Effect of Dietary Linoleic Acid (18:2n-6) Supplementation on the Growth Performance, Fatty Acid Profile, and Lipid Metabolism Enzyme Activities of Coho Salmon (Oncorhynchus kisutch) Alevins
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets
2.2. Experimental Fish and Feeding Trial
2.3. Sampling Procedures
2.4. Calculations and Analytical Methods
2.4.1. Growth Performance2.4.2. Body Composition Analyses
2.4.2. Body Composition Analyses
2.4.3. Muscle Fatty Acid Analysis
2.4.4. Liver Biochemical Analysis
2.5. Statistical Analyses
3. Results
3.1. Growth Performance
3.2. Whole-Body Compositions and Muscle Fatty Acid Profile
3.3. Liver Lipid Metabolic Enzymes Activities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NRC (National Research Council). Nutrient Requirements of Fish and Shrimp; The National Academy Press: Washington, DC, USA, 2011. [Google Scholar]
- Tocher, D.R. Metabolism and functions of lipids and fatty acids in teleost fish. Rev. Fish. Sci. 2003, 11, 107–184. [Google Scholar] [CrossRef]
- Tocher, D.R.; Zheng, X.; Schlechtriem, C.; Hastings, N.; Dick, J.R.; Teale, A.J. Highly unsaturated fatty acid synthesis in marine fish: Cloning, functional characterization, and nutritional regulation of fatty acyl delta 6 desaturase of Atlantic cod (Gadus morhua L.). Lipids 2006, 41, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Senadheera, S.D.; Turchini, G.M.; Thanuthong, T.; Francis, D.S. Effects of Dietary α-Linolenic Acid (18:3n-3) / Linoleic Acid (18:2n-6) Ratio on Fatty Acid Metabolism in Murray Cod (Maccullochella peelii peelii). J. Agric. Food Chem. 2011, 59, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Pamungkas, W.; Jusadi, D.; Zairin, M., Jr.; Setiawati, M.; Supriyono, E.; Imron, I. Effect of dietary essential fatty acids on level of oestradiol-17β and vitellogenin, reproductive performance and larval quality of striped catfish (Pangasianodon hypophthalmus) in out of spawning season. Aquac. Res. 2020, 51, 3900–3909. [Google Scholar] [CrossRef]
- Sargent, J.R.; Tocher, D.R.; Bell, J.G. The Lipids. In Fish Nutrition, 3rd ed.; Halver, J.E., Hardy, R.W., Eds.; Academic Press: San Diego, CA, USA, 2002; pp. 181–257. [Google Scholar] [CrossRef]
- Ng, W.K.; Andin, V.C. The Malaysian mahseer, Tor tambroides (Bleeker), requires low dietary lipid levels with a preference for lipid sources with high omega-6 and low omega-3 polyunsaturated fatty acids. Aquaculture 2011, 322, 82–90. [Google Scholar] [CrossRef]
- Orlando, T.M.; Fontes, T.V.; Paulino, R.R.; Solis Murgas, L.D.; López-Olmeda, J.F.; Rosa, P.V. Effects of the dietary linoleic acid to linolenic acid ratio for Nile tilapia (Oreochromis niloticus) breeding females. Aquaculture 2019, 516, 734625. [Google Scholar] [CrossRef]
- Das, U.N. Essential fatty acid-a review. Curr. Pharm. Biotechnol. 2006, 7, 467–482. [Google Scholar] [CrossRef]
- Torstensen, B.E.; Tocher, D.R. The effects of fish. In Fish Oil Replacement and Alternative Lipid Sources in Aquaculture Feeds; Turchini, G.M., Ng, W.K., Tocher, D.R., Eds.; CRC Press: Boca Raton, FL, USA; Taylor and Francis Group: Boca Raton, FL, USA, 2010. [Google Scholar]
- Sargent, J.; Bell, G.; McEvoy, L.; Tocher, D.; Estevez, A. Recent developments in the essential fatty acid nutrition of fish. Aquaculture 1999, 177, 191–199. [Google Scholar] [CrossRef]
- Tocher, D.R.; Glencross, B.D. Lipids and fatty acids. In Dietary Nutrients, Additives and Fish Health; Cheng-Sheng, L., Chhorn, L., Delbert, M.G., Webster, C.D., Eds.; CRC Press: Hoboken, NJ, USA, 2015; p. 47. [Google Scholar]
- Paulino, R.R.; Pereira, R.T.; Fontes, T.V.; Oliva-Teles, A.; Peres, H.; Carneiro, D.J.; Rosa, P.V. Optimal dietary linoleic acid to linolenic acid ratio improved fatty acid profile of the juvenile tambaqui (Colossoma macropomum). Aquaculture 2018, 488, 9–16. [Google Scholar] [CrossRef]
- Dai, Y.J.; Jiang, G.Z.; Liu, W.B.; Abasubong, K.P.; Zhang, D.D.; Li, X.F.; Chi, C.; Liu, W.B. Evaluation of dietary linoleic acid on growth as well as hepatopancreatic index, lipid accumulation oxidative stress and inflammation in Chinese mitten crabs (Eriocheir sinensis). Aquac. Rep. 2022, 22, 100983. [Google Scholar] [CrossRef]
- Bogut, I.; Has-Schön, E.; Čačić, M.; Milaković, Z.; Novoselić, D.; Brkić, S. Linolenic acid supplementation in the diet of European catfish (Silurus glanis). J. Appl. Ichthyol. 2002, 18, 1–6. [Google Scholar] [CrossRef]
- Chen, Y.F.; Sun, Z.Z.; Liang, Z.; Xie, Y.D.; Su, J.L.; Luo, Q.L.; Zhu, J.Y.; Liu, Q.Y.; Han, T.; Wang, A. Effects of dietary fish oil replacement by soybean oil and L-carnitine supplementation on growth performance, fatty acid composition, lipid metabolism and liver health of juvenile largemouth bass, Micropterus salmoides. Aquaculture 2020, 516, 734596. [Google Scholar] [CrossRef]
- Du, J.; Chen, Q.; Li, Y.; Xiang, X.; Xu, W.; Mai, K.; Ai, Q. Activation of the farnesoid X receptor (FXR) suppresses linoleic acid-induced inflammation in the large yellow croaker (Larimichthys crocea). J. Nutr. 2020, 150, 2469–2477. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhou, Y.; Wu, M.; Li, X.; Mai, K.; Ai, Q. ω-6 Polyunsaturated fatty acids (linoleic acid) activate both autophagy and antioxidation in a synergistic feedback loop via TOR-dependent and TOR-independent signaling pathways. Cell Death Dis. 2020, 11, 607. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, H.; Li, E.; Qin, J.; Chen, L. Growth performance, lipid requirement and antioxidant capacity of juvenile Russian sturgeon Acipenser gueldenstaedti fed various levels of linoleic and linolenic acids. Aquac. Res. 2016, 48, 3216–3229. [Google Scholar] [CrossRef]
- Guo, Z.; Zhu, X.; Liu, J.; Han, D.; Yang, Y.; Xie, S.; Lan, Z. Dietary lipid requirement of juvenile hybrid sturgeon, Acipenser baerii♀ × A. gueldenstaedtii♂. J. Appl Ichthyol. 2011, 27, 743–748. [Google Scholar] [CrossRef]
- Bautista, M.; De la Cruz, M. Linoleic (x6) and linolenic (x3) acids in the diet of fingerling milkfish (Chanos chanos Forsskal). Aquaculture 1988, 71, 347–358. [Google Scholar] [CrossRef]
- Chou, B.S.; Shiau, S.Y. Both n-6 and n-3 fatty acids are required for maximal growth of juvenile hybrid tilapia. N. Am. J. Aquacult. 1999, 61, 13–20. [Google Scholar] [CrossRef]
- Henderson, R.J.; Tocher, D.R. The lipid composition and biochemistry of freshwater fish. Prog. Lipid. Res. 1987, 26, 281–347. [Google Scholar] [CrossRef]
- Takeuchi, T.S.; Arais, T.; Watanabe, Y. Shimma. Requirements of the eel Anguilla japonicas for essential fatty acids. Bull. Jpn. Soc. Sci. Fish. 1980, 46, 345–353. [Google Scholar] [CrossRef]
- Takeuchi, T.; Watanabe, T.N. Requirement for essential fatty acids of chum salmon (Oncorhynchus keta) in freshwater environment. Bull. Jpn. Soc. Sci. Fish. 1979, 45, 1319–1323. [Google Scholar] [CrossRef]
- Yu, T.C.; Sinnhuber, R.O. Effect of dietary w3 and w6 fatty acids on growth and feed conversion efficiencies of coho salmon (Oncorhynchus kisutch). Aquaculture 1979, 16, 31–38. [Google Scholar] [CrossRef]
- Li, E.; Lim, C.; Klesius, P.H.; Welker, T.L. Growth, body fatty acid composition, immune response, and resistance to Streptococcus iniae of hybrid tilapia, Oreochromis niloticus × Oreochromis Aureus, fed diets containing various levels of linoleic and linolenic acids. J. World Aquacult. Soc. 2013, 44, 42–55. [Google Scholar] [CrossRef]
- Yamada, W.K.; Clemans, G.W.; Hutchinson, M.C. Essential fatty acids deficiency in humans. Prog. Lipid Res. 1981, 19, 187–215. [Google Scholar] [CrossRef]
- Sargent, J.R.; Parker, R.J.; Mueller-Harvey, I.; Henderson, R.J. Lipid Biomarkers in Marine Ecology. In Microbes and the Sea; Sleigh, M.A., Ed.; Ellis Harwood Ltd.: Chichester, UK, 1987; pp. 119–138. [Google Scholar]
- Castell, J.D.; Lee, D.J.; Sinnhuber, R.O. Essential fatty acids in the diet of rainbow trout (Salmo gairdneri), Lipid metabolism and fatty acid composition. J. Nutr. 1972, 102, 93–100. [Google Scholar] [CrossRef]
- Watanabe, T.; Takashima, F.; Ogino, C. Effect of dietary methyl linolenate on growth of rainbow trout. Bull. Jpn. Soc. Sci. Fish. 1974, 40, 181–188. [Google Scholar] [CrossRef]
- Olsen, R.E.; Henderson, R.J.; Ringø, E. Lipids in Arctic charr, Salvenius alpinus (L). I. Dietary induced changes in lipid class and fatty acid composition. Fish Physiol. Biochem. 1991, 9, 151–164. [Google Scholar] [CrossRef]
- Hart, J.L. Pacific fishes of Canada. J. Fish. Res. Bd. Can. Bull. 1973, 180, 740. [Google Scholar]
- Food and Agriculture Organisation of the United Nations. The State of World Fisheries and Aquaculture-Meeting the Sustainable Development Goals; Food and Agriculture Organisation of the United Nations: Rome, Italy, 2018. [Google Scholar]
- Tocher, D.R.; Dick, J.R. Essential fatty acid deficiency in freshwater fish: The effects of linoleic, alpha-linolenic, gamma-linolenic and stearidonic acids on the metabolism of [1-14c]18:3n-3 in a carp cell culture model. Fish Physiol. Biochem. 2000, 22, 67–75. [Google Scholar] [CrossRef]
- Izquierdo, M.S.; Fernández-Palacios, H.; Tacon, A. Effect of broodstock nutrition on reproductive performance of fish. Aquaculture 2001, 197, 25–42. [Google Scholar] [CrossRef]
- Quintero, H.; Durland, E.; Davis, D.A.; Dunham, R. Effects of lipid supplementation on reproductive performance of female channel catfish Ictalurus punctatus, induced and strip-spawned for hybridization. Aquacult. Nutr. 2010, in press. [Google Scholar] [CrossRef]
- Abi-Ayad, E.A.; Melard, C.; Kestemont, P. Effects of n-3 fatty acids in eurasian perch broodstock diet on egg fatty acid composition and larvae stress resistance. Aquac. Int. 1997, 5, 161–168. Available online: http://hdl.handle.net/2268/77296 (accessed on 2 July 2022).
- Shields, R.J. Larviculture of marine finfish in Europe. Aquaculture 2001, 200, 55–88. [Google Scholar] [CrossRef]
- Yu, H.R.; Li, L.Y.; Xu, C.M.; Li, M.; Li, F.H.; Guo, M.J.; Qiu, X.Y.; Shan, L.L. Effect of dietary eicosapentaenoic acid (20:5n-3) on growth performance, fatty acid profile and lipid metabolism in coho salmon (Oncorhynchus kisutch) alevins. Aquac. Rep. 2022, 23, 101084. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of Official Analytical Chemists International, 16th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2005. [Google Scholar]
- Xu, H.; Ai, Q.; Mai, K.; Xu, W.; Wang, J.; Ma, H.; Zhang, W.; Wang, X.; Liufu, Z. Effects of dietary arachidonic acid on growth performance, survival, immune response and tissue fatty acid composition of juvenile Japanese sea bass, Lateolabrax japonicus. Aquaculture 2010, 307, 75–82. [Google Scholar] [CrossRef]
- Glencross, B.D.; Smith, D.M.; Thomas, M.R. The effect of dietary n-3 and n-6 fatty acid balance on the growth of the prawn Penaeus monodo. Aquacult. Nutr. 2002, 8, 43–51. [Google Scholar] [CrossRef]
- Stickney, R.R.; McGeachin, R.B.; Robinson, E.H.; Arnold, G.; Suter, L. Growth of Tilapia aurea as a function of degree of dietary lipid saturation. In Proceedings of the Annual Conference Southeastern Association of Fish and Wildlife Agencies, Montgomery, AK, USA, 1982; Volume 36, pp. 172–181. [Google Scholar]
- Kanazawa, A.; Teshima, S.; Sakamoto, M.; Awal, M.A. Requirements of Tilapia zillii for essential fatty acids. Bull. Jpn. Soc. Sci. Fish. 1980, 46, 1353–1356. [Google Scholar] [CrossRef][Green Version]
- Takeuchi, T.; Satoh, S.; Watanabe, T. Requirement of Tilapia nilotica for essential fatty acids. Bull. Jpn. Soc. Sci. Fish. 1983, 49, 1127–1134. [Google Scholar] [CrossRef]
- Emre, Y.; Kurtoğlu, A.; Emre, N.; Güroy, B.; Güroy, D. Effect of replacing dietary fish oil with soybean oil on growth performance, fatty acid composition and haematological parameters of juvenile meagre, Argyrosomus regius. Aquac. Res. 2016, 47, 2256–2265. [Google Scholar] [CrossRef]
- Mu, H.; Shen, H.; Liu, J.; Xie, F.; Zhang, W.; Mai, K. High level of dietary soybean oil depresses the growth and anti-oxidative capacity and induces inflammatory response in large yellow croaker Larimichthys crocea. Fish Shellfish Immunol. 2018, 77, 465–473. [Google Scholar] [CrossRef]
- Chellappa, S.; Huntingford, F.A.; Strang, R.; Thomson, R.Y. Condition factor and hepatosomatic index as estimates of energy status in male three-spined stickleback. J. Fish Biol. 1995, 47, 775–787. [Google Scholar] [CrossRef]
- Campbell, S.; Love, R.M. Energy reserves of male and female haddock (Melanogrammus aeglefinus L.) from the Moray Firth. ICES J. Mar. Sci. 1978, 38, 120–121. [Google Scholar] [CrossRef]
- Ahmed, M.; Liang, H.; Kasiya, H.C.; Ji, K.; Ge, X.; Ren, M.; Liu, B.; Zhu, X.; Sun, A. Complete replacement of fish meal by plant protein ingredients with dietary essential amino acids supplementation for juvenile blunt snout bream (Megalobrama amblycephala). Aquac. Nutr. 2019, 25, 205–214. [Google Scholar] [CrossRef]
- Tian, J.J.; Lei, C.X.; Ji, H. Influence of dietary linoleic acid (18:2n-6) and α-linolenic acid (18:3n-3) ratio on fatty acid composition of different tissues in freshwater fish Songpu mirror carp Cyprinus Carpio. Aquac. Res. 2016, 47, 3811–3825. [Google Scholar] [CrossRef]
- Wu, F.C.; Chen, H.Y. Effects of dietary linolenic acid to linoleic acid ratio on growth, tissue fatty acid profile and immune response of the juvenile grouper Epinephelus malabaricus. Aquaculture 2012, 324, 111–117. [Google Scholar] [CrossRef]
- Thanuthong, T.; Francis, D.S.; Senadheera, S.D.; Jones, P.L.; Turchini, G.M. Fish oil replacement in rainbow trout diets and total dietary PUFA content: I) Effects on feed efficiency, fat deposition and the efficiency of a finishing strategy. Aquaculture 2011, 320, 82–90. [Google Scholar] [CrossRef]
- Bell, J.G.; Tocher, D.R.; MacDonald, F.M.; Sargent, J.R. Effects of dietary borage oil [enriched in α-linolenic acid, 18:3(n-6)] or marine fish oil [enriched in eicosapentaenoic acid,20:5(n-3)] on growth, mortalities, liver histopathology and lipid composition of juvenile turbot (Scophthalmus maximus). Fish Physiol. Biochem. 1995, 14, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Caballero, M.; Obach, A.; Rosenlund, G.; Montero, D.; Gisvold, M.; Izquierdo, M. Impact of different dietary lipid sources on growth, lipid digestibility, tissue fatty acid composition and histology of rainbow trout, Oncorhynchus mykiss. Aquaculture 2002, 214, 253–271. [Google Scholar] [CrossRef]
- Seong, M.; Lee, S.; Lee, S.; Song, Y.; Bae, J.; Chang, K.; Bai, S.C. The effects of different levels of dietary fermented plant-based protein concentrate on growth, hematology and non-specific immune responses in juvenile olive flounder, Paralichthys olivaceus. Aquaculture 2018, 483, 196–202. [Google Scholar] [CrossRef]
- Bell, J.G.; Henderson, R.J.; Tocher, D.R.; McGhee, F.; Dick, J.R.; Porter, A.; Smullen, R.P.; Sargent, J.R. Substituting fish oil with crude palm oil in the diet of Atlantic salmon (Salmo salar) affects muscle fatty acid composition and hepatic fatty acid metabolism. J. Nutr. 2002, 132, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Bransden, M.P.; Carter, C.G.; Nichols, P.D. Replacement of fish oil with sunflower oil in feeds for Atlantic salmon (Salmo salar L.): Effect on growth performance, tissue fatty acid composition and disease resistance. Comp. Biochem. Physiol. 2003, 135, 611–625. [Google Scholar] [CrossRef]
- Francis, D.S.; Turchini, G.M.; Jones, P.L.; De Silva, S.S. Effects of dietary oil source on growth and fillet fatty acid composition of Murray cod, Maccullochella peelii peelii. Aquaculture 2006, 253, 547–556. [Google Scholar] [CrossRef]
- Luo, Z.; Li, X.; Bai, H.; Gong, S. Effects of dietary fatty acid composition on muscle composition and hepatic fatty acid profile in juvenile Synechogobius hasta. J. Appl. Ichthyol. 2008, 24, 116–119. [Google Scholar] [CrossRef]
- Castro, C.; Couto, A.; Pérez-Jiménez, A.; Serra, C.R.; Díaz-Rosales, P.; Fernandes, R.; Corraze, G.; Panserat, S.; Oliva-Teles, A. Effects of fish oil replacement by vegetable oil blend on digestive enzymes and tissue histomorphology of European sea bass (Dicentrarchus labrax) juveniles. Fish Physiol. Biochem. 2016, 42, 203–217. [Google Scholar] [CrossRef]
- El–Husseiny, O.M.; Abdul-Aziz, G.M.; Goda, A.M.A.S.; Suloma, A. Effect of altering linoleic acid and linolenic acid dietary levels and ratios on the performance and tissue fatty acid profiles of Nile tilapia Oreochromis niloticus fry. Aquacult. Int. 2010, 18, 1105–1119. [Google Scholar] [CrossRef]
- Tan, X.Y.; Luo, Z.; Xie, P.; Liu, X.J. Effect of dietary linolenic acid/linoleic acid ratio on growth performance, hepatic fatty acid profiles and intermediary metabolism of juvenile yellow catfish Pelteobagrus fulvidraco. Aquaculture 2009, 296, 96–101. [Google Scholar] [CrossRef]
- Koven, W.M.; Barr, Y.; Lutzky, S.; Ben-Atia, I.; Weiss, R.; Harel, M.; Behrens, P.; Tandler, A. The effect of dietary arachidonic acid (20:4n-6) on growth, survival and resistance to handling stress in gilthead seabream (Sparus aurata) larvae. Aquaculture 2001, 193, 107–122. [Google Scholar] [CrossRef]
- Koven, W.M.; Van Anholt, R.D.; Lutzky, S.; Ben Atia, I.; Nixon, O.; Ron, B.; Tandler, A. The effect of dietary arachidonic acid on growth, survival, and cortisol levels in different-age gilthead seabream larvae (Sparus auratus) exposed to handling or daily salinity change. Aquaculture 2003, 228, 307–320. [Google Scholar] [CrossRef]
- Auwerx, J.; Leroy, P.; Schoonjans, K. Lipoprotein lipase: Recent contributions from molecular biology. Crit. Rev. Clin. Lab. Sci. 1992, 29, 243–268. [Google Scholar] [CrossRef]
- Torstensen, B.E.; Lie, Ø.; Frøyland, L. Lipid metabolism and tissue composition in Atlantic salmon (Salmo salar L.) effects of capelin oil, palm oil, and oleic acid-enriched sunflower oil as dietary lipid sources. Lipids 2000, 35, 653–664. [Google Scholar] [CrossRef]
- Richard, N.; Kaushik, S.; Larroquet, L.; Panserat, S.; Corraze, G. Replacing dietary fish oil by vegetable oils has little effect on lipogenesis, lipid transport and tissue lipid uptake in rainbow trout (Oncorhynchus mykiss). Br. J. Nutr. 2006, 96, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.L.; Luo, Z.; Pan, Y.X.; Zheng, J.L.; Zhu, Q.L.; Sun, L.D.; Zhuo, M.Q.; Hu, W. Differential induction of enzymes and genes involved in lipid metabolism in liver and visceral adipose tissue of juvenile yellow catfish Pelteobagrus fulvidraco exposed to copper. Aquat. Toxicol. 2013, 136–137, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.T.; Liu, Y.J.; Tian, L.X.; Mai, K.S.; Du, Z.Y.; Wang, Y.; Yang, H.J. Effect of dietary lipid level on growth performance, lipid deposition, hepatic lipogenesis in juvenile cobia (Rachycentron canadum). Aquaculture 2005, 249, 439–447. [Google Scholar] [CrossRef]
- Kolditz, C.; Borthaire, M.; Richard, N.; Corraze, G.; Panserat, S.; Vachot, C.; Lefèvre, F.; Médale, F. Liver and muscle metabolic changes induced by dietary energy content and genetic selection in rainbow trout (Oncorhynchus mykiss). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.K.; Zhu, X.M.; Han, D.; Yang, Y.X.; Lei, W.; Xie, S.Q. Effects of dietary lipid levels on growth, survival and lipid metabolism during early ontogeny of Pelteobagrus vachelli larvae. Aquaculture 2010, 299, 121–127. [Google Scholar] [CrossRef]
- Menoyo, D.; Lopez-Bote, C.J.; Bautista, J.M.; Obach, A. Growth, digestibility and fatty acid utilization in large Atlantic salmon (Salmo salar) fed varying levels of n-3 and saturated fatty acids. Aquaculture 2003, 225, 295–307. [Google Scholar] [CrossRef]
- Smith, S.; Witkowski, A.; Joshi, A.K. Structural and functional organization of the animal fatty acid synthase. Prog. Lipid Res. 2003, 42, 289–317. [Google Scholar] [CrossRef]
- Wang, A.; Han, G.; Qi, Z.; Lv, F.; Yu, Y.; Huang, J.; Wang, T.; Xu, P. Cloning of lipoprotein lipase (LPL) and the effects of dietary lipid levels on LPL expression in GIFT tilapia (Oreochromis niloticus). Aquacult. Int. 2013, 21, 1219–1232. [Google Scholar] [CrossRef]
- Alvarez, M.J.; Dıez, A.; Lopez-Bote, C.; Gallego, M.; Bautista, J.M. Short-term modulation of lipogenesis by macronutrients in rainbow trout (Oncorhynchus mykiss) hepatocytes. Br. J. Nutr. 2000, 84, 619–628. [Google Scholar] [CrossRef]
- Hudgins, L.C.; Badaya, A.; Hellerstein, M.K.; Parker, T.S.; Levine, D.M.; Seidman, C.E.; Neese, R.A.; Tremaroli, J.D.; Hirsch, J. The effect of dietary carbohydrate on genes for fatty acid synthase and inflammatory cytokines in adipose tissues from lean and obese subjects. J. Nutr. Biochem. 2008, 19, 237–245. [Google Scholar] [CrossRef]
- Leng, X.J.; Wu, X.F.; Tian, J.; Li, X.Q.; Guan, L.; Weng, D.C. Molecular cloning of fatty acid synthase from grass carp (Ctenopharyngodon idella) and the regulation of its expression by dietary fat level. Aquacult. Nutr. 2012, 18, 551–558. [Google Scholar] [CrossRef]
- Najjar, S.M.; Yang, Y.; Fernstrom, M.A.; Lee, S.J.; DeAngelis, A.M.; Rjaily, G.A.; Al-Share, Q.Y.; Dai, T.; Miller, T.A.; Ratnam, S.; et al. Insulin acutely decreases hepatic fatty acid synthase activity. Cell Metab. 2005, 2, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Boujard, T.; Gelineau, A.; Coves, D.; Corraze, G.; Dutto, G.; Gasset, E.; Kaushik, S. Regulation of feed intake, growth, nutrient and energy utilisation in European sea bass (Dicentrarchus labrax) fed high fat diets. Aquaculture 2004, 231, 529–545. [Google Scholar] [CrossRef]
- Peng, M.; Xu, W.; Mai, K.; Zhou, H.; Zhang, Y.; Liufu, Z.; Zhang, K.K.; Ai, Q. Growth performance, lipid deposition and hepatic lipid metabolism related gene expression in juvenile turbot (Scophthalmus maximus L.) fed diets with various fish oil substitution levels by soybean oil. Aquaculture 2014, 433, 442–449. [Google Scholar] [CrossRef]
- Peng, S.; Shi, Z.; Gao, Q.; Zhang, C.; Wang, J. Dietary n-3 LC-PUFAs affect lipoprotein lipase (LPL) and fatty acid synthase (FAS) activities and mRNA expression during vitellogenesis and ovarian fatty acid composition of female silver pomfret (Pampus argenteus) broodstock. Aquacult. Nutr. 2016, 23, 692–701. [Google Scholar] [CrossRef]
- Zhang, Z.; Garzotto, M.; Beer, T.M.; Thuillier, P.; Lieberman, S.; Mori, M.; Shannon, J. Effects of ω-3 fatty acids and catechins on fatty acid synthase in the prostate: A randomized controlled trial. Nutr. Cancer 2016, 68, 1309–1319. [Google Scholar] [CrossRef]


| Ingredients (%) | Dietary Linoleic Acid Level (%) | |||||
|---|---|---|---|---|---|---|
| 0.11 | 0.74 | 1.37 | 2.00 | 2.63 | 3.26 | |
| Degreasing fish meal 1 | 40.00 | 40.00 | 40.00 | 40.00 | 40.00 | 40.00 |
| Soybean protein concentrate 1 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Soybean meal 1 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 |
| Peanut meal 1 | 9.80 | 9.80 | 9.80 | 9.80 | 9.80 | 9.80 |
| α-Starch 1 | 12.00 | 12.00 | 12.00 | 12.00 | 12.00 | 12.00 |
| Sodium alginate 1 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Soybean lecithin 1 | 1.80 | 1.80 | 1.80 | 1.80 | 1.80 | 1.80 |
| Fish oil 1 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Coconut oil 1 | 6.00 | 4.80 | 3.60 | 2.40 | 1.20 | 0.00 |
| Corn oil | 0.00 | 1.20 | 2.40 | 3.60 | 4.80 | 6.00 |
| Mineral premix 2 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Vitamin premix 3 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Vitamin C phosphate | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Choline chloride | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| Ethoxyquin | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Proximate composition | ||||||
| Moisture (%) | 7.33 | 7.29 | 7.60 | 7.20 | 7.32 | 7.38 |
| Crude protein (%) | 46.65 | 46.90 | 46.98 | 46.73 | 47.00 | 47.01 |
| Crude lipid (%) | 13.67 | 14.16 | 13.79 | 13.94 | 14.01 | 13.84 |
| Ash (%) | 9.11 | 9.29 | 9.38 | 9.39 | 9.27 | 9.47 |
| Fatty Acids | Coconut Oil | Corn Oil | Dietary LA Level (%) | |||||
|---|---|---|---|---|---|---|---|---|
| 0.11 | 0.74 | 1.37 | 2.00 | 2.63 | 3.26 | |||
| C12:0 | 28.34 | 0.02 | 0.783 | 0.627 | 0.471 | 0.315 | 0.159 | 0.002 |
| C14:0 | 15.22 | 0.01 | 0.317 | 0.254 | 0.191 | 0.127 | 0.064 | 0.001 |
| C16:0 | 13.28 | 10.90 | 0.252 | 0.242 | 0.232 | 0.221 | 0.211 | 0.201 |
| C16:1n-7 | 0.02 | 0.11 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 |
| C18:0 | 3.46 | 1.81 | 0.026 | 0.022 | 0.019 | 0.016 | 0.012 | 0.009 |
| C18:1n-9 | 2.13 | 25.2 | 0.023 | 0.164 | 0.304 | 0.445 | 0.586 | 0.727 |
| C18:3n-3(ALA) | 0.83 | 1.35 | 0.007 | 0.007 | 0.008 | 0.009 | 0.009 | 0.010 |
| C20:5n-3(EPA) | - | - | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 |
| C22:6n-3(DHA) | - | - | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 |
| C20:0 | 1.23 | 0.20 | 0.014 | 0.012 | 0.010 | 0.008 | 0.006 | 0.004 |
| C20:1 | 1.03 | 0.21 | 0.013 | 0.011 | 0.010 | 0.008 | 0.006 | 0.004 |
| C18:2n-6(LA) | 1.55 | 54.4 | 0.110 | 0.740 | 1.370 | 2.000 | 2.630 | 3.260 |
| C20:4n-6(ARA) | 0.05 | - | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
| ∑n-6PUFA | 12.36 | 55.2 | 0.214 | 0.835 | 1.420 | 2.006 | 2.962 | 3.631 |
| Dietary LA Level (%) | 0.11 | 0.74 | 1.37 | 2.00 | 2.63 | 3.26 | p Value |
|---|---|---|---|---|---|---|---|
| Survival rate (%) | 95.00 ± 2.89 | 97.50 ± 2.50 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 0.146 |
| Initial body weight (g) | 0.365 ± 0.001 | 0.362 ± 0.002 | 0.363 ± 0.001 | 0.364 ± 0.001 | 0.364 ± 0.002 | 0.366 ± 0.002 | 0.609 |
| Final body weight (g) | 3.86 ± 0.02 b | 4.24 ± 0.04 c | 4.46 ± 0.05 d | 4.07 ± 0.07 c | 3.54 ± 0.10 a,b | 3.27 ± 0.07 a | 0.011 |
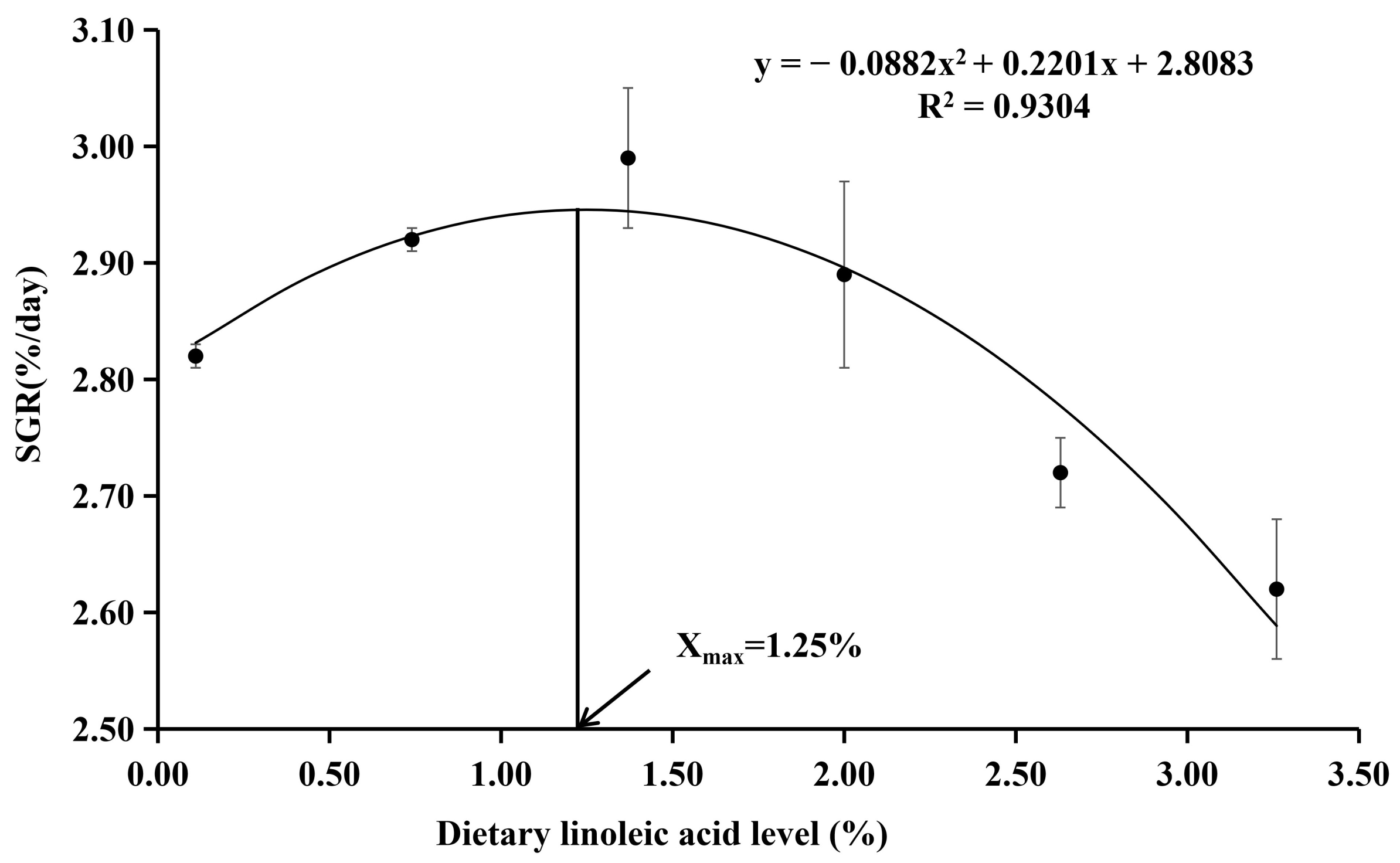
| SGR (% day−1) | 2.82 ± 0.01 b | 2.92 ± 0.01 c | 2.99 ± 0.06 d | 2.89 ± 0.08 c | 2.72 ± 0.03 a,b | 2.62 ± 0.06 a | 0.010 |
| FCR | 1.73 ± 0.01 c | 1.58 ± 0.02 b | 1.48± 0.01 a | 1.63 ± 0.02 b | 1.90 ± 0.03 c,d | 2.09 ± 0.04 d | 0.010 |
| CF | 1.27 ± 0.11 | 1.13 ± 0.17 | 1.02 ± 0.03 | 1.17 ± 0.10 | 1.04 ± 0.09 | 1.11 ± 0.02 | 0.582 |
| HSI | 1.27 ± 0.03 | 1.27 ± 0.01 | 1.22 ± 0.02 | 1.27 ± 0.05 | 1.26 ± 0.07 | 1.36 ± 0.14 | 0.790 |
| VSI | 1.73 ± 0.07 | 1.72 ± 0.05 | 1.51 ± 0.20 | 1.43 ± 0.07 | 1.50 ± 0.02 | 1.46 ± 0.04 | 0.194 |
| Dietary Linoleic Acid Level (%) | Moisture (%) | Crude Protein (%) | Crude Lipid (%) | Ash (%) |
|---|---|---|---|---|
| 0.11 | 77.89 ± 0.13 | 12.63 ± 0.17 | 4.40 ± 0.01 b | 3.64 ± 0.03 |
| 0.74 | 77.46 ± 0.01 | 12.99 ± 0.20 | 4.36 ± 0.61 b | 3.55 ± 0.01 |
| 1.37 | 77.91 ± 0.24 | 12.71 ± 0.33 | 4.12 ± 0.59 a | 3.45 ± 0.03 |
| 2.00 | 77.98 ± 0.06 | 12.23 ± 0.10 | 4.24 ± 0.30 ab | 3.45 ± 0.06 |
| 2.63 | 77.44 ± 0.27 | 13.11 ± 0.30 | 4.55 ± 0.33 b | 3.60 ± 0.02 |
| 3.26 | 77.62 ± 0.21 | 12.78 ± 0.37 | 4.53 ± 0.31 b | 3.65 ± 0.03 |
| One-way ANOVA | ||||
| p value | 0.293 | 0.843 | 0.049 | 0.206 |
| Fatty Acids | Dietary LA Level (%) | |||||
|---|---|---|---|---|---|---|
| 0.11 | 0.74 | 1.37 | 2.00 | 2.63 | 3.26 | |
| C14:0 | 2.24 ± 0.03 d | 2.23 ± 0.01 d | 2.22 ± 0.02 d | 1.88 ± 0.02 c | 1.77 ± 0.03 b | 1.56 ± 0.02 a |
| C16:0 | 22.02 ± 0.24 f | 20.6 ± 0.17 e | 18.44 ± 0.06 d | 16.06 ± 0.17 c | 14.52 ± 0.08 b | 12.51 ± 0.03 a |
| C18:0 | 6.09 ± 0.04 b | 5.68 ± 0.16 a,b | 5.56 ± 0.06 a | 5.37 ± 0.25 a | 5.28 ± 0.16 a | 5.25 ± 0.06 a |
| ∑SFA | 30.34 ± 0.27 f | 28.53 ± 0.31 e | 26.22 ± 0.02 d | 23.3 ± 0.44 c | 21.58 ± 0.16 b | 19.32 ± 0.10 a |
| C16:1n-7 | 7.27 ± 0.10 e | 7.14 ± 0.06 e | 6.67 ± 0.13 d | 5.47 ± 0.82 c | 4.23 ± 0.04 b | 3.33 ± 0.04 a |
| C18:1n-9 | 33.58 ± 0.11 | 32.54 ± 0.62 | 31.53 ± 0.27 | 32.6 ± 0.20 | 33.54 ± 0.03 | 31.47 ± 0.22 |
| C20:1 | 1.17 ± 0.03 c | 1.14 ± 0.06 c | 0.86 ± 0.01 b | 0.82 ± 0.01 a,b | 0.76 ± 0.02 a | 0.75 ± 0.02 a |
| ∑MUFA | 42.02 ± 0.20 c | 40.82 ± 0.74 b,c | 39.06 ± 0.39 b | 38.89 ± 0.28 b | 38.52 ± 0.08 b | 35.55 ± 0.27 a |
| C18:3n-3(ALA) | 0.43 ± 0.012 | 0.42 ± 0.005 | 0.46 ± 0.005 | 0.41 ± 0.006 | 0.47 ± 0.005 | 0.43 ± 0.006 |
| C20:5n-3(EPA) | 0.80 ± 0.005 | 0.75 ± 0.005 | 0.76 ± 0.006 | 0.82 ± 0.005 | 0.81 ± 0.006 | 0.80 ± 0.008 |
| C22:6n-3(DHA) | 2.23 ± 0.01 | 2.22 ± 0.01 | 2.18 ± 0.03 | 2.20 ± 0.01 | 2.22 ± 0.01 | 2.22 ± 0.03 |
| ∑n-3 PUFA | 3.26 ± 0.02 | 3.19 ± 0.01 | 3.25 ± 0.03 | 3.39 ± 0.02 | 3.46 ± 0.01 | 3.51 ± 0.02 |
| C18:2n-6 (LA) | 4.92 ± 0.02 a | 6.08 ± 0.03 b | 10.38 ± 0.04 c | 15.6 ± 0.02 d | 19.64 ± 0.01 e | 23.46 ± 0.20 f |
| C18:3n-6 | 0.49 ±0.003 a,b | 0.51 ± 0.03 c,d | 0.51 ± 0.003 d | 0.49 ± 0.008 b,c | 0.49 ± 0.008 b,c | 0.47 ± 0.003 a |
| C20:4n-6(ARA) | 2.05 ± 0.015 a | 2.08 ± 0.006 b | 2.11 ± 0.003 c | 2.12 ± 0.008 c | 2.14 ± 0.006 c | 2.17 ± 0.006 d |
| ∑n-6 PUFA | 5.54 ± 0.05 a | 8.19 ± 0.01 b | 12.61 ± 0.01 c | 18.08 ± 0.02 d | 23.21 ± 0.01 e | 26.28 ± 0.02 f |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Li, L.; Yu, L.; Xu, C.; Zhang, J.; Qiu, X.; Zhang, Y.; Shan, L. Effect of Dietary Linoleic Acid (18:2n-6) Supplementation on the Growth Performance, Fatty Acid Profile, and Lipid Metabolism Enzyme Activities of Coho Salmon (Oncorhynchus kisutch) Alevins. Animals 2022, 12, 2631. https://doi.org/10.3390/ani12192631
Yu H, Li L, Yu L, Xu C, Zhang J, Qiu X, Zhang Y, Shan L. Effect of Dietary Linoleic Acid (18:2n-6) Supplementation on the Growth Performance, Fatty Acid Profile, and Lipid Metabolism Enzyme Activities of Coho Salmon (Oncorhynchus kisutch) Alevins. Animals. 2022; 12(19):2631. https://doi.org/10.3390/ani12192631
Chicago/Turabian StyleYu, Hairui, Lingyao Li, Leyong Yu, Congmei Xu, Jiayi Zhang, Xiangyi Qiu, Yijing Zhang, and Lingling Shan. 2022. "Effect of Dietary Linoleic Acid (18:2n-6) Supplementation on the Growth Performance, Fatty Acid Profile, and Lipid Metabolism Enzyme Activities of Coho Salmon (Oncorhynchus kisutch) Alevins" Animals 12, no. 19: 2631. https://doi.org/10.3390/ani12192631
APA StyleYu, H., Li, L., Yu, L., Xu, C., Zhang, J., Qiu, X., Zhang, Y., & Shan, L. (2022). Effect of Dietary Linoleic Acid (18:2n-6) Supplementation on the Growth Performance, Fatty Acid Profile, and Lipid Metabolism Enzyme Activities of Coho Salmon (Oncorhynchus kisutch) Alevins. Animals, 12(19), 2631. https://doi.org/10.3390/ani12192631







