Multimodal Cues Do Not Improve Predator Recognition in Green Toad Tadpoles
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals Collection
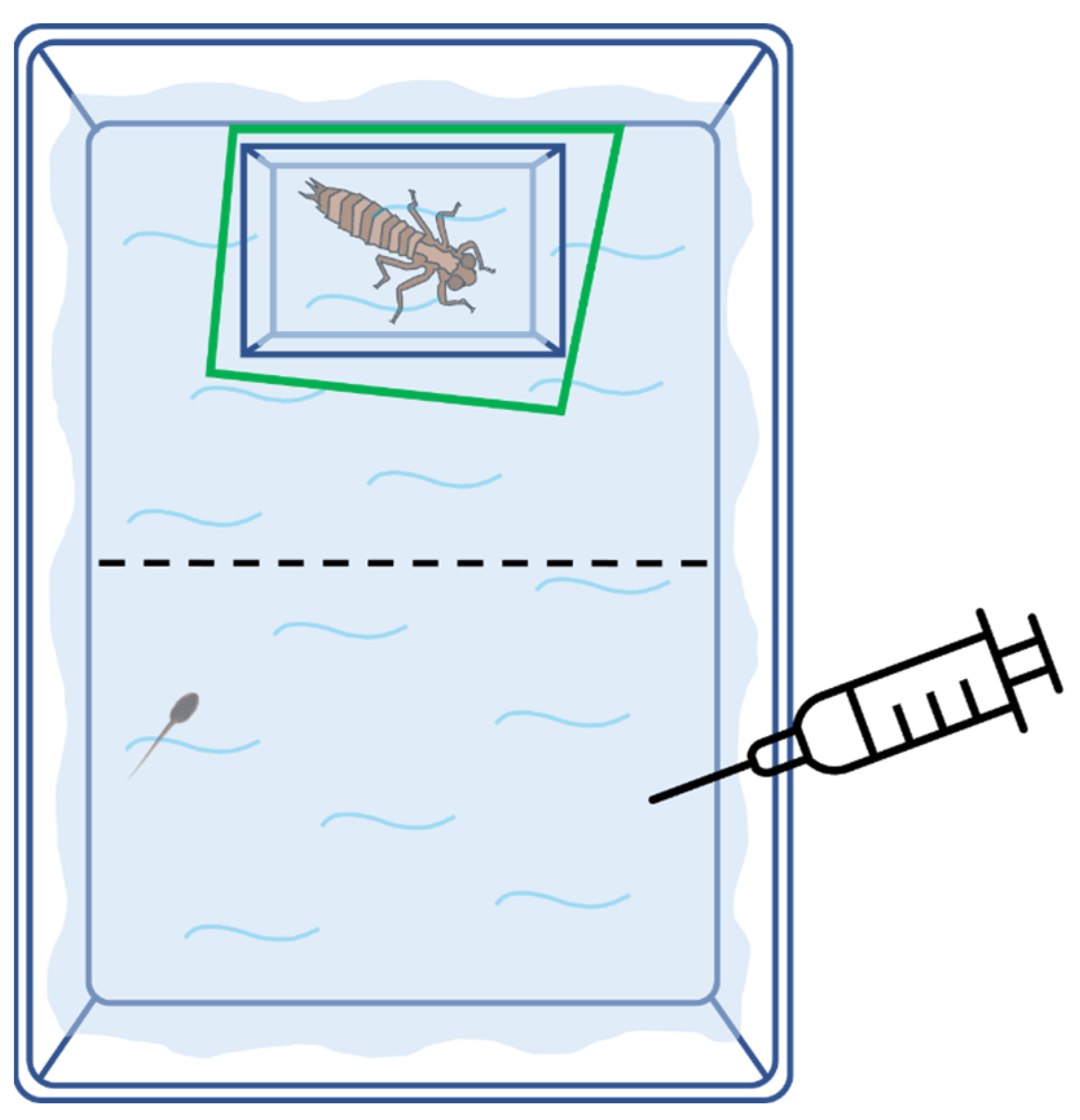
2.2. Experimental Design
2.3. Data Collection and Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lima, S.L.; Dill, L.M. Behavioral Decisions Made under the Risk of Predation: A Review and Prospectus. Can. J. Zool. 1990, 68, 619–640. [Google Scholar] [CrossRef]
- Lima, S.L.; Steury, T.D. Perception of Risk: The Foundation of Non-Lethal Predator-Prey Interactions. In The Ecology of Predator-Prey Interactions; Barbosa, P., Castellanos, I., Eds.; Oxford University Press: Oxford, UK, 2005; pp. 166–188. [Google Scholar]
- Kats, L.B.; Dill, L.M. The Scent of Death: Chemosensory Assessment of Predation Risk by Prey Animals. Écoscience 1998, 5, 361–394. [Google Scholar] [CrossRef]
- Brown, G.E.; Ferrari, M.C.O.; Chivers, D.P. Learning about Danger: Chemical Alarm Cues and Threat-Sensitive Assessment of Predation Risk by Fishes. In Fish Cognition and Behavior; Brown, C., Laland, K., Krause, J., Eds.; Wiley: Hoboken, NJ, USA, 2011; pp. 59–80. [Google Scholar] [CrossRef]
- Blumstein, D.T.; Bouskila, A. Assessment and Decision Making in Animals: A Mechanistic Model Underlying Behavioral Flexibility Can Prevent Ambiguity. Oikos 1996, 77, 569. [Google Scholar] [CrossRef]
- Schul, J.; Patterson, A.C. What Determines the Tuning of Hearing Organs and the Frequency of Calls? A Comparative Study in the Katydid Genus Neoconocephalus (Orthoptera, Tettigoniidae). J. Exp. Biol. 2003, 206, 141–152. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Surlykke, A.; Skals, N.; Rydell, J.; Svensson, M. Sonic Hearing in a Diurnal Geometrid Moth, Archiearis parthenias, Temporally Isolated From Bats. Naturwissenschaften 1998, 85, 36–37. [Google Scholar] [CrossRef]
- Brechbühl, J.; Moine, F.; Klaey, M.; Nenniger-Tosato, M.; Hurni, N.; Sporkert, F.; Giroud, C.; Broillet, M.-C. Mouse Alarm Pheromone Shares Structural Similarity with Predator Scents. Proc. Natl. Acad. Sci. USA 2013, 110, 4762–4767. [Google Scholar] [CrossRef]
- Peacor, S.D. Behavioural Response of Bullfrog Tadpoles to Chemical Cues of Predation Risk Are Affected by Cue Age and Water Source. Hydrobiologia 2006, 573, 39–44. [Google Scholar] [CrossRef]
- Mogali, S.M.; Shanbhag, B.A.; Saidapur, S.K. Strong Food Odours Mask Predation Risk and Affect Evocation of Defence Behaviours in the Tadpoles of Sphaerotheca breviceps. J. Ethol. 2015, 33, 41–46. [Google Scholar] [CrossRef]
- Scribano, G.; Balestrieri, A.; Gazzola, A.; Pellitteri-Rosa, D. Strong Behavioural Defensive Responses of Endemic Rana latastei Tadpoles Induced by a Native Predator’s Odour. Ethology 2020, 126, 922–930. [Google Scholar] [CrossRef]
- Ferrari, M.C.O.; Wisenden, B.D.; Chivers, D.P. Chemical Ecology of Predator–Prey Interactions in Aquatic Ecosystems: A Review and Prospectus. Can. J. Zool. 2010, 88, 698–724. [Google Scholar] [CrossRef]
- Hettyey, A.; Tóth, Z.; Thonhauser, K.E.; Frommen, J.G.; Penn, D.J.; Van Buskirk, J. The Relative Importance of Prey-Borne and Predator-Borne Chemical Cues for Inducible Antipredator Responses in Tadpoles. Oecologia 2015, 179, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Hettyey, A.; Rölli, F.; Thürlimann, N.; Zürcher, A.-C.; Van Buskirk, J. Visual Cues Contribute to Predator Detection in Anuran Larvae. Biol. J. Linn. Soc. Lond. 2012, 106, 820–827. [Google Scholar] [CrossRef]
- Dicke, M.; Grostal, P. Chemical Detection of Natural Enemies by Arthropods: An Ecological Perspective. Annu. Rev. Ecol. Syst. 2001, 32, 1–23. [Google Scholar] [CrossRef]
- Manteuffel, G.; Wess, O.; Himstedt, W. Messungen Am Dioptrischen Apparat von Amphibienaugen Und Berechnung Der Sehrschärfe Im Wasser Und Luft. Zool. Jahrb. Abt. Allg. Zool. Physiol. Tierre 1977, 81, 395–406. [Google Scholar]
- Von Seckendorff Hoff, K.; Blaustein, A.R.; McDiarmid, R.W.; Altig, R. Behavior: Interactions and Their Consequences. In Tadpoles: The Biology of Anuran Larvae; McDarmid, R., Altig, R., Eds.; The University of Chicago Press: Chicago, IL, USA, 1999; pp. 215–239. [Google Scholar]
- Mathis, A.; Vincent, F. Differential Use of Visual and Chemical Cues in Predator Recognition and Threat-Sensitive Predator-Avoidance Responses by Larval Newts (Notophthalmus viridescens). Can. J. Zool. 2000, 78, 1646–1652. [Google Scholar] [CrossRef]
- Wassersug, R.J.; Lum, A.M.; Potel, M.J. An Analysis of School Structure for Tadpoles (Anura: Amphibia). Behav. Ecol. Sociobiol. 1981, 9, 15–22. [Google Scholar] [CrossRef]
- Gouchie, G.M.; Roberts, L.F.; Wassersug, R.J. The Effect of Mirrors on African Clawed Frog (Xenopus laevis) Larval Growth, Development, and Behavior. Behav. Ecol. Sociobiol. 2008, 62, 1821–1829. [Google Scholar] [CrossRef]
- Balestrieri, A.; Gazzola, A.; Pellitteri-Rosa, D.; Vallortigara, G. Discrimination of Group Numerousness under Predation Risk in Anuran Tadpoles. Anim. Cogn. 2019, 22, 223–230. [Google Scholar] [CrossRef]
- Fischer, S.; Oberhummer, E.; Cunha-Saraiva, F.; Gerber, N.; Taborsky, B. Smell or Vision? The Use of Different Sensory Modalities in Predator Discrimination. Behav. Ecol. Sociobiol. 2017, 71, 143. [Google Scholar] [CrossRef]
- Van Buskirk, J. Specific Induced Responses to Different Predator Species in Anuran Larvae. J. Evolut. Biol. 2001, 14, 482–489. [Google Scholar] [CrossRef]
- Brown, T.A.; Fraker, M.E.; Ludsin, S.A. Space Use of Predatory Larval Dragonflies and Tadpole Prey in Response to Chemical Cues of Predation. Am. Midl. Nat. 2019, 181, 53. [Google Scholar] [CrossRef]
- Gazzola, A.; Russo, G.; Balestrieri, A. Embryonic and Larval Defensive Responses of Agile Frog (Rana dalmatina) to Alien Crayfish. Ethology 2018, 124, 347–356. [Google Scholar] [CrossRef]
- Gazzola, A.; Balestrieri, A.; Scribano, G.; Fontana, A.; Pellitteri-Rosa, D. Contextual Behavioural Plasticity in Italian Agile Frog (Rana latastei) Tadpoles Exposed to Native and Alien Predator Cues. J. Exp. Biol. 2021, 224, jeb240465. [Google Scholar] [CrossRef] [PubMed]
- Smithson, M.; Verkuilen, J. A Better Lemon Squeezer? Maximum-Likelihood Regression with Beta-Distributed Dependent Variables. Psychol. Methods 2006, 11, 54–71. [Google Scholar] [CrossRef] [PubMed]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Mächler, M.; Bolker, B.M. GlmmTMB Balances Speed and Flexibility Among Packages for Zero-Inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378. [Google Scholar] [CrossRef]
- Lenth, R.V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.7.3. Available online: https://CRAN.R–project.org/package=emmeans (accessed on 22 August 2022).
- Guadin, B.; Gazzola, A.; Balestrieri, A.; Scribano, G.; Martín, J.; Pellitteri-Rosa, D. Effects of a Group-Living Experience on the Antipredator Responses of Individual Tadpoles. Anim. Behav. 2021, 180, 93–99. [Google Scholar] [CrossRef]
- Mitchell, M.D.; Bairos-Novak, K.R.; Ferrari, M.C.O. Mechanisms Underlying the Control of Responses to Predator Odours in Aquatic Prey. J. Exp. Biol. 2017, 220, 1937–1946. [Google Scholar] [CrossRef]
- Stauffer, H.-P.; Semlitsch, R.D. Effects of Visual, Chemical and Tactile Cues of Fish on the Behavioural Responses of Tadpoles. Anim. Behav. 1993, 46, 355–364. [Google Scholar] [CrossRef]
- Kiesecker, J.M.; Chivers, D.P.; Blaustein, A.R. The Use of Chemical Cues in Predator Recognition by Western Toad Tadpoles. Anim. Behav. 1996, 52, 1237–1245. [Google Scholar] [CrossRef]
- Jowers, M.J.; Campbell-Palmer, R.; Walsh, P.T.; Downie, J.R. Intraspecific Variation in the Avoidance Response of Stream Frog (Mannophryne trinitatis) Tadpoles to Fish and Prawn Predators. Herpetol. J. 2006, 16, 337–346. [Google Scholar]
- Parris, M.J.; Reese, E.; Storfer, A. Antipredator Behavior of Chytridiomycosis-Infected Northern Leopard Frog (Rana pipiens) Tadpoles. Can. J. Zool. 2006, 84, 58–65. [Google Scholar] [CrossRef]
- Saidapur, S.K.; Veeranagoudar, D.K.; Hiragond, N.C.; Shanbhag, B.A. Mechanism of Predator–Prey Detection and Behavioral Responses in Some Anuran Tadpoles. Chemoecology 2009, 19, 21–28. [Google Scholar] [CrossRef]
- Szabo, B.; Mangione, R.; Rath, M.; Pašukonis, A.; Reber, S.A.; Oh, J.; Ringler, M.; Ringler, E. Naive Poison Frog Tadpoles Use Bi-Modal Cues to Avoid Insect Predators but Not Heterospecific Predatory Tadpoles. J. Exp. Biol. 2021, 224, jeb243647. [Google Scholar] [CrossRef] [PubMed]
- Lanoo, M.J. Integration: Nervous and Sensory Systems. In Tadpoles: The Biology of Anuran Larvae; McDarmid, R., Altig, R., Eds.; The University of Chicago Press: Chicago, IL, USA, 1999; pp. 148–169. [Google Scholar]
- Gonzalo, A.; López, P.; Martín, J. Iberian Green Frog Tadpoles May Learn to Recognize Novel Predators From Chemical Alarm Cues of Conspecifics. Anim. Behav. 2007, 74, 447–453. [Google Scholar] [CrossRef]
- Ferrari, M.C.O. Short-Term Environmental Variation in Predation Risk Leads to Differential Performance in Predation-Related Cognitive Function. Anim. Behav. 2014, 95, 9–14. [Google Scholar] [CrossRef]
- Mogali, S.M.; Saidapur, S.K.; Shanbhag, B.A. Tadpoles of the Bronze Frog (Rana temporalis) Assess Predation Risk before Evoking Antipredator Defense Behavior. J. Ethol. 2012, 30, 379–386. [Google Scholar] [CrossRef]
- Gelman, A.; Hill, J. Data Analysis Using Regression and Multilevel/Hierarchical Models, 1st ed.; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar] [CrossRef]
- Yu, T.L.; Lambert, M.R. Conspecific Visual Cues: The Relative Importance of Interference and Exploitation Competition among Tadpoles of Rana kukunoris. Ethol. Ecol. Evol. 2017, 29, 193–199. [Google Scholar] [CrossRef]
- Rot-Nikcevic, I.; Taylor, C.N.; Wassersug, R.J. The Role of Images of Conspecifics as Visual Cues in the Development and Behavior of Larval Anurans. Behav. Ecol. Sociobiol. 2006, 60, 19–25. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gazzola, A.; Guadin, B.; Balestrieri, A.; Pellitteri-Rosa, D. Multimodal Cues Do Not Improve Predator Recognition in Green Toad Tadpoles. Animals 2022, 12, 2603. https://doi.org/10.3390/ani12192603
Gazzola A, Guadin B, Balestrieri A, Pellitteri-Rosa D. Multimodal Cues Do Not Improve Predator Recognition in Green Toad Tadpoles. Animals. 2022; 12(19):2603. https://doi.org/10.3390/ani12192603
Chicago/Turabian StyleGazzola, Andrea, Bianca Guadin, Alessandro Balestrieri, and Daniele Pellitteri-Rosa. 2022. "Multimodal Cues Do Not Improve Predator Recognition in Green Toad Tadpoles" Animals 12, no. 19: 2603. https://doi.org/10.3390/ani12192603
APA StyleGazzola, A., Guadin, B., Balestrieri, A., & Pellitteri-Rosa, D. (2022). Multimodal Cues Do Not Improve Predator Recognition in Green Toad Tadpoles. Animals, 12(19), 2603. https://doi.org/10.3390/ani12192603






