T.; Wang, C.; Zhong, Z.; Zhang, Z. Solid lipid nanoparticles loaded with insulin by sodium cholate-phosphatidylcholine-based mixed micelles: Preparation and characterization. Int. J. Pharm. 2007, 340, 153–162. [Google Scholar] [CrossRef]
- Bachmann, M.F.; Jennings, G.T. Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010, 10, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems—A review (Part 1). Trop. J. Pharm. Res. 2013, 12, 255–264. [Google Scholar] [CrossRef]
- Rigotti, A.; Acton, S.L.; Krieger, M. The class B scavenger receptors SR-BI and CD36 are receptors for anionic phospholipids. J. Biol. Chem. 1995, 270, 16221–16224. [Google Scholar] [CrossRef]
- Mamo, W.; Jonsson, P.; Flock, J.I.; Lindberg, M.; Müller, H.P.; Wadström, T.; Nelson, L. Vaccination against Staphylococcus aureus mastitis: Immunological response of mice vaccinated with fibronectin-binding protein (FnBP-A) to challenge with S. aureus. Vaccine 1994, 12, 988–992. [Google Scholar] [CrossRef]
- Gómez, M.I.; García, V.E.; Gherardi, M.M.; Cerquetti, M.C.; Sordelli, D.O. Intramammary immunization with live-attenuated Staphylococcus aureus protects mice from experimental mastitis. FEMS Immunol. Med. Microbiol. 1998, 20, 21–27. [Google Scholar] [CrossRef]
- Diarra, M.S.; Petitclerc, D.; Deschênes, É.; Lessard, N.; Grondin, G.; Talbot, B.G.; Lacasse, P. Lactoferrin against Staphylococcus aureus Mastitis: Lactoferrin alone or in combination with penicillin G on bovine polymorphonuclear function and mammary epithelial cells colonisation by Staphylococcus aureus. Vet. Immunol. Immunopathol. 2003, 95, 33–42. [Google Scholar] [CrossRef]
- Leitner, G.; Lubashevsky, E.; Trainin, Z. Staphylococcus aureus vaccine against mastitis in dairy cows, composition and evaluation of its immunogenicity in a mouse model. Vet. Immunol. Immunopathol. 2003, 93, 159–167. [Google Scholar] [CrossRef]
- Brouillette, E.; Grondin, G.; Lefebvre, C.; Talbot, B.G.; Malouin, F. Mouse mastitis model of infection for antimicrobial compound efficacy studies against intracellular and extracellular forms of Staphylococcus aureus. Vet. Microbiol. 2004, 101, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Castagliuolo, I.; Piccinini, R.; Beggiao, E.; Palù, G.; Mengoli, C.; Ditadi, F.; Vicenzoni, G.; Zecconi, A. Mucosal genetic immunization against four adhesins protects against Staphylococcus aureus-induced mastitis in mice. Vaccine 2006, 24, 4393–4402. [Google Scholar] [CrossRef] [PubMed]
- Gong, R.; Hu, C.; Xu, H.; Guo, A.; Chen, H.; Zhang, G.; Shi, L. Evaluation of clumping factor a binding region a in a subunit vaccine against Staphylococcus aureus-induced mastitis in mice. Clin. Vaccine Immunol. 2010, 17, 1746–1752. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Hu, C.; Gong, R.; Chen, Y.; Ren, N.; Xiao, G.; Xie, Q.; Zhang, M.; Liu, Q.; Guo, A.; et al. Evaluation of a novel chimeric B cell epitope-based vaccine against mastitis induced by either Streptococcus agalactiae or Staphylococcus aureus in mice. Clin. Vaccine Immunol. 2011, 18, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Watson, A.D.; Kerr, D.E. Genome-wide expression analysis of lipopolysaccharide-induced mastitis in a mouse model. Infect. Immun. 2006, 74, 1907–1915. [Google Scholar] [CrossRef] [PubMed]
- Glynn, D.J.; Hutchinson, M.R.; Ingman, W.V. Toll-like receptor 4 regulates lipopolysaccharide-induced inflammation and lactation insufficiency in a mouse model of mastitis. Biol. Reprod. 2014, 90, 91. [Google Scholar] [CrossRef]
- Xiao, H.B.; Wang, C.R.; Liu, Z.K.; Wang, J.Y. LPS induces pro-inflammatory response in mastitis mice and mammary epithelial cells: Possible involvement of NF-κB signaling and OPN. Pathol. Biol. 2015, 63, 11–16. [Google Scholar] [CrossRef]
- Camussone, C.M.; Reidel, I.G.; Molineri, A.I.; Cicotello, J.; Miotti, C.; Suarez Archilla, G.A.; Curti, C.C.; Veaute, C.; Calvinho, L.F. Efficacy of immunization with a recombinant S. aureus vaccine formulated with liposomes and ODN-CpG against natural S. aureus intramammary infections in heifers and cows. Res. Vet. Sci. 2022, 145, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Reidel, I.G.; Camussone, C.; Suarez Archilla, G.A.; Calvinho, L.F.; Veaute, C. Liposomal and CpG-ODN formulation elicits strong humoral immune responses to recombinant Staphylococcus aureus antigens in heifer calves. Vet. Immunol. Immunopathol. 2019, 212, 1–8. [Google Scholar] [CrossRef]
- Reidel, I.; Giorello, A.; Calvinho, L.F.; Gennaro, A.M. Effects of the liposomal co-encapsulation of antigen and PO-CpG oligonucleotide on immune response in mice. Int. J. Res. Appl. Nat. Sci. 2017, 3, 1–19. [Google Scholar]
- Burton, J.L.; Erskine, R.J. Immunity and mastitis Some new ideas for an old disease. Vet. Clin. North Am. Food Anim. Pract. 2003, 19, 1–45. [Google Scholar] [CrossRef]
- Tizard, I. Antibodies. In Veterinary Immunology; Tizard, I., Ed.; Elsevier: St. Louis, MO, USA, 2018; pp. 474–503. ISBN 978-0-323-52349-3. [Google Scholar]
- Trinchieri, G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003, 3, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015, 16, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004, 5, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Suresh, R.; Mosser, D.M. Pattern recognition receptors in innate immunity, host defense, and immunopathology. Am. J. Physiol. Adv. Physiol. Educ. 2013, 37, 284–291. [Google Scholar] [CrossRef]
- Kim, D.; Kwon, S.; Ahn, C.S.; Lee, Y.; Choi, S.Y.; Park, J.; Kwon, H.Y.; Kwon, H.J. Adjuvant effect of liposome-encapsulated natural phosphodiester CpG-DNA. BMB Rep. 2011, 44, 758–763. [Google Scholar] [CrossRef]
- García, V.; Gómez, M.; Iglesias, M.; Sanjuan, N.; Gherardi, M.; Cerquetti, M.C.; Sordelli, D. Intramammary immunization with live-attenuated Staphylococcus Aureus: Microbiological and immunological studies in a mouse mastitis model. FEMS Immunol. Med. Microbiol. 1996, 14, 45–51. [Google Scholar] [CrossRef]
- Middleton, J.R.; Ma, J.; Rinehart, C.L.; Taylor, V.N.; Luby, C.D.; Steevens, B.J. Efficacy of different LysiginTM formulations in the prevention of Staphylococcus aureus intramammary infection in dairy heifers. J. Dairy Res. 2006, 73, 10–19. [Google Scholar] [CrossRef]
- Burvenich, C.; Bannerman, D.D.; Lippolis, J.D.; Peelman, L.; Nonnecke, B.J.; Kehrli, M.E., Jr.; Paape, M.J. Cumulative physiological events influence the inflammatory response of the bovine udder to Escherichia coli infections during the transition period. J. Dairy Sci. 2007, 90, E39–E54. [Google Scholar] [CrossRef]
- Elazar, S.; Gonen, E.; Livneh-Kol, A.; Rosenshine, I.; Shpigel, N.Y. Neutrophil recruitment in endotoxin-induced murine mastitis is strictly dependent on mammary alveolar macrophages. Vet. Res. 2010, 41, 10. [Google Scholar] [CrossRef] [PubMed]
- Elazar, S.; Gonen, E.; Livneh-Kol, A.; Rosenshine, I.; Shpigel, N.Y. Essential role of neutrophils but not mammary alveolar macrophages in a murine model of acute Escherichia coli mastitis. Vet. Res. 2010, 41, 53. [Google Scholar] [CrossRef] [PubMed]
- Porcherie, A.; Gilbert, F.B.; Germon, P.; Cunha, P.; Trotereau, A.; Rossignol, C.; Winter, N.; Berthon, P.; Rainard, P. IL-17A is an important effector of the immune response of the mammary gland to Escherichia coli infection. J. Immunol. 2016, 196, 803–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Molecule | Quantification Result |
|---|---|
| Proteins (µg/mL) | 5596.14 ± 509.05 |
| DNA (ng/mL) | 1102.22 ± 114.72 |
| Lipopolysaccharide (LPS) (mg/mL) | 97.89 ± 7.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quiroga, J.; Vidal, S.; Siel, D.; Caruffo, M.; Valdés, A.; Cabrera, G.; Lapierre, L.; Sáenz, L. Novel Proteoliposome-Based Vaccine against E. coli: A Potential New Tool for the Control of Bovine Mastitis. Animals 2022, 12, 2533. https://doi.org/10.3390/ani12192533
Quiroga J, Vidal S, Siel D, Caruffo M, Valdés A, Cabrera G, Lapierre L, Sáenz L. Novel Proteoliposome-Based Vaccine against E. coli: A Potential New Tool for the Control of Bovine Mastitis. Animals. 2022; 12(19):2533. https://doi.org/10.3390/ani12192533
Chicago/Turabian StyleQuiroga, John, Sonia Vidal, Daniela Siel, Mario Caruffo, Andrea Valdés, Gonzalo Cabrera, Lissette Lapierre, and Leonardo Sáenz. 2022. "Novel Proteoliposome-Based Vaccine against E. coli: A Potential New Tool for the Control of Bovine Mastitis" Animals 12, no. 19: 2533. https://doi.org/10.3390/ani12192533






