Intraovarian Injection of Reconstituted Lyophilized Growth-Promoting Factor Extracted from Horse Blood Platelets (L-GFequina) Increases Oocytes Recovery and In Vitro Embryo Production in Holstein Cows
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Lyophilized L-GFequina
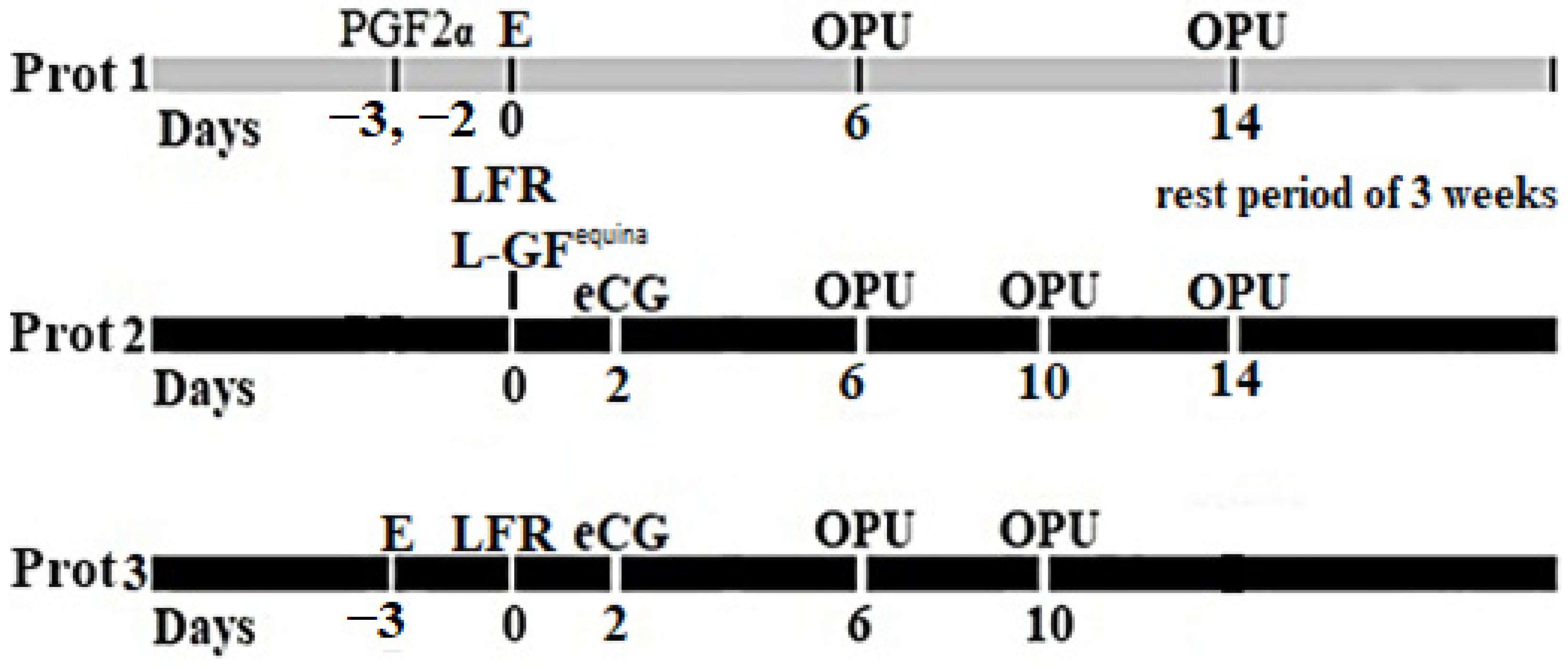
2.3. Assignment of Groups and Protocol of Administration
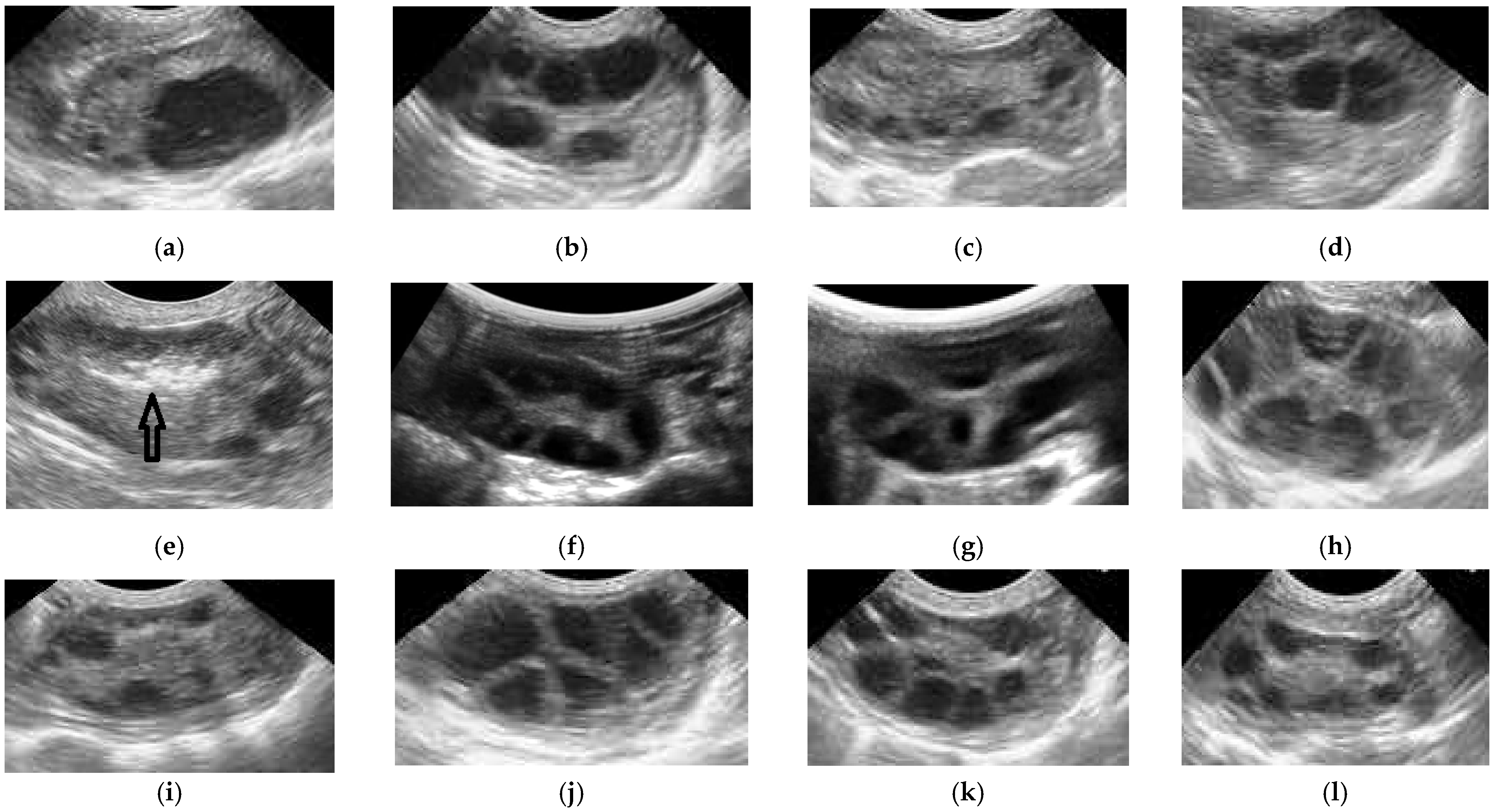
2.4. Ovum Pick-Up
2.5. In Vitro Embryo Production Protocol
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pontes, J.H.; Nonato-Junior, I.; Sanches, B.V.; Ereno-Junior, J.C.; Uvo, S.; Barreiros, T.R.; Oliveira, J.A.; Hasler, J.F.; Seneda, M.M. Comparison of embryo yield and pregnancy rate between in vivo and in vitro methods in the same Nelore (Bos indicus) donor cows. Theriogenology 2009, 71, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Viana, J.H.M. 2020 Statistics of embryo collection and transfer in domestic farm animals. Embryo Technol. Newsl. 2021, 39, 4. [Google Scholar]
- Bó, G.A.; Cedeño, A.; Mapletoft, R.J. Strategies to increment in vivo and in vitro embryo production and transfer in cattle. Anim. Reprod. 2019, 16, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Vieira, L.M.; Rodrigues, C.A.; Castro Netto, A.; Guerreiro, B.M.; Silveira, C.R.; Moreira, R.J.; Sá Filho, M.F.; Bó, G.A.; Mapletoft, R.J.; Baruselli, P.S. Superstimulation prior to the ovum pick-up to improve in vitro embryo production in lactating and non-lactating Holstein cows. Theriogenology 2014, 82, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Petyim, S.; Bage, R.; Hallap, T.; Bergqvist, A.S.; Rodriguez-Martinez, H.; Larsson, B. Two different schemes of twice-weekly ovum pick-up in dairy heifers: Effect on oocyte recovery and ovarian function. Theriogenology 2003, 60, 175–188. [Google Scholar] [CrossRef]
- Demissie, T.; Yilma, T.; Degefa, T.; Wirtu, G.; Lemma, A. Effect of follicular ablation and gonadotropin priming on the recovery and quality of oocytes in Boran cows. Int. J. Vet. Sci. Res. 2021, 7, 138–143. [Google Scholar] [CrossRef]
- Takuma, T.; Otsubo, T.; Kurokawa, Y.; Ichimaru, H.; Otoi, T. Effects of twice-weekly follicular punctures of ovaries with or without the corpus luteum on follicular and luteal dynamics. Reprod. Domest. Anim. 2010, 45, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.; Salaheddine, M. Effects of repeated ultrasound-guided transvaginal follicular aspiration on bovine oocyte recovery and subsequent follicular development. Theriogenology 1998, 50, 575–585. [Google Scholar] [CrossRef]
- Chasombat, J.; Nagai, T.; Parnpai, R.; Vongpralub, T. Ovarian Follicular Dynamics, Ovarian Follicular Growth, Oocyte Yield, In vitro Embryo Production and Repeated Oocyte Pick Up in Thai Native Heifers Undergoing Superstimulation. Asian-Australas. J. Anim. Sci. 2013, 26, 488–500. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Foster, T.E.; Puskas, B.L.; Mandelbaum, B.R.; Gerhardt, M.B.; Rodeo, S.A. Platelet-Rich Plasma: From Basic Science to Clinical Applications. Am. J. Sports Med. 2009, 37, 2259–2272. [Google Scholar] [CrossRef]
- Wu, P.I.; Diaz, R.; Borg-Stein, J. Platelet-Rich Plasma. Phys. Med. Rehabil. Clin. N. Am. 2016, 27, 825–853. [Google Scholar] [CrossRef] [PubMed]
- Martínez, C.E.; Smith, P.C.; Palma Alvarado, V.A. The influence of platelet-derived products on angiogenesis and tissue repair: A concise update. Front. Physiol. 2015, 6, 290. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Andia, I.; Ardanza, B.; Nurden, P.; Nurden, A.T. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb. Haemost. 2004, 91, 4–15. [Google Scholar] [CrossRef]
- Cremonesi, F.; Bonfanti, S.; Idda, A.; Anna, L.C. Improvement of Embryo Recovery in Holstein Cows Treated by Intra-Ovarian Platelet Rich Plasma before Superovulation. Vet. Sci. 2020, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Cremonesi, F.; Stefano Bonfanti, S.; Idda, A.; Lange-Consiglio, A. Platelet Rich Plasma for Regenerative Medicine Treatment of Bovine Ovarian Hypofunction. Front. Vet. Sci. 2020, 7, 517. [Google Scholar] [CrossRef]
- Dawod, A.; Miro, J.; Elbaz, H.T.; Fahmy, H.; Abdoon, A.S. Effect of Intrauterine Infusion of Equine Fresh Platelets-Rich Plasma (PRP) or Lyophilized PRP (L-GFequina) on Ovarian Activity and Pregnancy Rate in Repeat Breeder Purebred Arabian Mares. Animals 2021, 11, 1123. [Google Scholar] [CrossRef]
- Everts, P.; Onishi, K.; Jayaram, P.; Lana, J.F.; Mautner, K. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. Int. J. Mol. Sci. 2020, 21, 7794. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Pinto, N.R.; Pereda, A.; Jiménez, P.; Corso, M.D.; Kang, B.S.; Nally, M.; Lanata, N.; Wang, H.L.; Quirynen, M. The impact of the centrifuge characteristics and centrifugation protocols on the cells, growth factors, and fibrin architecture of a leukocyte- and platelet-rich fibrin (L-PRF) clot and membrane. Platelets 2018, 29, 171–184. [Google Scholar] [CrossRef]
- Abd El-Rahman, S.S.; Amer, M.S.; Hassan, M.H.; Fahmy, H.M.; Shama, A.A. Repair of experimentally induced femoral chondral defect in a rabbit model using Lyophilized growth promoting factor extracted from horse blood platelets (L-GFequina). Injury 2022, 53, 1375–1384. [Google Scholar] [CrossRef]
- Roche, J.R.; Friggens, N.C.; Kay, J.K.; Fisher, M.W.; Stafford, K.J.; Berry, D.P. Invited review: Body condition score and its association with dairy cow productivity, health, and welfare. J. Dairy Sci. 2009, 92, 5769–5801. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, T.; Fukumoto, Y.; Yamamoto, Y.; Ogata, Y.; Horiuchi, T. Estradiol benzoate treatment before ovum pick-up increases the number of good quality oocytes retrieved and improves the production of transferable embryos in Japanese Black cattle. Vet. Anim. Sci. 2018, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Patrie, A.; Watson, P. Statistics for Veterinary and Animal Science; Blackwell Science Ltd.: Oxford, UK, 1999; pp. 83–103. [Google Scholar]
- Hosseini, L.; Shirazi, A.; Naderi, M.M.; Shams-Esfandabadi, N.; Borjian Boroujeni, S.; Sarvari, A.; Sadeghnia, S.; Behzadi, B.; Akhondi, M.M. Platelet-rich plasma promotes the development of isolated human primordial and primary follicles to the preantral stage. Reprod. Biomed. Online 2017, 35, 343–350. [Google Scholar] [CrossRef]
- Callejo, J.; Salvador, C.; González-Nuñez, S.; Almeida, L.; Rodriguez, L.; Marqués, L.; Valls, A.; Lailla, J.M. Live birth in a woman without ovaries after autograft of frozen-thawed ovarian tissue combined with growth factors. J. Ovarian Res. 2013, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Pazoki, H.; Eimani, H.; Farokhi, F.; Shahverdi, A.; Yazdi, R.S.; Tahaei, L.S. Comparing the growth and the development of mouse pre-antral follicle in medium with PL (Platelet Layset) and with FBS. Middle East Fertil. Soc. J. 2015, 20, 231–236. [Google Scholar] [CrossRef]
- Rajabzadeh, A.R.; Eimani, H.; Koochesfahani, H.M.; Shahvardi, A.H.; Fathi, R. Morphological study of isolated ovarian preantral follicles using fibrin gel plus platelet lysate after subcutaneous transplantation. Cell J. 2015, 17, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Schams, D.; Papkoff, H. Chemical and immunochemical studies on pregnant mare serum gonadotropin. Biochim. Biophys. Acta 1971, 263, 139–148. [Google Scholar] [CrossRef]
- Schams, D.; Menzer, C.H.; Schallenberger, E.; Hoffmann, B.; Hahn, J.; Hahn, R. Some studies on pregnant mare serum gonadotrophin (PMSG) and on endocrine responses after application for superovulation in cattle. In Control of Reproduction in the Cow; Sreenan, J.M., Ed.; Martinus Nijhoff: The Hague, The Netherlands, 1978; pp. 122–143. [Google Scholar]
- Goulding, D.; Williams, D.; Roche, J.; Boland, M. Factors affecting superovulation in heifers treated with PMSG. Theriogenology 1996, 45, 765–773. [Google Scholar] [CrossRef]
- Pessoa, G.A.; Martini, A.P.; Carloto, G.W.; Rodrigues, M.C.C.; Claro Júnior, I.; Baruselli, P.S.; Brauner, C.C.; Rubin, M.I.B.; Corrêa, M.N.; Leivas, F.G.; et al. Different doses of equine chorionic gonadotropin on ovarian follicular growth and pregnancy rate of suckled Bos taurus beef cows subjected to timed artificial insemination protocol. Theriogenology 2016, 85, 792799. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.H.; Sanches, C.P.; Seddon, A.S.; Veras, M.B.; Lima, F.A.; Monteiro, P.L.J., Jr.; Wiltbank, M.C.; Sartori, R. Short communication: Follicle superstimulation before ovum pick-up for in vitro embryo production in Holstein cows. J. Dairy Sci. 2016, 99, 9307–9312. [Google Scholar] [CrossRef] [PubMed]
- Blondin, P.; Bousquet, D.; Twagiramungu, H.; Barnes, F.; Sirard, M.A. Manipulation of follicular development to produce developmentally competent bovine oocytes. Biol. Reprod. 2002, 66, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Mundim, T.C.D.; Ramos, A.F.; Sartori, R.; Dode, M.A.N.; Melo, E.O.; Gomes, L.F.S.; Rumpf, R.; Franco, M.M. Changes in gene expression profiles of bovine embryos produced in vitro, by natural ovulation, or hormonal superstimulation. Genet. Mol. Res. 2009, 8, 1398–1407. [Google Scholar] [CrossRef] [PubMed]
- Sills, E.S.; Rickers, N.S.; Li, X.; Palermo, G. First data on in vitro fertilization and blastocyst formation after intraovarian injection of calcium gluconate-activated autologus platelet rich plasma. Gynecol. Endocrinol. 2018, 34, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Seckin, S.; Ramadan, H.; Mouanness, M.; Kohansieh, M.; Merhi, Z. Ovarian response to intraovarian platelet-rich plasma (PRP) administration: Hypotheses and potential mechanisms of action. J. Assist. Reprod. Genet. 2022, 39, 37–61. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Canning, J.; Kaneko, T.; Pru, J.K.; Tilly, J.L. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature 2004, 428, 145–150. [Google Scholar] [CrossRef]
- Pantos, K.; Simopoulou, M.; Pantou, A.; Rapani, A.; Tsioulou, P.; Nitsos, N.; Syrkos, S.; Pappas, A.; Koutsilieris, M.; Sfakianoudis, K. A Case Series on Natural Conceptions Resulting in Ongoing Pregnancies in Menopausal and Prematurely Menopausal Women Following Platelet-Rich Plasma Treatment. Cell Transpl. 2019, 28, 1333–1340. [Google Scholar] [CrossRef]
- Lubkowska, A.; Dolegowska, B.; Banfi, G. Growth factor content in PRP and their applicability in medicine. J. Biol. Regul. Homeost. Agents 2012, 26 (Suppl. S1), 3S–22S. [Google Scholar]
| Items | Day 6 | Day 10 | Day 14 | ||||
|---|---|---|---|---|---|---|---|
| Prot. 1 (n = 6) | Prot. 2 (n = 6) | Prot. 3 (n = 5) | Prot. 2 (n = 6) | Prot. 3 (n = 5) | Prot. 1 (n = 6) | Prot. 2 (n = 6) | |
| Total no. follicles | 5.5 ± 0.7 b | 20.2 ± 0.7 a | 18.0 ± 1.7 a | 11.5 ± 1.3 a | 6.0 ± 0.3 b | 7.2 ± 0.4 b | 13.8 ± 1.2 a |
| Follicles 3–4.9 mm | 2.3 ± 0.2 c | 4.8 ± 0.4 b | 6.6 ± 0.9 a | 4.2 ± 0.3 a | 3.4 ± 0.2 a | 3.7 ± 0.2 a | 4.0 ± 0.4 a |
| Follicles 5–6.9 mm | 1.2 ± 0.2 b | 4.7 ± 0.3 a | 4.2 ± 0.3 a | 3.0 ± 0.5 a | 1.6 ± 0.2 b | 1.7 ± 0.2 b | 4.3 ± 0.3 a |
| >7 mm follicles | 2.0 ± 0.4 c | 10.7 ± 0.3 a | 7.2 ± 0.8 b | 4.3 ± 1.0 a | 1.0 ± 0.3 b | 1.8 ± 0.3 b | 5.5 ± 0.9 a |
| No COCs recovered | 2.0 ± 0.3 c | 7.7 ± 0.7 b | 9.4 ± 0.5 a | 7.7 ± 0.4 a | 1.6 ± 0.2 b | 1.8 ± 0.3 b | 6.8 ± 0.7 a |
| Recovery rate | 35.5 ± 4.6 b | 38.1 ± 3.2 b | 53.6 ± 8.4 a | 70.6 ± 8.4 a | 26.4 ± 3.4 b | 25.4 ± 3.8 b | 51.8 ± 7.8 a |
| Number of blastocyst/OPU session | 0.7 ± 0.2 b | 3.2 ± 0.3 a | 3.4 ± 0.4 a | 3.2 ± 0.4 a | 0.6 ± 0.2 b | 0.5 ± 0.2 b | 3.5 ± 0.2 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borş, S.-I.; Dascălu, D.-L.; Borş, A.; Fahmy, H.M.; Kandil, O.M.; Abdoon, A.S.S. Intraovarian Injection of Reconstituted Lyophilized Growth-Promoting Factor Extracted from Horse Blood Platelets (L-GFequina) Increases Oocytes Recovery and In Vitro Embryo Production in Holstein Cows. Animals 2022, 12, 2618. https://doi.org/10.3390/ani12192618
Borş S-I, Dascălu D-L, Borş A, Fahmy HM, Kandil OM, Abdoon ASS. Intraovarian Injection of Reconstituted Lyophilized Growth-Promoting Factor Extracted from Horse Blood Platelets (L-GFequina) Increases Oocytes Recovery and In Vitro Embryo Production in Holstein Cows. Animals. 2022; 12(19):2618. https://doi.org/10.3390/ani12192618
Chicago/Turabian StyleBorş, Silviu-Ionuț, Dan-Lucian Dascălu, Alina Borş, Hossam M. Fahmy, Omaima M. Kandil, and Ahmed Sabry S. Abdoon. 2022. "Intraovarian Injection of Reconstituted Lyophilized Growth-Promoting Factor Extracted from Horse Blood Platelets (L-GFequina) Increases Oocytes Recovery and In Vitro Embryo Production in Holstein Cows" Animals 12, no. 19: 2618. https://doi.org/10.3390/ani12192618
APA StyleBorş, S.-I., Dascălu, D.-L., Borş, A., Fahmy, H. M., Kandil, O. M., & Abdoon, A. S. S. (2022). Intraovarian Injection of Reconstituted Lyophilized Growth-Promoting Factor Extracted from Horse Blood Platelets (L-GFequina) Increases Oocytes Recovery and In Vitro Embryo Production in Holstein Cows. Animals, 12(19), 2618. https://doi.org/10.3390/ani12192618








