Chlorogenic Acid Improves Quality of Chilled Ram Sperm by Mitigating Oxidative Stress
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Semen Collection and Processing Procedures
2.2. Chemicals
2.3. Semen Processing and Evaluation
2.4. Sperm Kinematic Parameters
2.5. Sperm Plasma Membrane Integrity Assessment
2.6. Sperm Acrosomal Integrity Assessment
2.7. Determination of ROS in Sperm
2.8. Evaluation of Sperm Protein Concentration
2.9. Evaluation of T-AOC of Seminal Plasma
2.10. Evaluation of CAT Activity in Seminal Plasma
2.11. Assessment of MDA Content in Sperm
2.12. Determination of SOD Activity in Sperm
2.13. Determination of ATP Content
2.14. Statistical Analysis
3. Results
3.1. Effects of CGA on Sperm Viability and PM
3.2. Effects of CGA on Sperm Plasma and Acrosomal Membrane Integrity
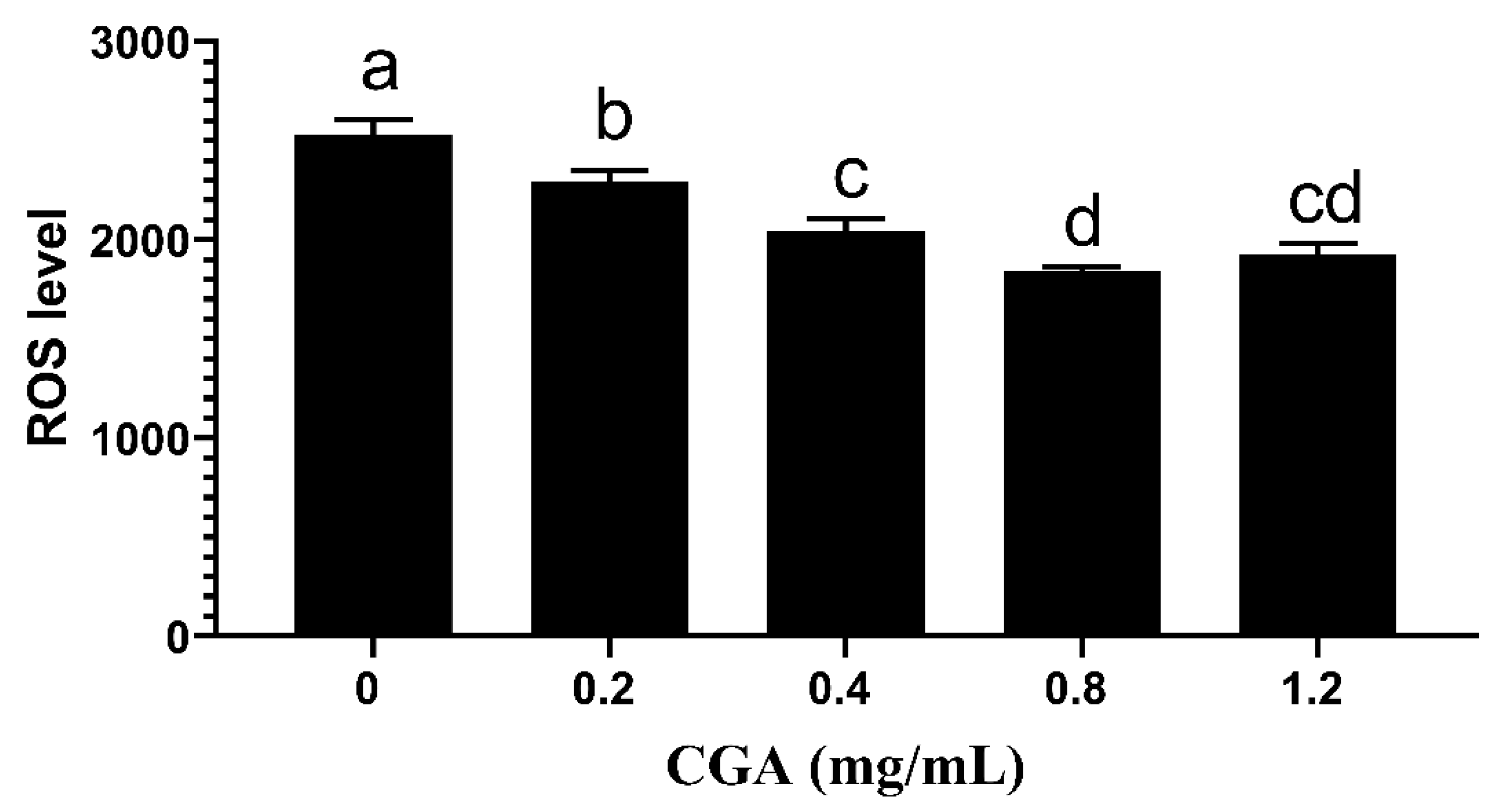
3.3. Effects of CGA Supplementation on Sperm ROS Content
3.4. Effects of CGA on CAT Activity in Seminal Plasma
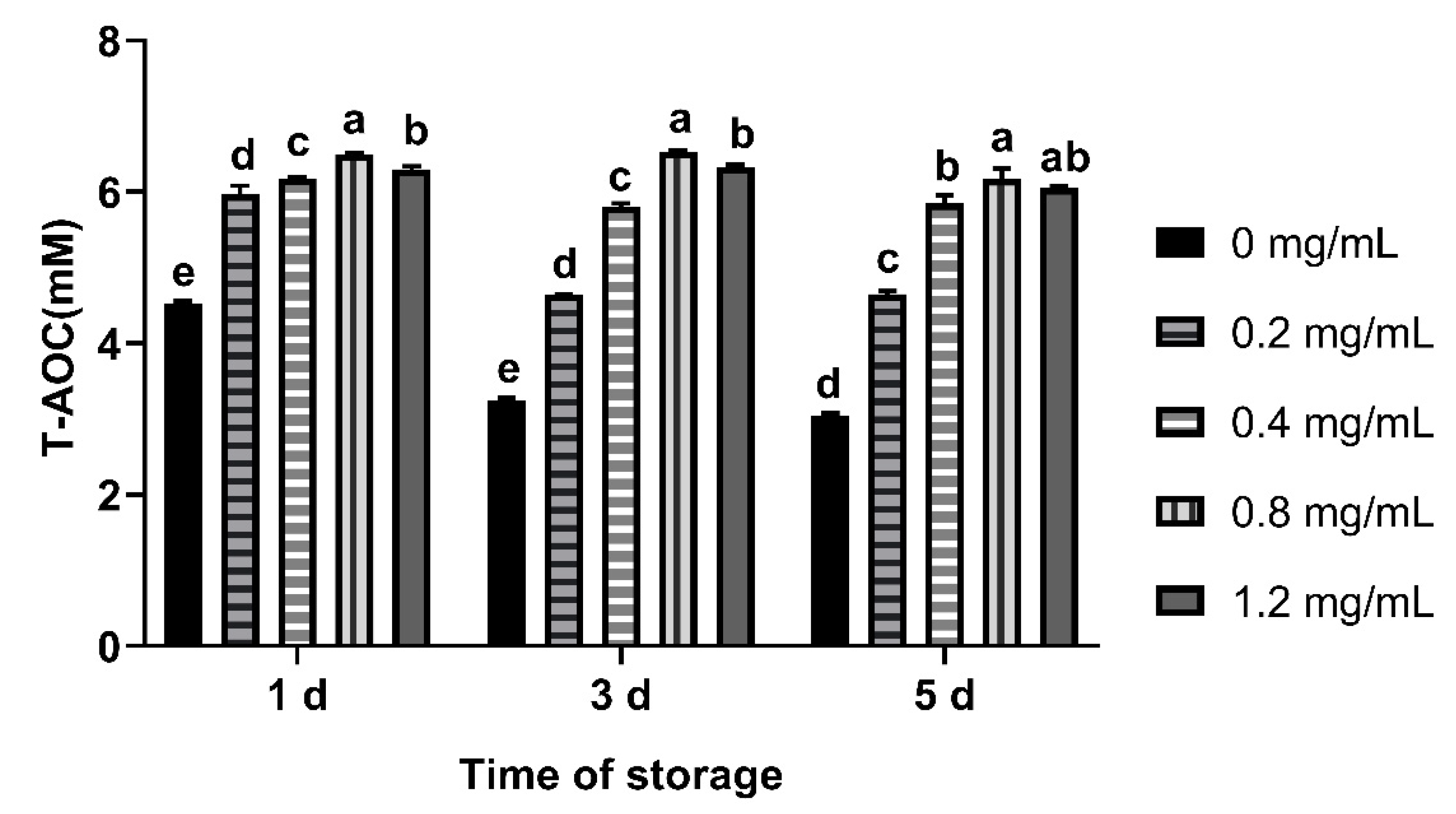
3.5. Effects of CGA on T-AOC in Seminal Plasma
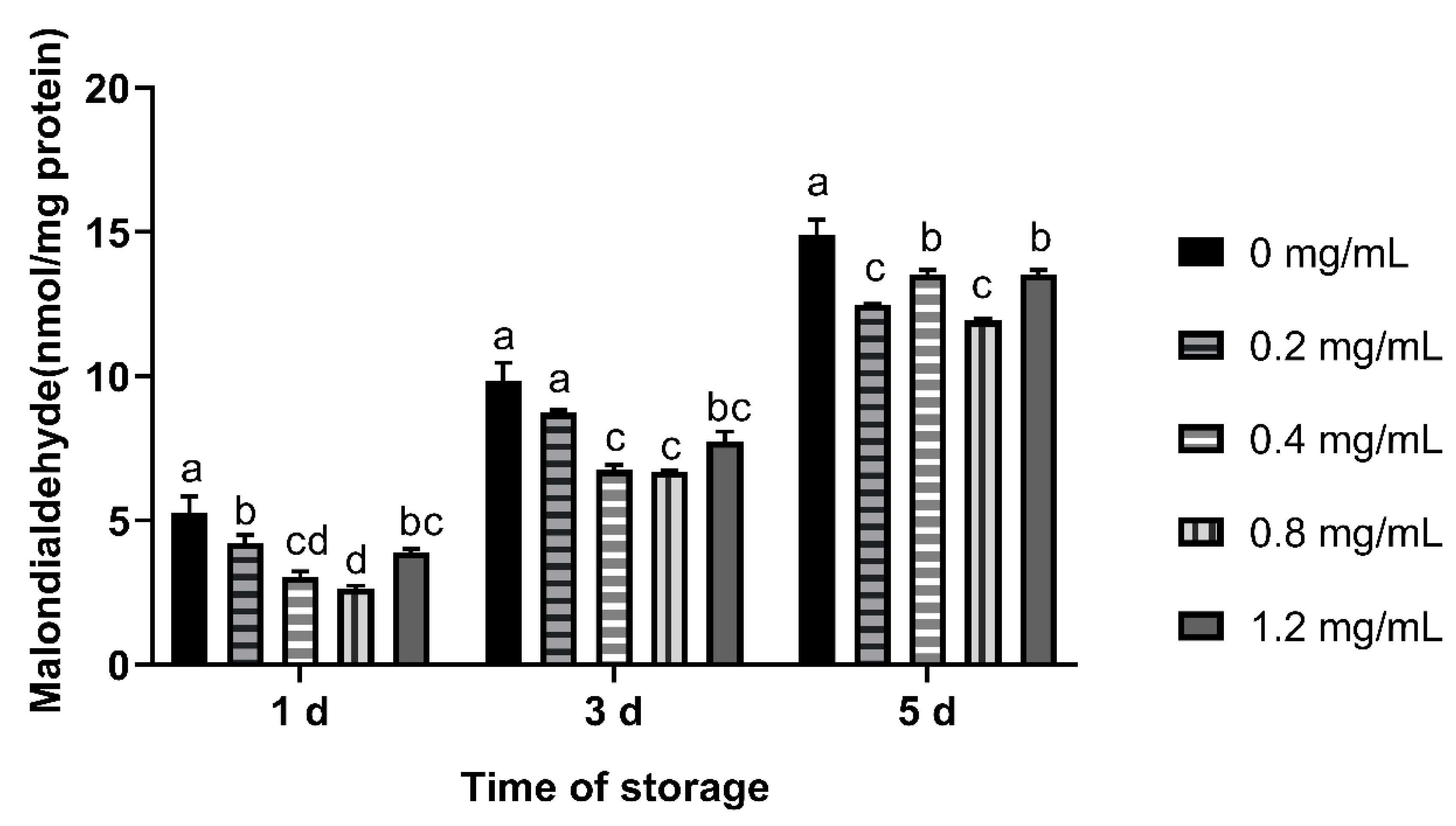
3.6. Effects of CGA on MDA in Sperm
3.7. Effects of CGA on SOD Activity in Sperm
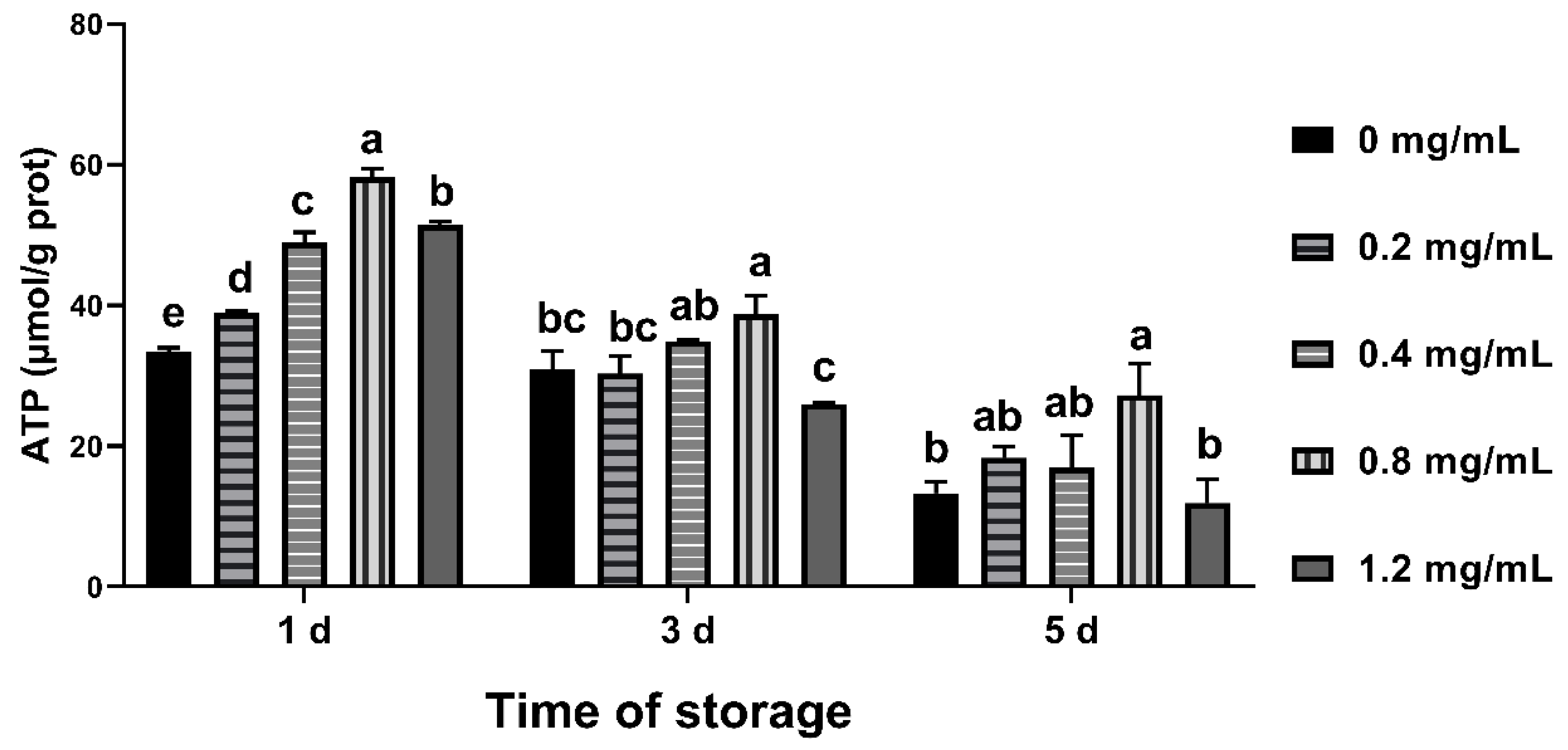
3.8. Effects of CGA on ATP Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shabbir, S.; Boruah, P.; Xie, L.; Kulyar, M.F.-E.-A.; Nawaz, M.; Yousuf, S.; Liu, T.; Jabeen, F.; Miao, X. Genome-wide transcriptome profiling uncovers differential miRNAs and lncRNAs in ovaries of Hu sheep at different developmental stages. Sci. Rep. 2021, 11, 5865. [Google Scholar] [CrossRef]
- Arando, A.; Gonzalez, A.; Delgado, J.; Arrebola, F.; Perez-Marín, C. Storage temperature and sucrose concentrations affect ram sperm quality after vitrification. Anim. Reprod. Sci. 2017, 181, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Layek, S.; Mohanty, T.; Kumaresan, A.; Parks, J. Cryopreservation of bull semen: Evolution from egg yolk based to soybean based extenders. Anim. Reprod. Sci. 2016, 172, 1–9. [Google Scholar] [CrossRef]
- Bailey, J.L.; Bilodeau, J.F.; Cormier, N. Semen cryopreservation in domestic animals: A damaging and capacitating phenomenon. J. Androl. 2000, 21, 1–7. [Google Scholar]
- Liu, G.; Pan, B.; Li, S.; Ren, J.; Wang, B.; Wang, C.; Su, X.; Dai, Y. Effect of bioactive peptide on ram semen cryopreservation. Cryobiology 2020, 97, 153–158. [Google Scholar] [CrossRef]
- Masoudi, R.; Shahneh, A.Z.; Towhidi, A.; Kohram, H.; Akbarisharif, A.; Sharafi, M. Fertility response of artificial insemination methods in sheep with fresh and frozen-thawed semen. Cryobiology 2017, 74, 77–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Abella, D.; Preve, M.; Villegas, N. Insemination time and dilution rate of cooled and chilled ram semen affects fertility. Theriogenology 2003, 60, 21–26. [Google Scholar] [CrossRef]
- Chauhan, M.S.; Kapila, R.; Gandhi, K.K.; Anand, S.R. Acrosome damage and enzyme leakage of goat spermatozoa during dilution, cooling and freezing. Andrologia 1994, 26, 21–26. [Google Scholar] [CrossRef]
- Leboeuf, B.; Restall, B.; Salamon, S. Production and storage of goat semen for artificial insemination. Anim. Reprod. Sci. 2000, 62, 113–141. [Google Scholar] [CrossRef]
- Aitken, R.J. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol. Reprod. Dev. 2017, 84, 1039–1052. [Google Scholar] [CrossRef]
- Teodoro, J.S.; Palmeira, C.M.; Rolo, A.P. Mitochondrial Membrane Potential (ΔΨ) Fluctuations Associated with the Metabolic States of Mitochondria; Humana Press: New York, NY, USA, 2018; Volume 1782, pp. 109–119. [Google Scholar] [CrossRef]
- Eslami, M.; Ghasemiyan, H.; Hashem, E.Z. Semen supplementation with palmitoleic acid promotes kinematics, microscopic and antioxidative parameters of ram spermatozoa during liquid storage. Reprod. Domest. Anim. 2016, 52, 49–59. [Google Scholar] [CrossRef]
- Yue, D.; Yan, L.; Luo, H.; Xu, X.; Jin, X. Effect of Vitamin E supplementation on semen quality and the testicular cell membranal and mitochondrial antioxidant abilities in Aohan fine-wool sheep. Anim. Reprod. Sci. 2010, 118, 217–222. [Google Scholar] [CrossRef]
- Festa, R.; Giacchi, E.; Raimondo, S.; Tiano, L.; Zuccarelli, P.; Silvestrini, A.; Meucci, E.; Littarru, G.P.; Mancini, A. Coenzyme Q10 supplementation in infertile men with low-grade varicocele: An open, uncontrolled pilot study. Andrologia 2013, 46, 805–807. [Google Scholar] [CrossRef]
- Andersen, A.H.; Thinnesen, M.; Failing, K.; Goericke-Pesch, S. Effect of reduced glutathione (GSH) supplementation to Tris-egg yolk extender on chilled semen variables of dogs. Anim. Reprod. Sci. 2018, 198, 145–153. [Google Scholar] [CrossRef]
- Kovacs-Nolan, J.; Rupa, P.; Matsui, T.; Tanaka, M.; Konishi, T.; Sauchi, Y.; Sato, K.; Ono, S.; Mine, Y. In Vitro and ex Vivo Uptake of Glutathione (GSH) across the Intestinal Epithelium and Fate of Oral GSH after in Vivo Supplementation. J. Agric. Food Chem. 2014, 62, 9499–9506. [Google Scholar] [CrossRef]
- Basioura, A.; Boscos, C.; Parrilla, I.; Tsousis, G.; Tsakmakidis, I. Effect of astaxanthin on the quality of boar sperm stored at 17 °C, incubated at 37 °C or under in vitro conditions. Reprod. Domest. Anim. 2018, 53, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Cheuquemán, C.; Arias, M.E.; Risopatrón, J.; Felmer, R.; Álvarez, J.; Mogas, T.; Sánchez, R. Supplementation of IVF medium with melatonin: Effect on sperm functionality and in vitro produced bovine embryos. Andrologia 2015, 47, 604–615. [Google Scholar] [CrossRef]
- Kurcer, Z.; Hekimoglu, A.; Aral, F.; Baba, F.; Sahna, E. Effect of melatonin on epididymal sperm quality after testicular ischemia/reperfusion in rats. Fertil. Steril. 2010, 93, 1545–1549. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Dai, J.; Zhang, S.; Sun, L.; Liu, Y.; Zhang, D. Effect of Thawing Rates and Antioxidants on Semen Cryopreservation in Hu Sheep. Biopreservation Biobanking 2021, 19, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Mandrone, M.; Lorenzi, B.; Venditti, A.; Guarcini, L.; Bianco, A.; Sanna, C.; Ballero, M.; Poli, F.; Antognoni, F. Antioxidant and anti-collagenase activity of Hypericum hircinum L. Ind. Crops Prod. 2015, 76, 402–408. [Google Scholar] [CrossRef]
- Ma, H.; Liu, D.; Wang, W.; Wang, L.; Fu, B.; Li, Z.; He, X. Effect of semen extender supplementation with trehalose, vitamin C and E on post-thaw min pig sperm qualities. Cryo Lett. 2015, 36, 308–312. [Google Scholar]
- Shin, H.S.; Satsu, H.; Bae, M.J.; Zhao, Z.; Ogiwara, H.; Totsuka, M.; Shimizu, M. Anti-inflammatory effect of chlorogenic acid on the IL-8 production in Caco-2 cells and the dextran sulphate sodium-induced coli-tis symptoms in C57BL/6 mice. Food Chem. 2015, 168, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Han, K. The spermatogenic effect of yacon extract and its constituents and their inhibition effect of testosterone metabolism. Biomol. Ther. 2013, 21, 153–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, B.A.; Chaves, B.R.; Teles, M.C.; Pontelo, T.P.; Oliveira, C.R.; de Souza, R.V.; Rodríguez-Gil, J.E.; Zangeronimo, M.G. Chlorogenic acid improves the quality of boar semen subjected to cooled storage at 15 °C. Andrologia 2018, 50, e12978. [Google Scholar] [CrossRef] [PubMed]
- Rabelo, S.S.; Resende, C.O.; Pontelo, T.P.; Chaves, B.R.; Pereira, B.A.; Da Silva, W.E.; Peixoto, J.V.; Pereira, L.J.; Zangeronimo, M.G. Chlorogenic acid improves the quality of boar semen processed in Percoll. Anim. Reprod. 2020, 17, e20190021. [Google Scholar] [CrossRef] [Green Version]
- Namula, Z.; Hirata, M.; Wittayarat, M.; Tanihara, F.; Nguyen, N.T.; Hirano, T.; Nii, M.; Otoi, T.; Thi, N.N. Effects of chlorogenic acid and caffeic acid on the quality of frozen-thawed boar sperm. Reprod. Domest. Anim. 2018, 53, 1600–1604. [Google Scholar] [CrossRef]
- Noto, D.; Collodel, G.; Cerretani, D.; Signorini, C.; Gambera, L.; Menchiari, A.; Moretti, E. Protective Effect of Chlorogenic Acid on Human Sperm: In Vitro Studies and Frozen–Thawed Protocol. Antioxidants 2021, 10, 744. [Google Scholar] [CrossRef]
- Allai, L.; Druart, X.; Contell, J.; Louanjli, N.; Ben Moula, A.; Badi, A.; Essamadi, A.; Nasser, B.; El Amiri, B. Effect of argan oil on liquid storage of ram semen in Tris or skim milk based extenders. Anim. Reprod. Sci. 2015, 160, 57–67. [Google Scholar] [CrossRef]
- Amini, S.; Masoumi, R.; Rostami, B.; Shahir, M.H.; Taghilou, P.; Arslan, H.O. Effects of supplementation of Tris-egg yolk extender with royal jelly on chilled and frozen-thawed ram semen characteristics. Cryobiology 2019, 88, 75–80. [Google Scholar] [CrossRef]
- Zarei, F.; Kia, H.D.; Masoudi, R.; Moghaddam, G.; Ebrahimi, M. Supplementation of ram’s semen extender with Mito-TEMPO I: Improvement in quality parameters and reproductive performance of cooled-stored semen. Cryobiology 2021, 98, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Y.; Guo, F.; Lu, B.; Sun, J.; Wang, J.; Ren, Z. iTRAQ-based proteomic analysis of sperm reveals candidate proteins that affect the quality of spermatozoa from boars on plateaus. Proteome Sci. 2021, 30, 9. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Rateb, S.A.; Khalifa, M.A.; Abd El-Hamid, I.S.; Shedeed, H.A. Enhancing liquid-chilled storage and cryo-preservation capacities of ram spermatozoa by supplementing the diluent with different additives. Asian Australas. J. Anim. Sci. 2020, 33, 1068–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, Y.; DesMarais, T.L.; Tong, Z.; Yao, Y.; Costa, M. Oxidative stress alters global histone modification and DNA methylation. Free Radic. Biol. Med. 2015, 82, 22–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jena, N.R. DNA damage by reactive species: Mechanisms, mutation and repair. J. Biosci. 2012, 37, 503–517. [Google Scholar] [CrossRef]
- Bucak, M.N.; Tuncer, P.B.; Sarıözkan, S.; Ulutaş, P.A.; Çoyan, K.; Başpınar, N.; Özkalp, B. Effects of hypotaurine, cysteamine and aminoacids solution on post-thaw microscopic and oxidative stress parameters of Angora goat semen. Res. Veter. Sci. 2009, 87, 468–472. [Google Scholar] [CrossRef]
- Elkhawagah, A.R.; Martino, N.A.; Ricci, A.; Storti, V.; Rumbolo, F.; Lange-Consiglio, A.; Vincenti, L. Effect of relaxin on cryopreserved beef bull semen characteristics. Cryobiology 2020, 95, 51–59. [Google Scholar] [CrossRef]
- Tian, X.; Li, D.; He, Y.; Zhang, W.; He, H.; Du, R.; Pang, W.; Yang, G.; Yu, T. Supplementation of salvianic acid A to boar semen extender to improve seminal quality and antioxidant capacity. Anim. Sci. J. 2019, 90, 1142–1148. [Google Scholar] [CrossRef]
- Gibb, Z.; Butler, T.J.; Morris, L.H.; Maxwell, W.M.; Grupen, C.G. Quercetin improves the postthaw characteristics of cryopreserved sex-sorted and nonsorted stallion sperm. Theriogenology 2013, 79, 1001–1009. [Google Scholar] [CrossRef]
- Merati, Z.; Farshad, A. Ginger and echinacea extracts improve the quality and fertility potential of frozen-thawed ram epididymal spermatozoa. Cryobiology 2020, 92, 138–145. [Google Scholar] [CrossRef]
- Robayo, I.; Montenegro, V.; Valdés, C.; Cox, J.F. CASA assessment of kinematic parameters of ram spermatozoa and their relationship to migration efficiency in ruminant cervical mucus. Reprod. Domest. Anim. 2008, 43, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Amirat, L.; Tainturier, D.; Jeanneau, L.; Thorin, C.; Gérard, O.; Courtens, J.L.; Anton, M. Bull semen in vitro fertility after cryopreservation using egg yolk LDL: A comparison with Optidyl®, a commercial egg yolk extender. Theriogenology 2004, 61, 895–907. [Google Scholar] [CrossRef]
- Moradi, A.R.; Malekinejad, H.; Farrokhi-Ardabili, F.; Bernousi, I. Royal Jelly improves the sperm parameters of ram semen during liquid storage and serves as an antioxidant source. Small Rumin. Researc. 2013, 2, 346–352. [Google Scholar] [CrossRef]
- Cheng, D.; Xi, Y.; Cao, J.; Cao, D.; Ma, Y.; Jiang, W. Protective effect of apple (Ralls) polyphenol extract against aluminum-induced cognitive impairment and oxidative damage in rat. Neurotoxicology 2014, 45, 111–120. [Google Scholar] [CrossRef]
- Larsen, L.; Scheike, T.; Jensen, T.K.; Bonde, J.P.; Ernst, E.; Hjollund, I.N.H.; Zhou, Y.; Skakkebæk, N.E.; Giwercman, A. Computer-assisted semen analysis parameters as predictors for fertility of men from the general population. The Danish First Pregnancy Planner Study Team. Hum. Reprod. 2000, 15, 1562–1567. [Google Scholar] [CrossRef] [Green Version]
- Hammerstedt, R.H.; Graham, J.K.; Nolan, J.P. Cryopreservation of mammalian sperm: What we ask them to survive. J. Androl. 1990, 11, 73–88. [Google Scholar]
- Kasimanickam, R.; Pelzer, K.D.; Kasimanickam, V.; Swecker, W.S.; Thatcher, C.D. Association of classical semen parameters, sperm DNA fragmentation index, lipid peroxidation and antioxidant enzymatic activity of semen in ram-lambs. Theriogenology 2006, 65, 1407–1421. [Google Scholar] [CrossRef]
- Windsor, D.P. Mitochondrial function and ram sperm fertility. Reprod. Fertil. Dev. 1997, 9, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Garrett, L.J.A.; Revell, S.G.; Leese, H.J. Adenosine Triphosphate Production by Bovine Spermatozoa and Its Relationship to Semen Fertilizing Ability. J. Androl. 2008, 29, 449–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swegen, A.; Lambourne, S.R.; Aitken, R.J.; Gibb, Z. Rosiglitazone Improves Stallion Sperm Motility, ATP Content, and Mitochondrial Function. Biol. Reprod. 2016, 95, 107. [Google Scholar] [CrossRef] [PubMed]
- Tremoen, N.H.; Gaustad, A.H.; Andersen-Ranberg, I.; van Son, M.; Zeremichael, T.T.; Frydenlund, K.; Grindflek, E.; Våge, D.I.; Myromslien, F.D. Relationship between sperm motility characteristics and ATP concentrations, and association with fertility in two different pig breeds. Anim. Reprod. Sci. 2018, 193, 226–234. [Google Scholar] [CrossRef] [Green Version]
- Berg, H.F.; Kommisrud, E.; Bai, G.; Gaustad, E.R.; Klinkenberg, G.; Standerholen, F.B.; Thorkildsen, L.T.; Waterhouse, K.E.; Ropstad, E.; Heringstad, B.; et al. Comparison of sperm adenosine triphosphate content, motility and fertility of immobilized and conventionally cryopreserved Norwegian Red bull semen. Theriogenology 2018, 121, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Q.; Wang, L.; Yang, G.; Hu, J. Effects of glutathione on sperm quality during liquid storage in boars. Anim. Sci. J. 2016, 87, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-G.; Yan, G.-J.; Hong, J.-Y.; Su, Z.-Z.; Yang, G.-S.; Li, Q.-W.; Hu, J.-H. Effects of Bovine Serum Albumin on Boar Sperm Quality during Liquid Storage at 17 °C. Reprod. Domest. Anim. 2015, 50, 263–269. [Google Scholar] [CrossRef] [PubMed]


| Index | Time (d) | 0 mg/mL | 0.2 mg/mL | 0.4 mg/mL | 0.8 mg/mL | 1.2 mg/mL |
|---|---|---|---|---|---|---|
| Viability (%) | 0 | 91.27 ± 0.68 | 90.96 ± 0.48 | 91.67 ± 0.44 | 91.01 ± 0.47 | 92.26 ± 0.43 |
| 1 | 83.53 ± 0.70 b | 83.08 ± 0.41 b | 83.74 ± 0.27 b | 86.52 ± 0.51 a | 85.24 ± 0.08 a | |
| 3 | 71.92 ± 0.64 | 74.30 ± 1.53 | 75.31 ± 1.13 | 75.13 ± 1.89 | 74.51 ± 0.95 | |
| 5 | 57.81 ± 0.06 c | 67.76 ± 0.49 b | 69.25 ± 0.38 a | 69.68 ± 0.76 a | 68.36 ± 0.10 ab | |
| 7 | 49.80 ± 1.08 c | 57.28 ± 0.9 b | 58.12 ± 0.36 b | 60.57 ± 0.51 a | 56.73 ± 0.46 b | |
| PM (%) | 0 | 83.73 ± 0.65 | 83.09 ± 1.24 | 83.24 ± 0.69 | 82.45 ± 0.42 | 84.43 ± 0.64 |
| 1 | 73.45 ± 0.98 b | 72.73 ± 0.82 b | 73.85 ± 0.51 b | 77.78 ± 1.16 a | 77.14 ± 0.08 a | |
| 3 | 60.51 ± 0.36 b | 64.35 ± 1.22 ab | 66.28 ± 1.48 a | 66.08 ± 2.79 a | 63.01 ± 0.90 ab | |
| 5 | 44.76 ± 0.81 c | 56.96 ± 0.23 ab | 58.04 ± 0.03 a | 58.66 ± 1.13 a | 54.97 ± 1.02 b | |
| 7 | 33.92 ± 3.01 b | 44.17 ± 1.45 a | 46.08 ± 0.02 a | 47.75 ± 2.13 a | 45.62 ± 1.94 a | |
| VSL(μm/s) | 0 | 47.80 ± 0.92 | 48.59 ± 0.44 | 50.11 ± 0.36 | 50.14 ± 0.69 | 50.14 ± 1.69 |
| 1 | 33.84 ± 1.06 c | 38.65 ± 1.18 ab | 35.78 ± 1.36 ab | 39.54 ± 1.18 a | 37.39 ± 0.32 ab | |
| 3 | 24.60 ± 0.33 | 24.89 ± 0.25 | 24.78 ± 0.56 | 24.62 ± 0.12 | 24.29 ± 0.24 | |
| 5 | 30.42 ± 0.61 b | 27.72 ± 0.10 c | 30.03 ± 0.41 b | 32.44 ± 0.39 a | 29.75 ± 0.74 b | |
| 7 | 23.52 ± 0.20 b | 23.77 ± 0.49 b | 24.94 ± 1.11 ab | 26.58 ± 0.10 a | 23.01 ± 0.76 b | |
| VCL(μm/s) | 0 | 82.19 ± 0.61 | 79.72 ± 0.64 | 82.62 ± 0.81 | 79.53 ± 0.76 | 79.85 ± 1.74 |
| 1 | 67.13 ± 2.19 c | 78.29 ± 2.42 a | 74.06 ± 2.59 ab | 80.11 ± 2.27 a | 77.51 ± 0.71 a | |
| 3 | 62.19 ± 1.92 | 61.23 ± 0.26 | 60.98 ± 0.30 | 61.42 ± 0.94 | 60.21 ± 1.54 | |
| 5 | 66.36 ± 0.94 | 62.07 ± 0.27 | 66.19 ± 0.88 | 69.63 ± 0.15 | 66.04 ± 1.40 | |
| 7 | 51.18 ± 0.26 b | 52.56 ± 1.80 b | 58.56 ± 1.63 a | 60.98 ± 0.01 a | 50.06 ± 2.10 b | |
| VAP(μm/s) | 0 | 58.12 ± 0.43 | 57.65 ± 0.89 | 58.42 ± 0.57 | 57.68 ± 0.93 | 57.29 ± 1.47 |
| 1 | 47.47 ± 1.55 b | 55.36 ± 1.71 a | 52.37 ± 1.83 ab | 56.64 ± 1.60 a | 54.81 ± 0.50 a | |
| 3 | 43.97 ± 1.36 | 43.29 ± 0.18 | 43.12 ± 0.22 | 43.43 ± 0.67 | 42.58 ± 1.09 |
| Index (%) | Time (d) | 0 mg/mL | 0.2 mg/mL | 0.4 mg/mL | 0.8 mg/mL | 1.2 mg/mL |
|---|---|---|---|---|---|---|
| Plasma membrane integrity | 0 | 77.69 ± 0.40 | 77.72 ± 0.60 | 76.78 ± 0.54 | 76.29 ± 0.08 | 76.38 ± 0.73 |
| 1 | 72.30 ± 0.08 b | 72.73 ± 0.16 b | 72.43 ± 0.55 b | 74.93 ± 0.18 a | 72.96 ± 0.56 b | |
| 3 | 60.60 ± 0.15 d | 63.21 ± 0.35 c | 65.40 ± 0.22 b | 69.00 ± 0.48 a | 68.45 ± 0.03 a | |
| 5 | 51.64 ± 0.16 d | 54.93 ± 0.19 c | 61.98 ± 0.38 b | 65.58 ± 0.55 a | 64.60 ± 0.54 a | |
| 7 | 46.72 ± 0.25 d | 55.21 ± 0.53 c | 57.50 ± 0.40 b | 61.15 ± 1.01 a | 58.07 ± 0.63 b | |
| Acrosomal integrity | 0 | 95.55 ± 0.05 | 95.39 ± 0.33 | 95.68 ± 0.23 | 95.42 ± 0.43 | 95.00 ± 0.20 |
| 1 | 79.14 ± 0.42 c | 81.22 ± 0.37 b | 82.28 ± 0.33 b | 84.19 ± 0.86 a | 78.73 ± 0.40 c | |
| 3 | 75.80 ± 0.73 b | 77.65 ± 0.40 b | 80.88 ± 0.53 a | 81.15 ± 0.89 a | 77.53 ± 0.24 b | |
| 5 | 69.15 ± 1.33 c | 74.09 ± 0.60 b | 76.09 ± 0.96 b | 80.61 ± 1.49 a | 75.30 ± 0.46 b | |
| 7 | 57.57 ± 2.31 c | 61.62 ± 1.42 c | 67.60 ± 0.86 b | 75.88 ± 0.58 a | 67.60 ± 0.75 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, L.; Sohail, T.; Kang, Y.; Sun, X.; Li, Y. Chlorogenic Acid Improves Quality of Chilled Ram Sperm by Mitigating Oxidative Stress. Animals 2022, 12, 163. https://doi.org/10.3390/ani12020163
Wang Y, Zhang L, Sohail T, Kang Y, Sun X, Li Y. Chlorogenic Acid Improves Quality of Chilled Ram Sperm by Mitigating Oxidative Stress. Animals. 2022; 12(2):163. https://doi.org/10.3390/ani12020163
Chicago/Turabian StyleWang, Yanhu, Liuming Zhang, Tariq Sohail, Yan Kang, Xiaomei Sun, and Yongjun Li. 2022. "Chlorogenic Acid Improves Quality of Chilled Ram Sperm by Mitigating Oxidative Stress" Animals 12, no. 2: 163. https://doi.org/10.3390/ani12020163






