Defining “Normal” in Pig Parturition
Abstract
Simple Summary
Abstract
1. Introduction
2. The Process of Parturition
2.1. Pregnancy in the Pig
2.2. Eutocia and Dystocia
| Reference | Dystocia Classification | Prevalence (%) |
|---|---|---|
| Nam and Sukon 2021 [30] | Inter-piglet interval greater than 45 min, application of obstetric assistance | 47 |
| Zamemba et al. 2019 [25] | Inter-piglet interval greater than 45 min | 11.5 |
| Oliviero et al. 2010 [26] | A farrowing greater than 300 min | N/A |
| Cowart 2007 [23] | Failure to deliver fetuses within 2 h from the onset of labour, an inter-piglet interval greater than 1 h, a gestation period beyond 116 days, sow illness, discoloured vulval discharge | 1 |
| Alonso-Spilsbury et al. 2004 [24] | Absence of uterine contractions, inter-piglet intervals greater than 1 h, application of obstetric assistance | N/A |
| Jones 1966 [27] | Labour lasting longer than 2 h in duration, application of obstetric assistance, uterine inertia | 0.25 |
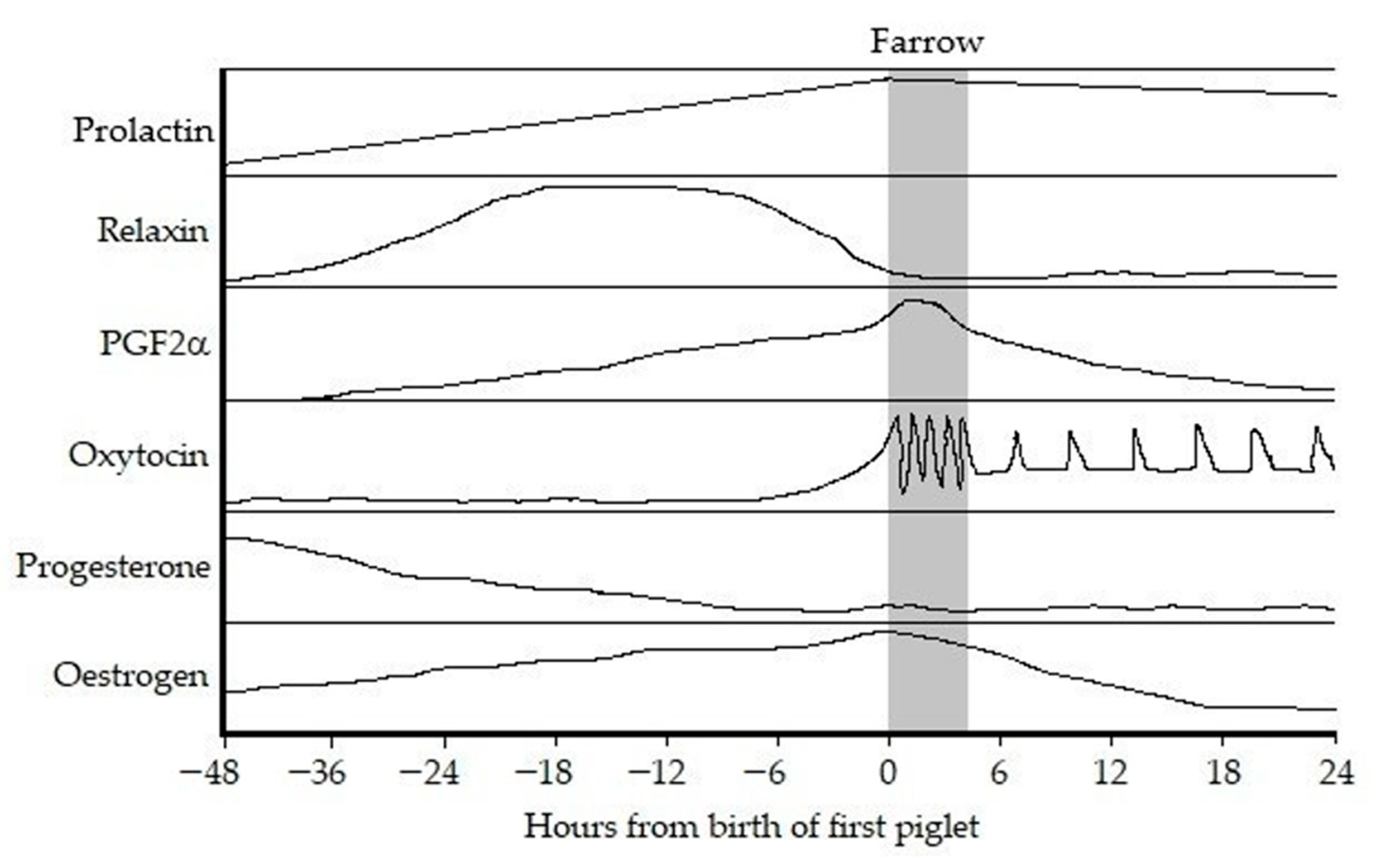
3. Endocrinology of Parturition
3.1. Periparturient Endocrinology
3.1.1. Oestrogen and Progesterone
3.1.2. Oxytocin
3.1.3. Prostaglandin F2α and Prostaglandin E2
3.1.4. Relaxin
3.1.5. Prolactin
4. Factors Affecting Parturition
4.1. Uterine Acticity and Contractility
4.2. Duration of Parturition
4.2.1. Overall Parturition
4.2.2. Inter-Piglet Interval
4.3. Farrowing Accommodation
4.3.1. Freedom of Movement
4.3.2. Availability of Nesting Material
4.3.3. Environmental Conditions
4.4. Sow Parity
4.5. Sow Nutrition
4.6. Piglet Factors
4.6.1. Piglet Size
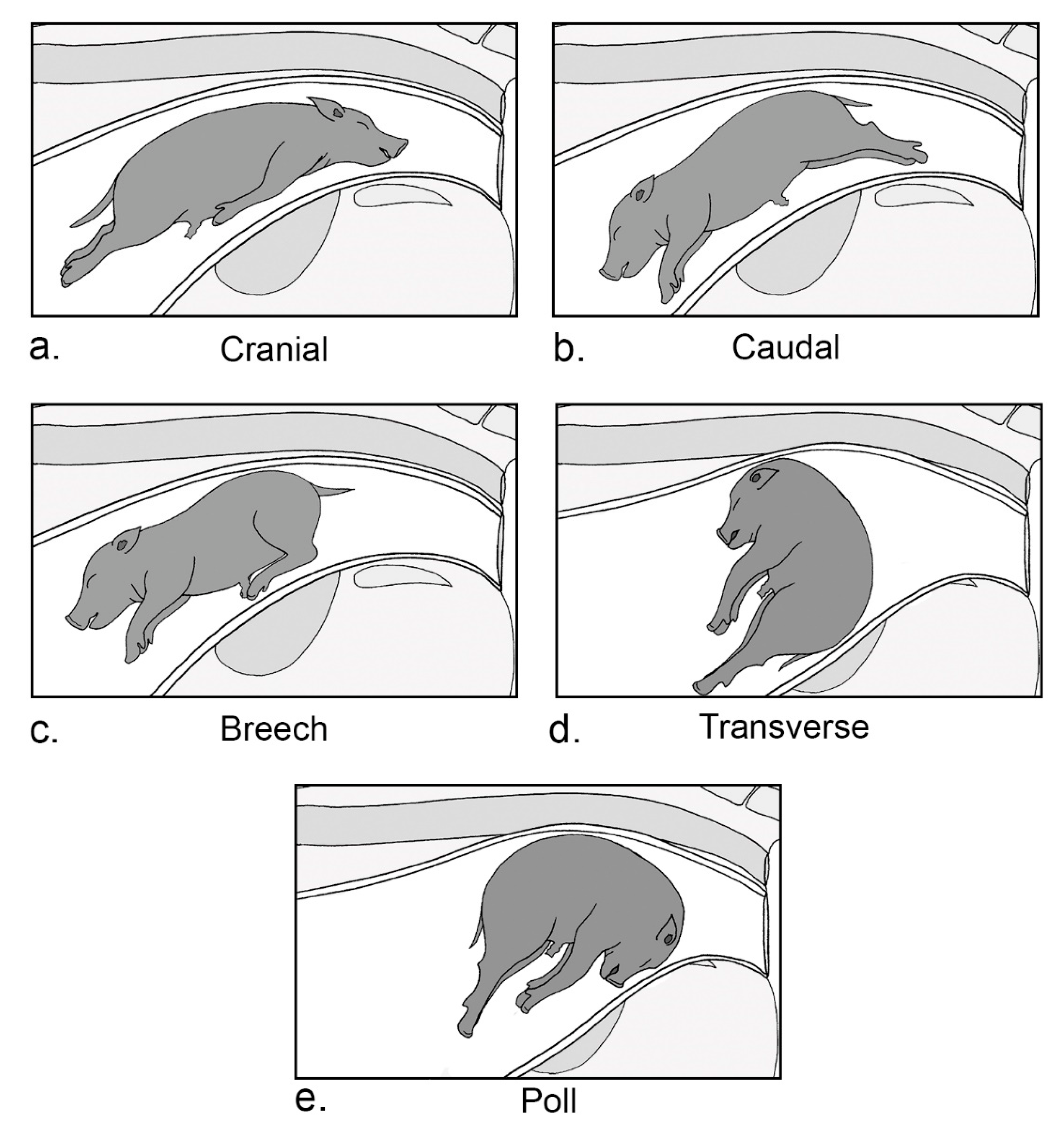
4.6.2. Piglet Presentation
4.6.3. Litter Size
5. Induction of Parturition
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Jacobson, C.; Bruce, M.; Kenyon, P.R.; Lockwood, A.; Miller, D.; Refshauge, G.; Masters, D.G. A review of dystocia in sheep. Small Rumin. 2020, 192, 106209. [Google Scholar] [CrossRef]
- Zaborski, D.; Grzesiak, W.; Szatkowska, I.; Dybus, A.; Muszynska, M.; Jedrzejczak, M. Factors affecting dystocia in cattle. Reprod. Domest. Anim. 2009, 44, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Peltoniemi, O.A.T.; Bjorkman, S.; Oliviero, C. Disorders of parturition and the puerperium in the gilt and sow. In Veterinary Reproduction and Obstetrics, 10th ed.; Noakes, D.E., Parkinson, T.J., England, G.C.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 315–325. [Google Scholar]
- Andersson, A.; Valros, A.; Rombin, J.; Jensen, P. Extensive infanticide in enclosed European wild boars (Sus scrofa). Appl. Anim. Behav. Sci. 2011, 134, 184–192. [Google Scholar] [CrossRef]
- Harris, M.J.; Bergeron, R.; Gonyou, H.W. Parturient behaviour and offspring-directed aggression in farmed wild boar of three genetic lines. Appl. Anim. Behav. Sci. 2001, 74, 153–163. [Google Scholar] [CrossRef]
- Peltoniemi, O.; Oliviero, C.; Yun, J.; Grahofer, A.; Björkman, S. Management practices to optimize the parturition process in the hyperprolific sow. J. Anim. Sci. 2020, 98, 96–106. [Google Scholar] [CrossRef]
- Kemp, B.; Da Silva, C.L.A.; Soede, N.M. Recent advances in pig reproduction: Focus on impact of genetic selection for female fertility. Reprod. Domest. Anim. 2018, 53, 28–36. [Google Scholar] [CrossRef]
- Kennedy, B.W.; Quinton, V.M.; Smith, C. Genetic changes in Canadian performance-tested pigs for fat depth and growth rate. Can. J. Anim. Sci. 1995, 76, 41–48. [Google Scholar]
- Oliviero, C.; Peltoniemi, O.A.T. Troubled process of parturition of the domestic pig. In Animal Reproduction in Veterinary Medicine; Aral, F., Ed.; InTech Open: London, UK, 2021; pp. 1–14. [Google Scholar]
- Oliviero, C.; Kothe, S.; Heinonen, M.; Valros, A.; Peltoniemi, O.A.T. Prolonged duration of farrowing is associated with subsequent decresed fertiltiy in sows. Theriogenology 2013, 79, 1095–1099. [Google Scholar] [CrossRef]
- Taverne, M.A.M. Physiology of parturition. Anim. Reprod. Sci. 1992, 28, 433–440. [Google Scholar] [CrossRef]
- Mainau, E.; Manteca, X. Pain and discomfort caused by parturition in cows and sows. Appl. Anim. Behav. Sci. 2011, 135, 241–251. [Google Scholar] [CrossRef]
- Lally, J.E.; Murtagh, M.M.; Macphail, S.; Thomson, R. More in hope than expectation: A systematic review of women’s expectations and experience of pain relief in labour. BMC Med. 2008, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Ison, S.H.; Clutton, R.E.; Di Giminiani, P.; Rutherford, K.M.D. A review of pain assessment in pigs. Front. Vet. Sci. 2016, 3, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ison, S.H.; Jarvis, S.; Rutherford, K.M.D. The identification of potential behavioural indicators of pain in periparturient sows. Res. Vet. Sci. 2016, 109, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Koketsu, Y. Variability and repeatability in gestation length related to litter performance in female pigs on commercial farms. Theriogenology 2007, 68, 123–127. [Google Scholar] [CrossRef]
- Anderson, L.L. Growth, protein content and distribution of early pig embryos. Anat. Rec. 1978, 190, 143–154. [Google Scholar] [CrossRef]
- Leiser, R.; Pfarrer, C.; Abd-Elnaeim, M.; Dantzer, V. Feto-maternal anchorage in epitheliochorial and endotheliochorial placental types studied by histology and microvascular corrosion casts. Placenta 1998, 12, 21–39. [Google Scholar] [CrossRef]
- First, N.L.; Bosc, M.J. Proposed mechanisms controlling parturition and the induction of parturition in swine. J. Anim. Sci. 1979, 48, 1407–1421. [Google Scholar] [CrossRef]
- Johansson, R.; Kerestes, D. Quarterly Hogs and Pigs December 2019; USDA-NASS: Washington, DC, USA, 2019. [Google Scholar]
- Claxton, G. How Danish pigs are averaging 30 piglets a year. Farmers Weekly. 27 September 2014. Available online: https://www.fwi.co.uk/livestock/how-danish-sows-are-averaging-30-piglets-a-year (accessed on 2 October 2022).
- Nam, N.H.; Sukon, P. Non-infectious risk factors for intrapartum stillbirth in a swine farm in the North of Vietnam. Vet. World 2021, 14, 1829–1834. [Google Scholar] [CrossRef]
- Cowart, R.P. Parturition and dystocia in swine. In Large Animal Theriogenology; Youngquist, R.S., Threlfall, W.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 778–784. [Google Scholar]
- Alonso-Spilsbury, M.; Mota-Rojas, D.; Martınez-Burnes, J.; Arch, E.; Mayagoitia, A.L.; Ramırez-Necoechea, R.; Olmos, A.; Trujillo, M.E. Use of oxytocin in penned sows and its effect on fetal intra-partum asphyxia. Anim. Reprod. Sci. 2004, 84, 157–167. [Google Scholar] [CrossRef]
- Zaremba, W.; Udluft, T.; Failing, K.; Bostedt, H. Analysis of the course of birth and the early postpartal period in pigs after hormonal partus induction with special consideration of complication rate. Anim. Vet. Sci. 2019, 7, 29–39. [Google Scholar] [CrossRef]
- Oliviero, C.; Heinonen, M.; Valros, A.; Peltoniemi, O.A.T. Environmental and sow-related factors affecting the duration of farrowing. Anim. Reprod. Sci. 2010, 119, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.E.T. Observations on parturition in the sow: Part II: The parturient and post-parturient phases. Br. Vet. J. 1966, 122, 471–478. [Google Scholar] [CrossRef]
- Jackson, P.G.G. Chapter 8—Dystocia in the sow. In Handbook of Veterinary Obstetrics, 2nd ed.; Jackson, P.G.G., Ed.; W.B. Saunders: Oxford, UK, 2004; pp. 129–140. [Google Scholar]
- Van Dijk, A.; Van Rens, B.T.T.M.; Van Der Lende, T.; Taverne, M.A.M. Factors affecting duration of the expulsive stage of parturition and piglet birth intervals in sows with uncomplicated, spontaneous farrowings. Theriogenology 2005, 64, 1573–1590. [Google Scholar] [CrossRef] [PubMed]
- Nam, N.H.; Sukon, P. Risk factors associated with dystocia in swine. Vet. World 2021, 14, 1835–1839. [Google Scholar] [CrossRef] [PubMed]
- Liggins, G.C.; Thorburn, G.D. Initiation of parturition. In Marshall’s Physiology of Reproduction, 4th ed.; Lamming, G.E., Ed.; Springer: Dordrecht, The Netherlands, 1994; Volume 3, pp. 863–1002. [Google Scholar]
- Bazer, F.W.; Thatcher, W.W. Theory of maternal recognition of pregnancy in swine based on estrogen controlled endocrine versus exocrine secretion of prostaglandin F2alpha by the uterine endometrium. Prostaglandins 1977, 14, 397–400. [Google Scholar] [CrossRef]
- Ellendorff, F.; Taverne, M.; Elsaesser, F.; Forsling, M.; Parvizi, N.; Naaktgeboren, C.; Smidt, D. Endocrinology of parturition in the pig. Anim. Reprod. Sci. 1979, 2, 323–334. [Google Scholar] [CrossRef]
- Sherwood, O.D.; Martin, P.A.; Chang, C.C.; Dzuik, P.J. Plasma relaxin levels during late pregnancy and at parturition in pigs with altered utero-ovarian connections. Biol. Reprod. 1977, 17, 101–103. [Google Scholar] [CrossRef]
- Gilbert, C.L. Endocrine regulation of peripartuient behaviour in pigs. Reprod. Suppl. 2001, 58, 263–266. [Google Scholar]
- Young, I.R. The comparative physiology of parturition in mammals. Front. Horm. Res. 2001, 27, 10–30. [Google Scholar] [CrossRef]
- Van der Meulen, J.; Helmond, F.A.; Oudenaarden, C.P.J. Corpus luteum function in the pig: Progesterone profiles of cyclic and pregnant gilts. Neth. J. Agri. Sci. 1990, 38, 45–52. [Google Scholar] [CrossRef]
- Miller, W.R.; Williams, R.; Pipes, G.W.; Turner, C.W. Conjugation, distribution, and biological half-life (t ½) of radioactive progesterone in plasma and red cells of bovine blood. J. Dairy Sci. 1963, 46, 1402–1404. [Google Scholar] [CrossRef]
- Randall, G.C.B.; Tsang, B.K. Influence of the fetal pituitary-adrenal axis on fetal and maternal progesterone and unconjugated oestrogen concentrations in the pig. J. Reprod. Fertil. 1986, 78, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Craig, V.A. Placental steroid metabolism in late pregnancy. In Control of Pig Reproduction; Cole, D.J.A., Foxcroft, G.R., Eds.; Butterworth Scientific: London, UK, 1982; pp. 405–418. [Google Scholar]
- Soloff, M.S.; Swartz, T.L. Characterization of a proposed oxytocin receptor in the uterus of the rat and sow. J. Biol. Chem. 1974, 249, 1376–1381. [Google Scholar] [CrossRef]
- Blanks, A.M.; Thorton, S. The role of oxytocin in parturition. BJOG: Int. J. Obstet. Gynaecol. 2003, 110, 46–51. [Google Scholar] [CrossRef]
- Carter, C.S.; Kenkel, W.M.; MacLean, E.L.; Wilson, S.R.; Perkeybile, A.M.; Yee, J.R.; Ferris, C.F.; Nazarloo, H.P.; Porges, S.W.; Davis, J.M.; et al. Is Oxytocin “Nature’s Medicine”? Pharmacol. Rev. 2020, 72, 829–861. [Google Scholar] [CrossRef]
- Gilbert, C.L. Oxytocin secretion and management of parturition in the pig. Reprod. Domest. Anim. 1999, 34, 193–200. [Google Scholar] [CrossRef]
- Ivell, R.; Kimura, T.; Müller, D.; Augustin, K.; Abend, N.; Bathgate, R.; Telgmann, R.; Balvers, M.; Tillmann, G.; Fuchs, A.-R. The structure and regulation of the oxytocin receptor. Exp. Physiol. 2001, 86, 289–296. [Google Scholar] [CrossRef]
- Russell, J.A.; Leng, G. Sex, parturition and motherhood without oxytocin? J. Endocrinol. 1998, 157, 343–359. [Google Scholar] [CrossRef]
- Kingsbury, M.A.; Bilbo, S.D. The inflammatory event of birth: How oxytocin signaling may guide the development of the brain and gastrointestinal system. Front. Endocrinol. 2019, 55, 100794. [Google Scholar] [CrossRef]
- Widowski, T.M.; Curtis, S.E.; Dziuk, P.J.; Wagner, W.C.; Sherwood, O.D. Behavioral and endocrine responses of sows to prostaglandin F2α and cloprostenol. Biol. Reprod. 1990, 43, 290–297. [Google Scholar] [CrossRef]
- Sherwood, O.D. Relaxin’s physiological roles and other diverse actions. Endocr. Rev. 2004, 25, 205–234. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-J.; Lu, J.-W.; Zhang, C.-Y.; Wang, W.; Ying, H.; Myatt, L.; Sun, K. PGE2 vs. PGF2α in human parturition. Placenta 2020, 104, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Bill, R.; Carmo, L.P.; Vidondo, B.; Nathues, H.; Grahofer, A. Effect of intramuscular and intravaginal PGE-2 treatment compared to intramuscular oxytocin treatment in eutocic sows on the farrowing performance in a free farrowing system. Theriogenology 2021, 161, 1–7. [Google Scholar] [CrossRef]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Watts, A.D.; Flint, A.P.F.; Foxcroft, G.R.; Porter, D.G. Plasma steroid, relaxin and dihydro-keto-prostaglandin F2a changes in the minipig in relation to myometrial electrical and mechanical activity in the prepartum period. J. Reprod. Fertil. 1988, 83, 553–564. [Google Scholar] [CrossRef]
- Sherwood, O.D.; Nara, B.S.; Crnekovic, V.E.; First, N.L. Relaxin concentrations in pig plasma after the administration of indomethacin and prostaglandin F2a during late pregnancy. Endocrinology 1979, 104, 1716–1721. [Google Scholar] [CrossRef] [PubMed]
- Porter, D.G.; Watts, A.D. Relaxin and progesterone are myometrial inhibitors in the ovariectomized non-pregnant mini-pig. J. Reprod. Fertil. 1986, 76, 205–213. [Google Scholar] [CrossRef]
- Kertiles, L.P.; Anderson, L.L. Effect of relaxin on cervical dilatation, parturition and lactiation in the pig. Biol. Reprod. 1979, 21, 57–68. [Google Scholar] [CrossRef]
- Castrén, H.; Algers, B.; de Passillé, A.-M.; Rushen, J.; Uvnäs-Moberg, K. Preparturient variation in progesterone, prolactin, oxytocin and somatostatin in relation to nest building in sows. Appl. Anim. Behav. Sci. 1993, 38, 91–102. [Google Scholar] [CrossRef]
- Boulton, M.I.; Wickens, A.; Goode, J.A.; Lawrence, A.B.; Gilbert, C.L. Does Prolactin Mediate Induced Nest-Building Behaviour inPseudopregnant Gilts Treated with PGF2a? J. Neuroendocrinol. 1998, 10, 601–609. [Google Scholar] [CrossRef]
- Farmer, C.; Petitclerc, D. Specific window of prolactin inhibition in late gestation decreases mammary parenchymal tissue development in gilts. J. Anim. Sci. 2003, 81, 1823–1829. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.B.; Wagner, W.C. Effect of dopamine agonists or antagonists, TRH, stress and piglet removal on plasma prolactin concentrations in lactating gilts. Theriogenology 1985, 23, 283–296. [Google Scholar] [CrossRef]
- Simões, J.; Stilwell, G. Dystocia and other abnormal occurrences during calving. In Calving Management and Newborn Calf Care; Gauly, M., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; pp. 81–111. [Google Scholar]
- Taverne, M.A.M.; Naaktgeboren, C.; Van Der Weyden, G.C. Myometrial activity and expulsion of fetuses. Anim. Reprod. Sci. 1979, 2, 117–131. [Google Scholar] [CrossRef]
- Taverne, M. Myometrial activity during pregnancy and parturition in the pig. In Proceedings-Easter School in Agricultural Science, University of Nottingham; Nottingham University Press: Nottingham, UK, 1982. [Google Scholar]
- Olmos-Hernández, A.; Trujillo-Ortega, M.E.; Alonso-Spilsbury, M.; Sánchez-Aparicio, P.; Ramírez-Necoechea, R.; Mota-Rojas, D. Foetal Monitoring, Uterine Dynamics and Reproductive Performance in Spontaneous Farrowings in Sows. J. Appl. Anim. Res 2008, 33, 181–185. [Google Scholar] [CrossRef]
- Taverne, M.A.M.; Naaktgeboren, C.; Elsaesser, F.; Forsling, M.L.; Vanderweyden, G.C.; Ellendorff, F.; Smidt, D. Myometrial electrical activity and plasma concentrations of progesterone, estrogens and oxytocin during late pregnancy and parturition in the miniature pig. Biol. Reprod. 1979, 21, 1125–1134. [Google Scholar] [CrossRef]
- Petricelli, C.D.; Resende, A.P.M.; Elito Júnior, J.; Araujo Júnior, E.; Alexandre, S.M.; Zanetti, M.R.D.; Nakamura, M.U. Distensibility and Strength of the Pelvic Floor Muscles of Women in the Third Trimester of Pregnancy. Biomed. Res. Int. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Nguyen, N.; Sukon, P. Associated factors for farrowing duration in sows with natural parturition in intensive conditions. J. World’s Poult. Res. 2020, 10, 320–324. [Google Scholar] [CrossRef]
- Oliviero, C.; Junnikkala, S.; Peltoniemi, O. The challenge of large litters on the immune system of the sow and the piglets. Reprod. Domest. Anim. 2019, 54, 12–21. [Google Scholar] [CrossRef]
- Langendijk, P.; Fleuren, M.; Van Hees, H.; Van Kempen, T. The course of parturition affects piglet condition at birth and survival and growth through the nursery phase. Animals 2018, 8, 60. [Google Scholar] [CrossRef]
- Tummaruk, P.; Sang-Gassanee, K. Effect of farrowing duration, parity number and the type of anti-inflammatory drug on postparturient disorders in sows: A clinical study. Trop. Anim. Health Prod. 2013, 45, 1071–1077. [Google Scholar] [CrossRef]
- Heber, L.; Petroman, C.; Petroman, I.; Bălan, I.; Marin, D.; Şandru, O.; Palade, S. Possibilities to Combat MMA Syndrome in Sows. Sci. Pap. Anim. Sci. Biotech. 2010, 43, 409–411. [Google Scholar]
- Hughes, P.; Smits, R.; Xie, Y.; Kirkwood, R. Relationships among gilt and sow live weight, P2 backfat depth, and culling rates. J. Swine Health Prod. 2010, 18, 301–305. [Google Scholar]
- Friendship, R.M.; Wilson, M.R.; Almond, G.W.; McMillan, I.; Hacker, R.R.; Pieper, R.; Swaminathan, S.S. Sow wastage: Reasons for and effect on productivity. Can. J. Vet. Res. 1986, 50, 205–208. [Google Scholar] [PubMed]
- Svendsen, J.; Nielsen, N.C.; Bille, N.; Riising, H.J. Causes of culling and death in sows. Nord Vet. Med. 1975, 27, 604–615. [Google Scholar]
- Dagorn, J.; Aumaitre, A. Sow culling: Reasons for and effect on productivity. Livest. Prod. Sci. 1979, 6, 167–177. [Google Scholar] [CrossRef]
- Jones, J.E.T. An Investigation of the Causes of Mortality and Morbidity in Sows in a Commercial Herd. Br. Vet. J. 1967, 123, 327–339. [Google Scholar] [CrossRef]
- Plush, K.; Weaver, A.; Staveley, L.; Van Wettere, W. Maternal magnesium sulfate supplementation in a pre-farrow diet improves factors important for piglet viability. Animals 2018, 8, 185. [Google Scholar] [CrossRef]
- Holyoake, P.K.; Dial, G.D.; Trigg, T.; King, V.L. Reducing pig mortality through supervision during the perinatal period. J. Anim. Sci. 1995, 73, 3543–3551. [Google Scholar] [CrossRef]
- Okinda, C.; Lu, M.; Nyalala, I.; Li, J.; Shen, M. Asphyxia occurrence detection in sows during the farrowing phase by inter-birth interval evaluation. Comput. Electron. Agric. 2018, 152, 221–232. [Google Scholar] [CrossRef]
- Fraser, D.; Phillips, P.A.; Thompson, B.K. Farrowing behaviour and stillbirth in two environments: An evaluation of the restraint-stillbirth hypothesis. Appl. Anim. Behav. Sci. 1997, 55, 51–66. [Google Scholar] [CrossRef]
- Canario, L.; Cantoni, E.; Le Bihan, E.; Caritez, J.C.; Billon, Y.; Bidanel, J.P.; Foulley, J.L. Between-breed variability of stillbirth and its relationship with sow and piglet characteristics. J. Anim. Sci. 2006, 84, 3185–3196. [Google Scholar] [CrossRef] [PubMed]
- Biensen, N.; Von Borell, E.H.; Ford, S.P. Effects of space allocation and temperature on periparturient maternal behaviors, steroid concentrations, and piglet growth rates. J. Anim. Sci. 1996, 74, 2641–2648. [Google Scholar] [CrossRef]
- Gu, Z.; Gao, Y.; Lin, B.; Zhong, Z.; Liu, Z.; Wang, C.; Li, B. Impacts of a freedom farrowing pen design on sow behaviours and performancem farrowing pen design on sow behaviours and performance. Prev. Vet. Med. 2011, 102, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, C.; Heinonen, M.; Valros, A.; Hälli, O.; Peltoniemi, O.A.T. Effect of the environment on the physiology of the sow during late pregnancy, farrowing and early lactation. Anim. Reprod. Sci. 2008, 105, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Cronin, G.M.; Lefebure, B.; McClintock, S. A comparison of piglet production and survival in the Werribee Farrowing Pen and conventional farrowing crates at a commercial farm. Aust. J. Exp. Agric. 2000, 40, 17–23. [Google Scholar] [CrossRef]
- Lawrence, A.; Lewis, L.; Hofmeyer, G.J.; Styles, C. Maternal positions and mobility during first stage labour (review). Cochrane Database Syst. Rev. 2013, 10. [Google Scholar] [CrossRef]
- Lawrence, A.B.; Petherick, J.C.; McLean, K.A.; Deans, L.A.; Chirnside, J.; Gaughan, A.; Clutton, E.; Terlouw, E.M.C. The effect of environment on behaviour, plasma cortisol and prolactin in parturient sows. Appl. Anim. Behav. Sci. 1994, 39, 313–330. [Google Scholar] [CrossRef]
- Hales, J.; Moustsen, V.A.; Nielsen, M.B.F.; Hansen, C.F. The effect of temporary confinement of hyperprolific sows in Sow Welfare and Piglet protection pens on sow behaviour and salivary cortisol concentrations. Appl. Anim. Behav. Sci. 2016, 183, 19–27. [Google Scholar] [CrossRef]
- Hansen, C.F.; Hales, J.; Weber, P.M.; Edwards, S.A.; Moustsen, V.A. Confinement of sows 24 h before expected farrowing affects the performance of nest building behaviours but not progress of parturition. Appl. Anim. Behav. Sci. 2017, 188, 1–8. [Google Scholar] [CrossRef]
- 2008/120/EC; Council Directive 2008/120/EC of 18 December 2008 Laying Down Minimum Standards for the Protection of Pigs. United Nations Environment Programme: Nairobi, Kenya, 2008.
- Lammers, G.J.; De Lange, A. Pre- and post-farrowing behaviour in primiparous domesticated pigs. Appl. Anim. Behav. Sci. 1986, 15, 31–43. [Google Scholar] [CrossRef]
- Gustafsson, M.; Jensen, P.; de Jonge, F.H.; Illmann, G.; Spinka, M. Maternal behaviour of domestic sows and crosses between domestic sows and wild boar. Appl. Anim. Behav. Sci. 1999, 65, 29–42. [Google Scholar] [CrossRef]
- Plush, K.J.; McKenny, L.A.; Nowland, T.L.; van Wettere, W.H.E.J. The effect of hessian and straw as nesting materials on sow behaviour and piglet survival and growth to weaning. Animal 2021, 15, 100273. [Google Scholar] [CrossRef] [PubMed]
- Malmkvist, J.; Damgaard, B.M.; Pedersen, L.J.; Jørgensen, E.; Thodberg, K.; Chaloupková, H.; Bruckmaier, R.M. Effects of thermal environment on hypothalamic-pituitary-adrenal axis hormones, oxytocin, and behavioral activity in periparturient sows. J. Anim. Sci. 2009, 87, 2796–2805. [Google Scholar] [CrossRef] [PubMed]
- Malmkvist, J.; Pedersen, L.J.; Kammersgaard, T.S.; Jørgensen, E. Influence of thermal environment on sows around farrowing and during the lactation period. J. Anim. Sci. 2012, 90, 3186–3199. [Google Scholar] [CrossRef] [PubMed]
- Muns, R.; Malmkvist, J.; Larsen, M.L.V.; Sørensen, D.; Pedersen, L.J. High environmental temperature around farrowing induced heat stress in crated sows. J. Anim. Sci. 2016, 94, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Lucy, M.C.; Safranski, T.J. Heat stress in pregnant sows: Thermal responses and subsequent performance of sows and their offspring. Mol. Reprod. Dev. 2017, 84, 946–956. [Google Scholar] [CrossRef]
- Pedersen, L.J.; Jensen, T. Effects of late introduction of sows to two farrowing environments on the progress of farrowing and maternal behavior. J. Anim. Sience 2008, 86, 2730–2737. [Google Scholar] [CrossRef][Green Version]
- Yun, J.; Swan, K.-M.; Oliviero, C.; Peltoniemi, O.; Valros, A. Effects of prepartum housing environment on abnormal behaviour, the farrowing process, and interactions with circulating oxytocin in sows. Appl. Anim. Behav. Sci. 2015, 162, 20–25. [Google Scholar] [CrossRef]
- Fahmy, M.H.; Friend, D.W. Factors influencing, and repeatabilty of the duration of farrowing in Yorkshire sows. Can. J. Anim. Sci. 1981, 61, 17–22. [Google Scholar] [CrossRef]
- Cronin, G.M.; Schirmer, B.N.; McCallum, T.H.; Smith, J.A.; Butler, K.L. The effects of providing sawdust to pre-parturient sows in farrowing crates on sow behaviour, the duration of parturition and the occurrence of intra-partum stillborn piglets. Appl. Anim. Behav. Sci. 1993, 36, 301–315. [Google Scholar] [CrossRef]
- Ju, M.; Wang, X.; Li, X.; Zhang, M.; Shi, L.; Hu, P.; Zhang, B.; Han, X.; Wang, K.; Li, X.; et al. Effects of Litter Size and Parity on Farrowing Duration of Landrace × Yorkshire Sows. Animals 2021, 12, 94. [Google Scholar] [CrossRef] [PubMed]
- Lucia, T.; Corrêa, M.N.; Deschamps, J.C.; Bianchi, I.; Donin, M.A.; Machado, A.C.; Meincke, W.; Matheus, J.E.M. Risk factors for stillbirths in two swine farms in the south of Brazil. Prev. Vet. Med. 2002, 53, 285–292. [Google Scholar] [CrossRef]
- Björkman, S.; Oliviero, C.; Rajala-Schultz, P.J.; Soede, N.M.; Peltoniemi, O.A.T. The effect of litter size, parity and farrowing duration on placenta expulsion and retention in sows. Theriogenology 2017, 92, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Rens, B.T.V.; Lende, T.V.D. Parturition in gilts: Duration of farrowing, birth intervals and placenta expulsion in relation to maternal, piglet and placental traits. Theriogenology 2004, 62, 331–352. [Google Scholar] [CrossRef] [PubMed]
- Ahlstrom, S.; Jarvis, S.; Lawrence, A.B. Savaging gilts are more restless and more responsive to piglets during the expulsive phase of parturition. Appl. Anim. Behav. 2002, 76, 83–91. [Google Scholar] [CrossRef]
- Lawrence, A.B.; Petherick, J.C.; McLean, K.A.; Deans, L.; Chirnside, J.; Vaughan, A.; Gilbert, C.L.; Forsling, M.L.; Russell, J.A. The effects of chronic environmental stress on parturition and on oxytocin and vasopressin secretion in the pig. Anim. Reprod. Sci. 1995, 38, 251–264. [Google Scholar] [CrossRef]
- Langendijk, P.; Plush, K. Parturition and its relationship with stillbirths and asphyxiated piglets. Animals 2019, 9, 885. [Google Scholar] [CrossRef]
- Pearodwong, P.; Muns, R.; Tummaruk, P. Prevalence of constipation and its influence on post-parturient disorders in tropical sows. Trop. Anim. Health Prod. 2016, 48, 525–531. [Google Scholar] [CrossRef]
- Tabeling, R.; Schwier, S.; Kamphues, J. Effects of different feeding and housing conditions on dry matter content and consistency of faeces in sows. J. Anim. Physiol. Anim. Nutr. 2003, 87, 116–121. [Google Scholar] [CrossRef]
- Oliviero, C.; Kokkonen, T.; Heinonen, M.; Sankari, S.; Peltoniemi, O. Feeding sows with high fibre diet around farrowing and early lactation: Impact on intestinal activity, energy balance related parameters and litter performance. Res. Vet. Sci. 2009, 86, 314–319. [Google Scholar] [CrossRef]
- Mroz, Z.; Jongbloed, A.W.; Lenis, N.P.; Vreman, K. Water in pig nutrition: Physiology, allowances and environmental implications. Nutr. Res. Rev. 1995, 8, 137–164. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fahmy, M.H.; Holtmann, W.B.; Macintyre, T.M.; Moxley, J.E. Evaluation of piglet mortality in 28 two-breed crosses among eight breeds of pig. Anim. Sci. 1978, 26, 277–285. [Google Scholar] [CrossRef]
- Motsi, P.; Sakuhuni, C.; Halimani, T.E.; Bhebhe, E.; Ndiweni, P.N.B.; Chimonyo, M. Influence of parity, birth order, litter size and birth weight on duration of farrowing and birth intervals in commercial exotic sows in Zimbabwe. Anim. Sci. 2006, 82, 569–574. [Google Scholar] [CrossRef]
- Quiniou, N.; Dagorn, J.; Gaudré, D. Variation of piglets’ birth weight and consequences on subsequent performance. Livest. Prod. Sci. 2002, 78, 63–70. [Google Scholar] [CrossRef]
- Herpin, P.; Le Dividich, J.; Hulin, J.C.; Fillaut, M.; De Marco, F.; Bertin, R. Effects of the level of asphyxia during delivery on viability at birth and early postnatal vitality of newborn pigs. J. Anim. Sci. 1996, 74, 2067–2075. [Google Scholar] [CrossRef] [PubMed]
- Mainau, E.; Dalmau, A.; Ruiz-De-La-Torre, J.L.; Manteca, X. A behavioural scale to measure ease of farrowing in sows. Theriogenology 2010, 74, 1279–1287. [Google Scholar] [CrossRef]
- Reimers, T.J.; Dziuk, P.J.; Bahr, J.; Sprecher, D.J.; Webel, S.K.; Harmon, B.G. Transuterine embryonal migration in sheep, anteroposterior orientation of pig and sheep fetuses and presentation of piglets at birth. J. Anim. Sci. 1973, 37, 1212–1217. [Google Scholar] [CrossRef]
- Andersson, E.; Frössling, J.; Engblom, L.; Algers, B.; Gunnarsson, S. Impact of litter size on sow stayability in Swedish commercial piglet producing herds. Acta Vet. Scand. 2015, 58, 31. [Google Scholar] [CrossRef]
- Thorsen, C.K.; Schild, S.-L.A.; Rangstrup-Christensen, L.; Bilde, T.; Pedersen, L.J. The effect of farrowing duration on maternal behavior of hyperprolific sows in organic outdoor production. Livest. Sci. 2017, 204, 92–97. [Google Scholar] [CrossRef]
- Ison, S.H.; Jarvis, S.; Rutherford, K.M.D. A survey of sow management at farrowing in the UK. Anim. Welf. 2016, 25, 309–317. [Google Scholar] [CrossRef]
- Monteiro, M.S.; Muro, B.B.D.; Poor, A.P.; Leal, D.F.; Carnevale, R.F.; Shiroma, M.P.; Almond, G.W.; Garbossa, C.A.P.; Moreno, A.M.; Viana, C.H.C. Effects of farrowing induction with prostaglandins on farrowing traits and piglet performance: A systematic review and meta-analysis. Theriogenology 2022, 180, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kirkden, R.D.; Broom, D.M.; Andersen, I.L. Piglet mortality: The impact of induction of farrowing using prostaglandins and oxytocin. Anim. Reprod. Sci. 2013, 138, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.M.; Shirley, L.K.; Sharp, K.; Garcia, R.; Suarez-Trujillo, A.; Stewart, K.R. Effects of induction on the farrowing process and piglet blood parameters at the time of farrowing. Transl. Anim. Sci. 2021, 5, txab032. [Google Scholar] [CrossRef]
- Papadopoulos, G.A.; Vanderhaeghe, C.; Janssens, G.P.J.; Dewulf, J.; Maes, D.G.D. Risk factors associated with postpartum dysgalactia syndrome in sows. Vet. J. 2010, 184, 167–171. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walls, A.; Hatze, B.; Lomax, S.; Bathgate, R. Defining “Normal” in Pig Parturition. Animals 2022, 12, 2754. https://doi.org/10.3390/ani12202754
Walls A, Hatze B, Lomax S, Bathgate R. Defining “Normal” in Pig Parturition. Animals. 2022; 12(20):2754. https://doi.org/10.3390/ani12202754
Chicago/Turabian StyleWalls, Alexandra, Bianca Hatze, Sabrina Lomax, and Roslyn Bathgate. 2022. "Defining “Normal” in Pig Parturition" Animals 12, no. 20: 2754. https://doi.org/10.3390/ani12202754
APA StyleWalls, A., Hatze, B., Lomax, S., & Bathgate, R. (2022). Defining “Normal” in Pig Parturition. Animals, 12(20), 2754. https://doi.org/10.3390/ani12202754






