Effects of Extra-Long-Acting Recombinant Bovine FSH (bscrFSH) on Cattle Superovulation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Reagents and Media
2.3. Geographical Location
2.4. Single-Chain Recombinant Bovine FSH (bscrFSH) Hormone Synthesis and Composition
2.5. Experimental Groups and Superovulation (SOV) Protocols
2.6. Ultrasonographic Assessment
2.7. Blood Sampling and Hormonal Analyses
2.8. Embryo Quality and Quantity Assessment
2.9. Primer Design and PCR Protocol
2.10. Statistical Analyses
3. Results
3.1. Determination of Recombinant Bovine Single-Chain FSH (bscrFSH) Half-Life over Time during the SOV Protocol
3.2. Ovarian Response: Ovary Dimensions, Follicles and Corpora Lutea Evaluation over Time; NIH-FSH-p vs. bscrFSH-Derived SOV Protocol Application
3.3. Hormone Level Profiles during NIH-FSH-p vs. bscrFSH-Derived SOV Protocol Application: Concentrations of P4 and Cortisol over Time
3.4. In Vivo Embryo Quantity and Quality after NIH-FSH-p and bscrFSH-Derived SOV Protocol Application
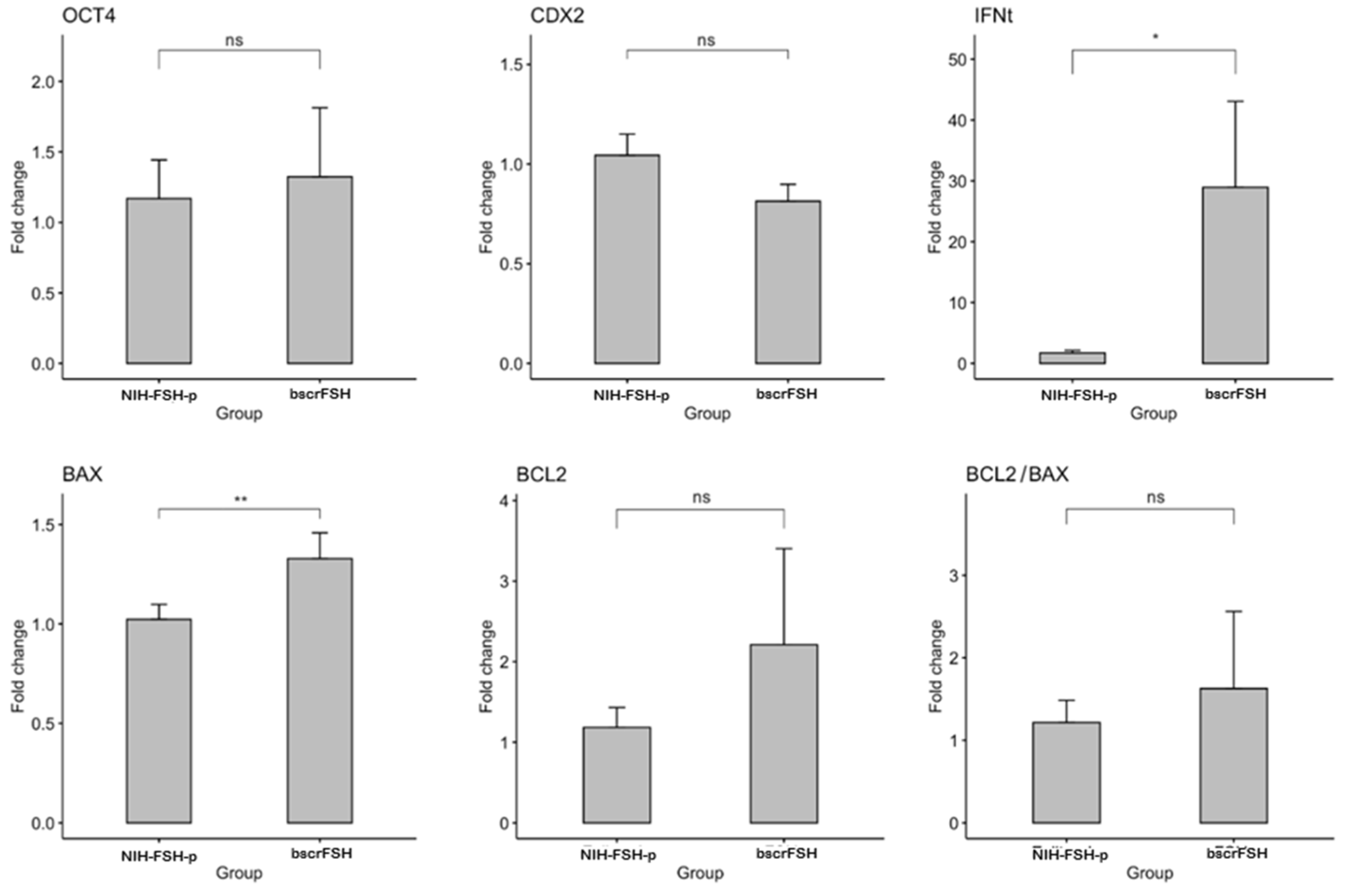
3.5. Gene Expression in In Vivo Produced Bovine Embryos Obtained after NIH-FSH-p and bscrFSH-Derived SOV Protocol Application
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hasler, J.F. Forty years of embryo transfer in cattle: A review focusing on the journal Theriogenology, the growth of the industry in North America, and personal reminisces. Theriogenology 2014, 81, 152–169. [Google Scholar] [CrossRef]
- Elsden, R.; Lewis, S.; Cumming, I.; Lawson, R. Proceedings: Superovulation in the cow following treatment with PMSG and prostaglandin F 2alpha. Reproduction 1974, 36, 455–456. [Google Scholar] [CrossRef]
- Phillippo, M.; Rowson, L.E.A. Prostaglandins and superovulation in the bovine. Ann. Biol. Anim. Biochim. Biophys. 1975, 15, 233–240. [Google Scholar] [CrossRef]
- Bó, G.A.; Mapletoft, R.J. Historical perspectives and recent research on superovulation in cattle. Theriogenology 2014, 81, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.G.; Hasler, J.F. A 100-Year Review: Reproductive technologies in dairy science. J. Dairy Sci. 2017, 100, 10314–10331. [Google Scholar] [CrossRef] [PubMed]
- Mikkola, M.; Hasler, J.F.; Taponen, J. Factors affecting embryo production in superovulated Bos taurus cattle. Reprod. Fertil. Dev. 2019, 32, 104–124. [Google Scholar] [CrossRef]
- Sanderson, N.; Martinez, M. A single administration of a long-acting recombinant ovine FSH (roFSH) for cattle superovulation. Theriogenology 2020, 154, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Deguettes, Q.; Fattal, E.; Moreau, M.; Lego, E.; Bochot, A. Controlled delivery of follicle-stimulating hormone in cattle. Int. J. Pharm. 2020, 590, 119904. [Google Scholar] [CrossRef]
- Kafi, M.; McGowan, M.R. Factors associated with variation in the superovulatory response of cattle. Anim. Reprod. Sci. 1997, 48, 137–157. [Google Scholar] [CrossRef]
- Mapletoft, R.J.; Steward, K.B.; Adams, G.P. Recent advances in the superovulation in cattle. Reprod. Nutr. Dev. 2002, 42, 601–611. [Google Scholar] [CrossRef] [Green Version]
- Pal, S. Superovulation: Advancements in cattle. Int. J. Sci. Eng. Res. 2019, 10, 131–134. [Google Scholar]
- Moor, R.M.; Kruip, T.A.M.; Green, D. Intraovarian control of folliculogenesis: Limits to superovulation? Theriogenology 1984, 21, 103–116. [Google Scholar] [CrossRef]
- Chupin, D.; Combarnous, Y.; Procureur, R. Antagonistic effect of LH on FSH-induced superovulation in cattle. Theriogenology 1984, 21, 229. [Google Scholar] [CrossRef]
- Braileanu, G.T.; Albanese, C.; Card, C.; Chedrese, P.J. FSH bioactivity in commercial preparations of gonadotropins. Theriogenology 1998, 49, 1031–1037. [Google Scholar] [CrossRef]
- Mapletoft, R.J.; Bó, G.A. The evolution of improved and simplified superovulation protocols in cattle. Reprod. Fertil. Dev. 2012, 24, 278–283. [Google Scholar] [CrossRef]
- Looney, C.R.; Bondioli, K.R.; Hill, K.G.; Massey, J.M. Superovulation of donor cows with bovine Follicle-Stimulating Hormone (bFSH) produced by recombinant DNA technology. Theriogenology 1988, 29, 271. [Google Scholar] [CrossRef]
- Wilson, J.M.; Jones, A.L.; Moore, K.; Looney, C.R.; Bondioli, K.R. Superovulation of cattle with a recombinant-DNA bovine follicle stimulating hormone. Anim. Reprod. Sci. 1993, 33, 71–82. [Google Scholar] [CrossRef]
- Carvalho, P.D.; Hackbart, K.S.; Bender, R.W.; Baez, G.M.; Dresch, A.R.; Guenther, J.N.; Souza, A.H.; Fricke, P.M. Use of a single injection of long-acting recombinant bovine FSH to superovulate Holstein heifers: A preliminary study. Theriogenology 2014, 82, 481–489. [Google Scholar] [CrossRef]
- Rodŕguez-Alvarez, L.; Cox, J.; Tovar, H.; Einspanier, R.; Castro, F.O. Changes in the expression of pluripotency-associated genes during preimplantation and peri-implantation stages in bovine cloned and in vitro produced embryos. Zygote 2010, 18, 269–279. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Dufort, I.; Caballero, J.; Moulavi, F.; Ghanaei, H.R.; Sirard, M.A. Transcriptome profiling of bovine inner cell mass and trophectoderm derived from in vivo generated blastocysts. BMC Dev. Biol. 2015, 15, 49. [Google Scholar] [CrossRef] [Green Version]
- Graf, A.; Krebs, S.; Heininen-Brown, M.; Zakhartchenko, V.; Blum, H.; Wolf, E. Genome activation in bovine embryos: Review of the literature and new insights from RNA sequencing experiments. Anim. Reprod. Sci. 2014, 149, 46–58. [Google Scholar] [CrossRef]
- Fulka, H.; St. John, J.C.; Fulka, J.; Hozák, P. Chromatin in early mammalian embryos: Achieving the pluripotent state. In Proceedings of the Differentiation; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2008; Volume 76, pp. 3–14. [Google Scholar]
- Barros, C.M.; Satrapa, R.A.; Castilho, A.C.S.; Fontes, P.K.; Razza, E.M.; Ereno, R.L.; Nogueira, M.F.G. Effect of superstimulatory treatments on the expression of genes related to ovulatory capacity, oocyte competence and embryo development in cattle. Reprod. Fertil. Dev. 2012, 25, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, C. Superovulation: Strategies, associated factors, and prediction of the superovulatory reponse in cows: [review]. Rev. Med. Vet. Zoot 2009, 56, 195–214. [Google Scholar]
- Bó, G.A.; Cedeño, A.; Mapletoft, R.J. Strategies to increment in vivo and in vitro embryo production and transfer in cattle. Anim. Reprod. 2019, 16, 411–422. [Google Scholar] [CrossRef]
- Bergfelt, D.R.; Bo, G.A.; Mapletoft, R.J.; Adams, G.P. Superovulatory response following ablation-induced follicular wave emergence at random stages of the oestrous cycle in cattle. Anim. Reprod. Sci. 1997, 49, 1–12. [Google Scholar] [CrossRef]
- Bó, G.A.; Baruselli, P.S.; Chesta, P.M.; Martins, C.M. The timing of ovulation and insemination schedules in superstimulated cattle. Theriogenology 2006, 65, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Bó, G.A.; Guerrero, D.C.; Tríbulo, A.; Tríbulo, H.; Tríbulo, R.; Rogan, D.; Mapletoft, R.J. New approaches to superovulation in the cow. Reprod. Fertil. Dev. 2010, 22, 106–112. [Google Scholar] [CrossRef] [Green Version]
- Phillips, P.E.; Jahnke, M.M. Embryo Transfer (Techniques, Donors, and Recipients). In Veterinary Clinics of North America—Food Animal Practice; WB Saunders: Philadelphia, PA, USA, 2016; Volume 32, pp. 365–385. [Google Scholar]
- Bó, G.A.; Mapletoft, R.J. Evaluation and classification of bovine embryos. Anim. Reprod. 2013, 10, 344–348. [Google Scholar]
- Hesser, M.W.; Morris, J.C.; Gibbons, J.R. Advances in Recombinant Gonadotropin Production for Use in Bovine Superovulation. Reprod. Domest. Anim. 2011, 46, 933–942. [Google Scholar] [CrossRef]
- Bo, G.A.; Hockley, D.K.; Nasser, L.F.; Mapletoft, R.J. Superovulatory response to a single subcutaneous injection of Folltropin-V in beef cattle. Theriogenology 1994, 42, 963–975. [Google Scholar] [CrossRef]
- Walsh, J.H.; Mantovani, R.; Duby, R.T.; Overstrom, E.W.; Dobrinsky, J.R.; Enright, W.J.; Roche, J.F.; Boland, M.P. The effects of once or twice daily injections of pFSH on superovulatory response in heifers. Theriogenology 1993, 40, 313–321. [Google Scholar] [CrossRef]
- Looney, C.R.; Boutte, B.W.; Archbald, L.F.; Godke, R.A. Comparison of once daily and twice daily FSH injections for superovulating beef cattle. Theriogenology 1981, 15, 13–22. [Google Scholar] [CrossRef]
- Yamamoto, M.; Ooe, M.; Kawaguchi, M.; Suzuki, T. Superovulation in the cow with a single intramuscular injection of FSH dissolved in polyvinylpyrrolidone. Theriogenology 1994, 41, 747–755. [Google Scholar] [CrossRef]
- Tríbulo, A.; Rogan, D.; Tríbulo, H.; Tríbulo, R.; Mapletoft, R.J.; Bó, G.A. Superovulation of beef cattle with a split-single intramuscular administration of Folltropin-V in two concentrations of hyaluronan. Theriogenology 2012, 77, 1679–1685. [Google Scholar] [CrossRef]
- Kimura, K.; Hirako, M.; Iwata, H.; Aoki, M.; Kawaguchi, M.; Seki, M. Successful superovulation of cattle by a single administration of FSH in aluminum hydroxide gel. Theriogenology 2007, 68, 633–639. [Google Scholar] [CrossRef]
- Gong, J.G.; Wilmut, I.; Bramley, T.A.; Webb, R. Pretreatment with recombinant bovine somatotropin enhances the superovulatory response to FSH in Heifers. Theriogenology 1996, 45, 611–622. [Google Scholar] [CrossRef]
- Looney, C.R.; Pryor, J.H. Novel bovine embryo transfer technologies in the United States. Anim. Reprod. 2012, 9, 404–413. [Google Scholar]
- Takagi, M.; Kim, I.H.; Izadyar, F.; Hyttel, P.; Bevers, M.M.; Dieleman, S.J.; Hendriksen, P.J.M.; Vos, P.L.A.M. Impaired final follicular maturation in heifers after superovulation with recombinant human FSH. Reproduction 2001, 121, 941–951. [Google Scholar] [CrossRef]
- Crowe, M.A.; Enright, W.J.; Boland, M.P.; Roche, J.F. Follicular growth and serum follicle-stimulating hormone (FSH) responses to recombinant bovine FSH in GnRH-immunized anoestrous heifers. Anim. Sci. 2001, 73, 115–122. [Google Scholar] [CrossRef]
- Kemper Green, C.N.; Hawkins, D.A.; Rocha, A.; Tanner, J.W.; Harms, P.G.; Forrest, D.W.; Welsh, T.H. Temporal aspects of ovarian follicular growth and steroidogenesis following exogenous follicle-stimulating hormone in Angus heifers. Anim. Reprod. Sci. 1996, 45, 157–176. [Google Scholar] [CrossRef]
- Mahmood, K.; Tahir, M.Z.; Butt, M.A.; Qureshi, S.M.; Riaz, A. GnRH or estradiol benzoate combination with CIDR improves in-vivo embryo production in bovines (Bos indicus and Bos taurus) under subtropics. PeerJ 2021, 9, e12077. [Google Scholar] [CrossRef] [PubMed]
- Laster, D.B. Disappearance and uptake of (125 I)FSH in the rat, rabbit, ewe and cow. J. Reprod. Fertil. 1972, 30, 407–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demoustier, M.M.; Beckers, J.F.; Van Der Zwalmen, P.; Closset, J.; Gillard, J.L.; Ectors, F. Determination of porcine plasma follitropin levels during superovulation treatment in cows. Theriogenology 1988, 30, 379–386. [Google Scholar] [CrossRef] [Green Version]
- Herrier, A.; Einspanier, R.; Schams, D.; Niemann, H. Effect of recombinant bovine somatotropin (rBST) on follicular IGF-I contents and the ovarian response following superovulatory treatment in dairy cows: A preliminary study. Theriogenology 1994, 41, 601–611. [Google Scholar] [CrossRef]
- Gong, J.G. Influence of metabolic hormones and nutrition on ovarian follicle development in cattle: Practical implications. Domest. Anim. Endocrinol. 2002, 23, 229–241. [Google Scholar] [CrossRef]
- Van De Wiel, D.F.M.; Van Rijn, P.A.; Meloen, R.H.; Moormann, R.J.M. High-level expression of biologically active recombinant bovine follicle stimulating hormone in a baculovirus system. J. Mol. Endocrinol. 1998, 20, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Barros, C.M.; Ereno, R.L.; Simões, R.A.L.; Fernandes, P.; Buratini, J.; Nogueira, M.F.G. Use of knowledge regarding LH receptors to improve superstimulatory treatments in cattle. Reprod. Fertil. Dev. 2010, 22, 132–137. [Google Scholar] [CrossRef]
- Price, C.A.; Carrière, P.D.; Gosselin, N.; Kohram, H.; Guilbault, L.A. Effects of superovulation on endogenous LH secretion in cattle, and consequences for embryo production. Theriogenology 1999, 51, 37–46. [Google Scholar] [CrossRef]
- Ireland, J.J.; Ward, F.; Jimenez-Krassel, F.; Ireland, J.L.H.; Smith, G.W.; Lonergan, P.; Evans, A.C.O. Follicle numbers are highly repeatable within individual animals but are inversely correlated with FSH concentrations and the proportion of good-quality embryos after ovarian stimulation in cattle. Hum. Reprod. 2007, 22, 1687–1695. [Google Scholar] [CrossRef] [Green Version]
- Jimenez-Krassel, F.; Folger, J.K.; Ireland, J.L.H.; Smith, G.W.; Hou, X.; Davis, J.S.; Lonergan, P.; Evans, A.C.O.; Ireland, J.J. Evidence that high variation in ovarian reserves of healthy young adults has a negative impact on the corpus luteum and endometrium during estrous cycles in cattle. Biol. Reprod. 2009, 80, 1272–1281. [Google Scholar] [CrossRef] [Green Version]
- Lerner, S.P.; Thayne, W.V.; Baker, R.D.; Henschen, T.; Meredith, S.; Inskeep, E.K.; Dailey, R.A.; Lewis, P.E.; Butcher, R.L. Age, dose of FSH and other factors affecting superovulation in Holstein cows. J. Anim. Sci. 1986, 63, 176–183. [Google Scholar] [CrossRef]
- Gonzalez, A.; Lussier, I.G.; Carruthers, T.D.; Murphy, B.D.; Mapletoft, R.J. Superovulation of beef heifers with Folltropin: A new FSH preparation containing reduced LH activity. Theriogenology 1990, 33, 519–529. [Google Scholar] [CrossRef]
- Barati, F.; Niasari-Naslaji, A.; Bolourchi, M.; Sarhaddi, F.; Razavi, K.; Naghzali, E.; Thatcher, W.W. Superovulatory response of Sistani cattle to three different doses of FSH during winter and summer. Theriogenology 2006, 66, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Bó, G.A.; Mapletoft, R.J. Superstimulation of ovarian follicles in cattle: Gonadotropin treatment protocols and FSH profiles. Theriogenology 2020, 150, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Diskin, M.G.; Morris, D.G. Embryonic and Early Foetal Losses in Cattle and Other Ruminants. Reprod. Domest. Anim. 2008, 43, 260–267. [Google Scholar] [CrossRef]
- Basir, G.S.; O, W.S.; So, W.W.K.; Ng, E.H.Y.; Ho, P.C. Evaluation of cycle-to-cycle variation of endometrial responsiveness using transvaginal sonography in women undergoing assisted reproduction. Ultrasound Obstet. Gynecol. 2002, 19, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Madoz, L.V.; Rabaglino, M.B.; Migliorisi, A.L.; Jaureguiberry, M.; Perez Wallace, S.; Lorenti, N.; Domínguez, G.; Giuliodori, M.J.; de la Sota, R.L. Association between progesterone concentration and endometrial gene expression in dairy cows. Domest. Anim. Endocrinol. 2021, 74, 106481. [Google Scholar] [CrossRef]
- Snider, A.P.; McLean, D.; Menino, A.R. Effects of feeding OmniGen-AF® on superovulatory response in donor beef cows: I. Serum progesterone and cortisol, embryo recovery and quality. Anim. Reprod. Sci. 2019, 210, 106174. [Google Scholar] [CrossRef] [PubMed]
- Alcivar, A.A.; Maurer, R.R.; Anderson, L.L. Endocrine changes in beef heifers superovulated with follicle-stimulating hormone (FSH-P) or human menopausal gonadotropin. J. Anim. Sci. 1992, 70, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Edwards, L.M.; Rahe, C.H.; Griffin, J.L.; Wolfe, D.F.; Marple, D.N.; Cummins, K.A.; Pitchett, J.F. Effect of transportation stress on ovarian function in superovulated Hereford heifers. Theriogenology 1987, 28, 291–299. [Google Scholar] [CrossRef]
- Macedo, G.G.; Zúccari, C.E.S.N.; de Abreu, U.G.P.; Negrão, J.A.; e Silva, E.V.D.C. Human–animal interaction, stress, and embryo production in Bos indicus embryo donors under tropical conditions. Trop. Anim. Health Prod. 2011, 43, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Dobson, H.; Smith, R.F. What is stress, and how does it affect reproduction? Anim. Reprod. Sci. 2000, 60–61, 743–752. [Google Scholar] [CrossRef]
- Espey, L.L. Current status of the hypothesis that mammalian ovulation is comparable to an inflammatory reaction. Biol. Reprod. 1994, 50, 233–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ideta, A.; Hayama, K.; Kawashima, C.; Urakawa, M.; Miyamoto, A.; Aoyagi, Y. Subjecting holstein heifers to stress during the follicular phase following superovulatory treatment may increase the female sex ratio of embryos. J. Reprod. Dev. 2009, 55, 529–533. [Google Scholar] [CrossRef] [Green Version]
- Acosta, T.J.; Tetsuka, M.; Matsui, M.; Shimizu, T.; Berisha, B.; Schams, D.; Miyamoto, A. In vivo evidence that local cortisol production increases in the preovulatory follicle of the cow. J. Reprod. Dev. 2005, 51, 483–489. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, M.C.C.; Bonotto, A.L.M.; Acosta, D.A.V.; Boligon, A.A.; Corrêa, M.N.; Brauner, C.C. Effect of oestrous synchrony between embryo donors and recipients, embryo quality and state on the pregnancy rate in beef cattle. Reprod. Domest. Anim. 2018, 53, 152–156. [Google Scholar] [CrossRef]
- Spencer, T.E.; Bazer, F.W. Conceptus signals for establishing and maintenance of pregnancy. Reprod. Biol. Endocrinol. 2004, 2, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erdem, H.; Karasahin, T.; Alkan, H.; Dursun, S.; Satilmis, F.; Guler, M. Effect of embryo quality and developmental stages on pregnancy rate during fresh embryo transfer in beef heifers. Trop. Anim. Health Prod. 2020, 52, 2541–2547. [Google Scholar] [CrossRef]
- García Guerra, A.; Tribulo, A.; Yapura, J.; Singh, J.; Mapletoft, R.J. Lengthening the superstimulatory treatment protocol increases ovarian response and number of transferable embryos in beef cows. Theriogenology 2012, 78, 353–360. [Google Scholar] [CrossRef]
- Nawaz, M.Y.; Jimenez-Krassel, F.; Steibel, J.P.; Lu, Y.; Baktula, A.; Vukasinovic, N.; Neuder, L.; Ireland, J.L.H.; Ireland, J.J.; Tempelman, R.J. Genomic heritability and genome-wide association analysis of anti-Müllerian hormone in Holstein dairy heifers. J. Dairy Sci. 2018, 101, 8063–8075. [Google Scholar] [CrossRef]
- Mossa, F.; Ireland, J.J. Physiology and endocrinology symposium: Anti-Müllerian hormone: A biomarker for the ovarian reserve, ovarian function, and fertility in dairy cows. J. Anim. Sci. 2019, 97, 1446–1455. [Google Scholar] [CrossRef]
- Abdel Aziz, R.L.; Abdel-Wahab, A.; Ibrahim, M.A.; Kasimanickam, R.K. Transcript abundance of anti-Mullérian hormone and follicle-stimulating hormone receptor predicted superstimulatory response in embryo donor Holstein cows. Reprod. Domest. Anim. 2021, 56, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Ruddock-D’Cruz, N.T.; Hall, V.J.; Tecirlioglu, R.T.; French, A.J. Gene expression analysis of single preimplantation bovine embryos and the consequence for developmental potential. Soc. Reprod. Fertil. Suppl. 2007, 64, 341–363. [Google Scholar] [CrossRef] [PubMed]
- D’Occhio, M.J.; Campanile, G.; Zicarelli, L.; Visintin, J.A.; Baruselli, P.S. Adhesion molecules in gamete transport, fertilization, early embryonic development, and implantation—Role in establishing a pregnancy in cattle: A review. Mol. Reprod. Dev. 2020, 87, 206–222. [Google Scholar] [CrossRef] [Green Version]
- Zolini, A.M.; Block, J.; Rabaglino, M.B.; Rincon, G.; Hoelker, M.; Bromfield, J.J.; Salilew-Wondim, D.; Hansen, P.J. Genes associated with survival of female bovine blastocysts produced in vivo. Cell Tissue Res. 2020, 382, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Simmet, K.; Zakhartchenko, V.; Philippou-Massier, J.; Blum, H.; Klymiuk, N.; Wolf, E. OCT4/POU5F1 is required for NANOG expression in bovine blastocysts. Proc. Natl. Acad. Sci. USA 2018, 115, 2770–2775. [Google Scholar] [CrossRef] [Green Version]
- Velásquez, A.E.; Veraguas, D.; Cabezas, J.; Manríquez, J.; Castro, F.O.; Rodríguez-Alvarez, L.L. The expression level of SOX2 at the blastocyst stage regulates the developmental capacity of bovine embryos up to day-13 of in vitro culture. Zygote 2019, 27, 398–404. [Google Scholar] [CrossRef]
- Kuijk, E.W.; Du Puy, L.; Van Tol, H.T.A.; Oei, C.H.Y.; Haagsman, H.P.; Colenbrander, B.; Roelen, B.A.J. Differences in early lineage segregation between mammals. Dev. Dyn. 2008, 237, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Molotkov, A.; Mazot, P.; Brewer, J.R.; Cinalli, R.M.; Soriano, P. Distinct Requirements for FGFR1 and FGFR2 in Primitive Endoderm Development and Exit from Pluripotency. Dev. Cell 2017, 41, 511–526.e4. [Google Scholar] [CrossRef] [Green Version]
- Leidenfrost, S.; Boelhauve, M.; Reichenbach, M.; Güngör, T.; Reichenbach, H.D.; Sinowatz, F.; Wolf, E.; Habermann, F.A. Cell arrest and cell death in mammalian preimplantation development: Lessons from the bovine model. PLoS ONE 2011, 6, e22121. [Google Scholar] [CrossRef] [Green Version]
- Bakri, N.M.; Ibrahim, S.F.; Osman, N.A.; Hasan, N.; Jaffar, F.H.F.; Rahman, Z.A.; Osman, K. Embryo apoptosis identification: Oocyte grade or cleavage stage? Saudi J. Biol. Sci. 2016, 23, S50–S55. [Google Scholar] [CrossRef] [Green Version]
- Melka, M.G.; Rings, F.; Hölker, M.; Tholen, E.; Havlicek, V.; Besenfelder, U.; Schellander, K.; Tesfaye, D. Expression of apoptosis regulatory genes and incidence of apoptosis in different Morphological Quality Groups of In Vitro-produced Bovine Pre-implantation Embryos. Reprod. Domest. Anim. 2010, 45, 915–921. [Google Scholar] [CrossRef]
- Gutiérrez-Adán, A.; Rizos, D.; Fair, T.; Moreira, P.N.; Pintado, B.; De La Fuente, J.; Boland, M.P.; Lonergan, P. Effect of speed of development on mRNA expression pattern in early bovine embryos cultured in vivo or in vitro. Mol. Reprod. Dev. 2004, 68, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Forde, N.; Lonergan, P. Transcriptomic analysis of the bovine endometrium: What is required to establish uterine receptivity to implantation in cattle? J. Reprod. Dev. 2012, 58, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, J.M.; Mathew, D.J.; Passaro, C.; Fair, T.; Lonergan, P. Embryonic maternal interaction in cattle and its relationship with fertility. Reprod. Domest. Anim. 2018, 53 (Suppl. 2), 20–27. [Google Scholar] [CrossRef] [PubMed]
- Kubisch, H.M.; Larson, M.A.; Roberts, R.M. Relationship between age of blastocyst formation and interferon-τ secretion by in vitro-derived bovine embryos. Mol. Reprod. Dev. 1998, 49, 254–260. [Google Scholar] [CrossRef]
- Neira, J.A.; Tainturier, D.; L’Haridon, R.M.; Martal, J. Comparative IFN-τ secretion after hatching by bovine blastocysts derived ex vivo and completely produced in vitro. Reprod. Domest. Anim. 2007, 42, 68–75. [Google Scholar] [CrossRef]
- Mundim, T.; Ramos, A.; Sartori, R.; Dode, M.; Melo, E.; Gomes, L.; Rumpf, R.; Franco, M. Changes in gene expression profiles of bovine embryos produced in vitro, by natural ovulation, or hormonal superstimulation. Genet. Mol. Res. 2009, 8, 1398–1407. [Google Scholar] [CrossRef] [PubMed]
- Fauque, P.; Jouannet, P.; Lesaffre, C.; Ripoche, M.A.; Dandolo, L.; Vaiman, D.; Jammes, H. Assisted reproductive technology affects developmental kinetics, H19 imprinting control region methylation and H19 gene expression in individual mouse embryos. BMC Dev. Biol. 2007, 7, 116. [Google Scholar] [CrossRef] [Green Version]
- Sato, A.; Otsu, E.; Negishi, H.; Utsunomiya, T.; Arima, T. Aberrant DNA methylation of imprinted loci in superovulated oocytes. Hum. Reprod. 2007, 22, 26–35. [Google Scholar] [CrossRef] [Green Version]
- Yao, N.; Wan, P.C.; Hao, Z.D.; Gao, F.F.; Yang, L.; Cui, M.S.; Wu, Y.; Liu, J.H.; Liu, S.; Chen, H.; et al. Expression of interferon-tau mRNA in bovine embryos derived from different procedures. Reprod. Domest. Anim. 2009, 44, 132–139. [Google Scholar] [CrossRef] [PubMed]



| Gene | Primer Forward Sequence (5′-3′) | Primer Reverse Sequence (5′-3′) | Amplicon Size (bp) | Annealing Temp. (°C) |
|---|---|---|---|---|
| OCT4 | TCGAGAACCGAGTGAGAGGC | ACACTCGGACCACGTCTTT | 120 | 55 |
| BAX | CGGGTTGTCGCCCTTTTCTAC | CAGCCGCTCTCGAAGGAAGT | 120 | 55 |
| CDX2 | AAACCCTACTGTCACCCAGT | TGAGGGTTCTAGCAGAGTCCA | 90 | 56 |
| INFt | ATGCTCCAGCAGTGCCTCAAC | TGTTGGAGCCCAGTGCAGAG | 95 | 57 |
| BCL2 | TGTGGAGCTGTATGGCCCTAGC | AGATAGGCACCCAGGGTGATGC | 114 | 56 |
| ACTB | TGCCCTGAGGCTCTCTTCCA | TTGGCGTAGAGGTCCTTGCG | 119 | 55 |
| GAPDH | AGGTCGGAGTGAACGGATTC | ATGGCGACGATGTCCACTTT | 85 | 56 |
| Time Points | Day 0 | Day 4 | Day 8 | Day 15 | |||||
|---|---|---|---|---|---|---|---|---|---|
| SOV | NIH-FSH-p | bscrFSH | NIH-FSH-p | bscrFSH | NIH-FSH-p | bscrFSH | NIH-FSH-p | bscrFSH | |
| Protocol | |||||||||
| Ovarian Dimensions | Length | 2.32 ± 0.07 a | 2.73 ± 0.08 a | 2.28 ± 0.05 a | 2.79 ± 0.07 a | 4.71 ± 0.11 b | 5.10 ± 0.17 b | 9.39 ± 0.16 c | 9.41 ± 0.18 c |
| Width | 1.74 ± 0.07 a | 1.83 ± 0.08 a | 1.61 ± 0.05 a | 1.77 ± 0.07 a | 4.28 ± 0.12 b | 4.86 ± 0.21 b | 8.73 ± 0.49 c | 8.94 ± 0.24 c | |
| Time Points | Day 0 | Day 4 | Day 8 | Day 15 | |||||
|---|---|---|---|---|---|---|---|---|---|
| SOV | NIH-FSH-p | bscrFSH | NIH-FSH-p | bscrFSH | NIH-FSH-p | bscrFSH | NIH-FSH-p | bscrFSH | |
| Protocol | |||||||||
| Hormone Levels | P4 | 4.14 ± 0.26 a | 3.85 ± 0.30 a | 5.21 ± 0.29 a | 6.07 ± 0.52 a | 1.00 ± 0.08 b | 0.92 ± 0.09 b | 61.27 ± 7.35 cA | 74.13 ± 8.93 cB |
| C | 3.43 ± 0.20 a | 4.13 ± 0.23 a | 4.67 ± 0.24 a | 6.05 ± 0.52 a | 13.14 ± 0.84 b | 12.51 ± 0.67 b | 6.27 ± 0.37 a | 5.87 ± 0.41 a | |
| In Vivo Embryo Production Parameters | SOV Protocols | |
|---|---|---|
| NIH-FSH-p (Mean ± SEM) | bscrFSH (Mean ± SEM) | |
| Total Number of Structures (TNS) | 8.00 ± 0.60 A | 10.32 ± 0.81 B |
| Unfertilized Oocytes (UFOs) | 0.50 ± 0.13 A | 0.71 ± 0.26 A |
| Morulae (M) | 2.85 ± 0.55 A | 5.47 ± 0.75 B |
| Early Blastocysts (EB) | 1.79 ± 0.31 A | 1.32 ± 0.27 A |
| Blastocysts (B) | 1.65 ± 0.30 A | 1.88 ± 0.54 A |
| Viable Embryos (VE) | 6.32 ± 0.56 A | 8.65 ± 0.67 B |
| Degenerated Embryos (DE) | 1.29 ± 0.25 A | 0.44 ± 0.16 B |
| Blocked Embryos (BE) | 0.12 ± 0.07 A | 0.47 ± 0.14 B |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Reinoso, M.A.; Aguilera, C.J.; Navarrete, F.; Cabezas, J.; Castro, F.O.; Cabezas, I.; Sánchez, O.; García-Herreros, M.; Rodríguez-Alvarez, L. Effects of Extra-Long-Acting Recombinant Bovine FSH (bscrFSH) on Cattle Superovulation. Animals 2022, 12, 153. https://doi.org/10.3390/ani12020153
Gutiérrez-Reinoso MA, Aguilera CJ, Navarrete F, Cabezas J, Castro FO, Cabezas I, Sánchez O, García-Herreros M, Rodríguez-Alvarez L. Effects of Extra-Long-Acting Recombinant Bovine FSH (bscrFSH) on Cattle Superovulation. Animals. 2022; 12(2):153. https://doi.org/10.3390/ani12020153
Chicago/Turabian StyleGutiérrez-Reinoso, Miguel A., Constanza J. Aguilera, Felipe Navarrete, Joel Cabezas, Fidel O. Castro, Ignacio Cabezas, Oliberto Sánchez, Manuel García-Herreros, and Lleretny Rodríguez-Alvarez. 2022. "Effects of Extra-Long-Acting Recombinant Bovine FSH (bscrFSH) on Cattle Superovulation" Animals 12, no. 2: 153. https://doi.org/10.3390/ani12020153







