B-Mode and Contrast-Enhanced Ultrasonography Aspects of Benign and Malignant Superficial Neoplasms in Dogs: A Preliminary Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Study Population
3.2. Imaging Results
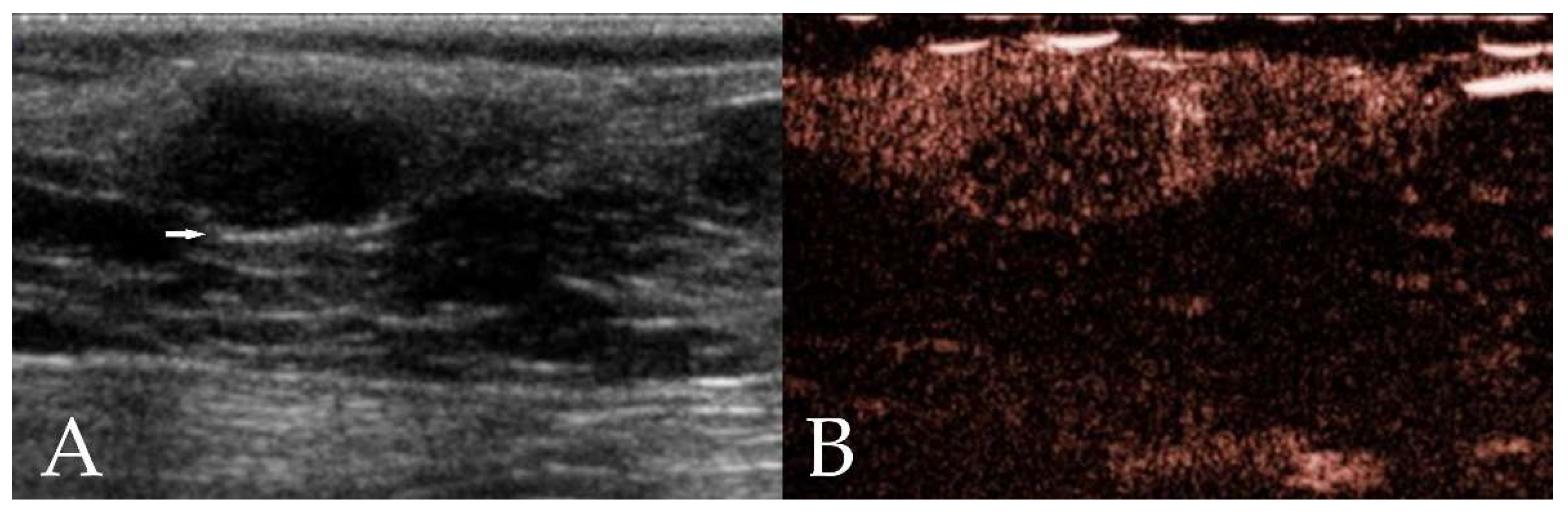
3.2.1. B-Mode Ultrasound
3.2.2. Qualitative CEUS
3.2.3. Quantitative CEUS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Histological Type | Number | Mean WoAUC (a.u.) |
|---|---|---|
| Benign neoplasms | ||
| Adenoma | 11 | 15,442 |
| Complex adenoma | 4 | 10,443 |
| Mastocytoma Grade I | 1 | 1167 |
| Malignant neoplasms | ||
| Complex adenocarcinoma Grade I | 2 | 9677 |
| Adenocarcinoma Grade I | 7 | 5016 |
| Adenocarcinoma Grade II | 2 | 7488 |
| Adenocarcinoma Grade III | 3 | 13,688 |
| Adenocarcinoma NG | 2 | 6931 |
| Mastocytoma Grade II | 5 | 2247 |
| Mastocytoma Grade III | 2 | 7539 |
| Mastocytoma NG | 1 | 8314 |
| Fibrosarcoma | 4 | 4156 |
| Neurofibrosarcoma | 1 | 807 |
| Schwannoma | 4 | 3207 |
| Osteosarcoma | 3 | 4778 |
| Extra skeletal osteosarcoma | 2 | 533 |
| Inflammatory carcinoma | 2 | 13,541 |
| Squamous cell carcinoma | 2 | 2307 |
| Cystadenocarcinoma | 1 | 14,820 |
| Complex carcinoma | 1 | 1491 |
| Melanoma | 1 | 3290 |
| Chondrosarcoma | 1 | 96 |
| Histiocytic sarcoma | 1 | 1675 |
References
- Dobson, J.M.; Lascelles, B.D. BSAVA Manual of Canine and Feline Oncology, 3rd ed.; British Small animal Veterinary Association: Quedgeley, UK, 2011; pp. 1–376. [Google Scholar]
- Kamstock, D.A.; Russell, D.S.; Powers, B.E. The pathology of neoplasia. In Withrow and MacEwen’s Small Animal Clinical Oncology-E-Book, 6th ed.; Vail, D.M., Thamm, D.H., Liptak, J.M., Eds.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2019; pp. 61–80. [Google Scholar]
- Nykamp, S.; Randall, E. Diagnostic imaging in oncology. In Withrow and MacEwen’s Small Animal Clinical Oncology-E-Book, 6th ed.; Vail, D.M., Thamm, D.H., Liptak, J.M., Eds.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2019; pp. 113–125. [Google Scholar]
- Dobson, J.M.; Samuel, S.; Milstein, H.; Rogers, K.; Wood, J.L.N. Canine neoplasia in the UK: Estimates of incidence rates from a population of insured dogs. J. Small Anim. Pract. 2002, 43, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Merlo, D.F.; Rossi, L.; Pellegrino, C.; Ceppi, M.; Cardellino, U.; Capurro, C.; Ratto, A.; Sambucco, P.L.; Sestito, V.; Tanara, G.; et al. Cancer Incidence in Pet Dogs: Findings of the Animal Tumor Registry of Genoa, Italy. J. Vet. Intern. Med. 2008, 22, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Brønden, L.B.; Nielsen, S.S.; Toft, N.; Kristensen, A.T. Data from the Danish veterinary cancer registry on the occurrence and distribution of neoplasms in dogs in Denmark. Vet. Rec. 2010, 166, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Wisner, E.R.; Pollard, R.E. Trends in veterinary cancer imaging. Vet. Comp. Oncol. 2004, 2, 49–74. [Google Scholar] [CrossRef]
- Mattoon, J.S.; Nyland, T.G. Fundamentals of diagnostic ultrasound. In Small Anim Diagnostic Ultrasound Mattoon, 3rd ed.; Mattoon, J.S., Nyland, T.G., Eds.; Elsevier: St. Louis, MO, USA, 2015; pp. 1–49. [Google Scholar]
- Nyman, H.T.; Nielsen, O.L.; McEvoy, F.J.; Lee, M.H.; Martinussen, T.; Hellmén, E.; Kristensen, A.T. Comparison of B-mode and Doppler ultrasonographic findings with histologic features of benign and malignant mammary tumors in dogs. Am. Coll. Vet. Radiol. 2006, 67, 985–991. [Google Scholar] [CrossRef]
- Nyman, H.T.; Kristensen, A.; Lee, M.H.; Martinussen, T.; McEvoy, F.J. Characterization of canine superficial tumors using gray-scale B mode, color flow mapping, and spectral doppler ultrasonography-A multivariate study. Vet. Radiol. Ultrasound 2006, 47, 192–198. [Google Scholar] [CrossRef]
- Brandão Guedes, P.E.; Bandeira Thame Daniel, H.; Oliveira Rosa Sampaio, K.M.; Barboza da Silva, E.; Ferreira, M.L.; Lima de Lavor, M.S.; De Oliveira Clark, R.M.; Wenceslau, A.A.; Abou Said, R.; Lessa Silva, F. Clinical and Ultrasonographic Aspects of Benign and Malignant Mammary Tumors in Female Dogs. Acta Sci. Vet. 2020, 48, 1–10. [Google Scholar] [CrossRef]
- Loh, Z.H.K.; Allan, G.S.; Nicoll, R.G.; Hunt, G.B. Ultrasonographic characteristics of soft tissue tumours in dogs. Aust. Vet. J. 2009, 87, 323–329. [Google Scholar] [CrossRef]
- Soler, M.; Dominguez, E.; Lucas, X.; Novellas, R.; Gomes-coelho, K.V.; Espada, Y.; Agut, A. Comparison between ultrasonographic findings of benign and malignant canine mammary gland tumours using B-mode, colour Doppler, power Doppler and spectral Doppler. Res. Vet. Sci. 2016, 107, 141–146. [Google Scholar] [CrossRef]
- Feliciano, M.A.R.; Ramirez, A.R.U.; Maronezi, M.C.; Maciel, R.A.; Avante, M.L.; Senhorello, I.L.S.; Mucédola, T.; Gasser, B.; Carvalho, C.F.; Vicente, W.R.R. Accuracy of four ultrasonography techniques in predicting histopathological classification of canine mammary carcinomas. Am. Coll. Vet. Radiol. 2018, 59, 444–452. [Google Scholar] [CrossRef]
- Balaci, I.M.; Ciupe, S.; Pop, A.R.; Parlapan, L.; Arion, A.; Vidrighinescu, R.; Groza, I.S. Ultrasonographig Aspects of Mammary Tumours in Bitches. Bull. UASVM Vet. Med. 2014, 71, 515–516. [Google Scholar] [CrossRef] [Green Version]
- Pietsch, H. Ultrasound contrast agents. In Small Animal Imaging, 2nd ed.; Kiessling, F., Pichler, B.J., Hauf, P., Eds.; Springer: Berlin, Germany, 2011; pp. 219–229. [Google Scholar]
- Correas, J.-M.; Bridal, L.; Lesavre, A.; Méjean, A.; Claudon, M.; Hélénon, O. Ultrasound contrast agents: Properties, principles of action, tolerance, and artifacts. Eur. Radiol. 2001, 11, 1316–1328. [Google Scholar] [CrossRef] [PubMed]
- Abma, E.; De Spiegelaere, W.; Vanderperren, K.; Stock, E.; Van Brantegem, L.; Cornelis, I.; Daminet, S.; Ni, Y.; Vynck, M.; Verstraete, G.; et al. A single dose of intravenous combretastatin A4-phosphate is reasonably well tolerated and significantly reduces tumour vascularization in canine spontaneous cancers. Vet. Comp. Oncol. 2018, 16, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Abma, E.; Stock, E.; De Spiegelaere, W.; Brantegem, L.V.; Vanderperren, K.; Ni, Y.; Vynck, M.; Daminet, S.; Clercq, K.D.; de Rooster, H. Power Doppler ultrasound and contrast-enhanced ultrasound demonstrate non-invasive tumour vascular response to anti-vascular therapy in canine cancer patients. Sci. Rep. 2019, 9, 9262. [Google Scholar] [CrossRef] [Green Version]
- Quartuccio, M.; Mangano, C.; Macri, F.; Rizzo, M.; Di Pietro, S.; Pugliese, M.; Mazzullo, G.; Cristarella, S.; De Majo, M. Contrast-enhanced ultrasound evaluation of testicular interstitial cell tumours in conscious non-sedated dogs. Vet. Med. (Praha) 2018, 63, 125–130. [Google Scholar] [CrossRef] [Green Version]
- Schärz, M.; Ohlerth, S.; Achermann, R.; Gardelle, O.; Roos, M.; Saunders, H.M.; Wergin, M.; Kaser-Hotz, B. Evaluation of quantified contrast-enhanced color and power Doppler ultrasonography for the assessment of vascularity and perfusion of naturally occuring tumors in dogs. Am. J. Vet. Res. 2005, 66, 21–29. [Google Scholar] [CrossRef]
- Feliciano, M.A.R.; Uscategui, R.A.R.; Maronezi, M.C.; Simões, A.P.R.; Silva, P.; Gasser, B.; Pavan, L.; Carvalho, C.F.; Canola, J.C.; Vicente, W.R.R. Ultrasonography methods for predicting malignancy in canine mammary tumors. PLoS ONE 2017, 12, e0178143. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Vara, J.A.; Borst, L.B. Immunohistochemistry: Fundamentals and applications in oncology. In Tumors in Domestic Animals, 5th ed.; Meuten, D.J., Ed.; John Wiley & Sons Inc.: Ames, IA, USA, 2017; pp. 44–87. [Google Scholar]
- Cicchelero, L.; Denies, S.; Vanderperren, K.; Stock, E.; Van Brantegem, L.; de Rooster, H.; Sanders, N.N. Immunological, anti-angiogenic and clinical effects of intratumoral interleukin 12 electrogene therapy combined with metronomic cyclophosphamide in dogs with spontaneous cancer: A pilot study. Cancer Lett. 2017, 400, 205–218. [Google Scholar] [CrossRef]
- Cicchelero, L.; Denies, S.; Haers, H.; Vanderperren, K.; Stock, E.; Van Brantegem, L.; de Rooster, H.; Sanders, N.N. Intratumoural interleukin 12 gene therapy stimulates the immune system and decreases angiogenesis in dogs with spontaneous cancer. Vet. Comp. Oncol. 2017, 15, 1187–1205. [Google Scholar] [CrossRef]
- Favril, S.; Abma, E.; Stock, E.; Devriendt, N.; Van Goethem, B.; Blasi, F.; Brioschi, C.; Polis, I.; De Cock, H.; Miragoli, L.; et al. Fluorescence-guided surgery using indocyanine green in dogs with superficial solid tumours. Vet. Rec. 2020, 187, 273. [Google Scholar] [CrossRef]
- Favril, S.; Brioschi, C.; Vanderperren, K.; Abma, E.; Devriendt, N.; Polis, I.; De Cock, H.; Cordaro, A.; Miragoli, L.; Olivia, P.; et al. Preliminary safety and imaging efficacy of the near-infrared fluorescent contrast agent DA364 during fluorescence-guided surgery in dogs with spontaneous superficial tumors. Oncotarget 2020, 11, 2310–2326. [Google Scholar] [CrossRef] [PubMed]
- Stock, E.; Vanderperren, K.; Van Der Vekens, E.; Haers, H.; Duchateau, L.; Polis, I.; Hesta, M.; Saunders, J.H. The effect of anesthesia with propofol and sedation with butorphanol on quantitative contrast-enhanced ultrasonography of the healthy feline kidney. Vet. J. 2014, 202, 637–639. [Google Scholar] [CrossRef] [PubMed]
- Sledge, D.G.; Webster, J.; Kiupel, M. Canine cutaneous mast cell tumors: A combined clinical and pathologic approach to diagnosis, prognosis, and treatment selection. Vet. J. 2016, 215, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Misdorp, W. Mast cells and canine mast cell tumours. A review. Vet. Q. 2004, 26, 156–169. [Google Scholar] [CrossRef] [PubMed]
- London, C.A.; Seguin, B. Mast cell tumors in the dog. Vet. Clin. North Am. Small Anim. Pract. 2003, 33, 473–489. [Google Scholar] [CrossRef]
- Patnaik, A.K.; Ehler, W.J.; MacEwen, E.G. Canine Cutaneous Mast Cell Tumor: Morphologic Grading and Survival Time in 83 Dogs. Vet. Pathol. 1984, 21, 469–474. [Google Scholar] [CrossRef]
- Ohlerth, S.; Wergin, M.; Bley, C.R.; Del Chicca, F.; Laluhová, D.; Hauser, B.; Roos, M.; Kaser-Hotz, B. Correlation of quantified contrast-enhanced power Doppler ultrasonography with immunofluorescent analysis of microvessel density in spontaneous canine tumours. Vet. J. 2010, 183, 58–62. [Google Scholar] [CrossRef] [Green Version]
- Vermeulen, P.E.; Gasparini, G.; Fox, S.B.; Toi, M.; Martin, L.; McCulloch, P.; Pezzella, F.; Viale, G.; Weidner, N.; Harris, A.L.; et al. Quantification of angiogenesis in solid human tumours: An international consensus on the methodology and criteria of evaluation. Eur. J. Cancer Part A 1996, 32, 2474–2484. [Google Scholar] [CrossRef]
- Ranieri, G.; Passantino, L.; Patruno, R.; Passantino, G.; Jirillo, F.; Catino, A.; Mattioli, V.; Gadaleta, C.; Ribatti, D. [Abstract] The dog mast cell tumour as a model to study the relationship between angiogenesis, mast cell density and tumour malignancy. Oncol. Rep. 2003, 10, 1189–1193. [Google Scholar]
- Preziosi, R.; Sarli, G.; Paltrinieri, M. Prognostic Value of Intratumoral Vessel Density in Cutaneous Mast Cell Tumours of the Dog. J. Comp. Pathol. 2004, 130, 143–151. [Google Scholar] [CrossRef]
- Diessler, M.E.; Castellano, M.C.; Portiansky, E.L.; Burns, S.; Idiart, J.R. Canine mammary carcinomas: Influence of histological grade, vascular invasion, proliferation, microvessel density and VEGFR2 expression on lymph node status and survival time. Vet. Comp. Oncol. 2017, 15, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Nagy, J.A.; Chang, S.-H.; Shih, S.-C.; Dvorak, A.M.; Dvorak, H.F. Heterogeneity of the Tumor Vasculature. Semin. Thromb. Hemost. 2010, 36, 321–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohlerth, S.; Kaser-Hotz, B. A review of Doppler sonography for the assessment of tumour vascularity. Vet. Comp. Oncol. 2003, 1, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, M.A.R.; Vicente, W.R.R.; Silva, M.A.M. Conventional and Doppler ultrasound for the differentiation of benign and malignant canine mammary tumours. J. Small Anim. Pract. 2012, 53, 332–337. [Google Scholar] [CrossRef]
- Wilson, S.; Greenbaum, L.; Goldberg, B. Contrast-enhanced ultrasound: What Is the Evidence and What Are the Obstacles? Am. J. Roentgenol. 2009, 193, 55–60. [Google Scholar] [CrossRef]
- Fleischer, A.C.; Wojcicki, W.E.; Donnelly, E.F.; Pickens, D.R.; Thirsk, G.; Thurman, G.B.; Hellerqvist, C.G. Quantified color Doppler sonography of tumor vascularity in an animal model. J. Ultrasound Med. 1999, 18, 547–551. [Google Scholar] [CrossRef] [Green Version]
- Tranquart, F.; Correas, J.M.; Ladam Marcus, V.; Manzoni, P.; Vilgrain, V.; Aube, C.; Elmaleh, A.; Chami, L.; Claudon, M.; Cuilleron, M.; et al. Real-time contrast-enhanced ultrasound in the evaluation of focal liver lesions: Diagnostic efficacy and economical issues from a French multicentric study. J. Radiol. 2009, 90, 109–122. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Fan, W.; Zhao, S.; Zhang, K.; Zhang, L.; Zhang, P.; Ma, R. Qualitative, quantitative and combination score systems in differential diagnosis of breast lesions by contrast-enhanced ultrasound. Eur. J. Radiol. 2016, 85, 48–54. [Google Scholar] [CrossRef]
- Mohammed, S.I.; Meloni, G.B.; Pinna Parpaglia, M.L.; Marras, V.; Burrai, G.P.; Meloni, F.; Pirino, S.; Antuofermo, E. Mammography and ultrasound imaging of preinvasive and invasive canine spontaneous mammary cancer and their similarities to human breast cancer. Cancer Prev. Res. 2011, 4, 1790–1798. [Google Scholar] [CrossRef] [Green Version]
- Zonderland, H.M. The role of ultrasound in the diagnosis of breast cancer. Semin. Ultrasound CT MRI 2000, 21, 317–324. [Google Scholar] [CrossRef]
- Baştan, A.; Özenç, E.; Yaǧci, I.P.; Baki Acar, D. Ultrasonographic evaluation of mammary tumors in bitches. Kafkas Univ. Vet. Fak. Derg. 2009, 15, 81–86. [Google Scholar] [CrossRef]
- Vannozzi, I.; Tesi, M.; Zangheri, M.; Innocenti, M.V.; Rota, A.; Citi, S.; Poli, A. B-mode ultrasound examination of canine mammary gland neoplastic lesions of small size (diameter <2 cm). Vet. Res. Commun. 2018, 42, 137–143. [Google Scholar]
- Tagawa, M.; Kanai, E.; Shimbo, G.; Kano, M.; Kayanuma, H. Ultrasonographic evaluation of depth–width ratio (D/W) of benign and malignant mammary tumors in dogs. J. Vet. Med. Sci. 2016, 78, 521–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulinelli, R.R.; Freitas-Júnior, R.; Moreira, M.A.R.; Alves de Moraes, V.; Bernardes-Júnior, J.R.M.; da Silva Rocha, C.; Naldi Ruiz, A.; Tostes Lucato, M. Risk of malignancy in solid breast nodules according to their sonographic features. J. Ultrasound Med. 2005, 24, 635–641. [Google Scholar] [CrossRef]
- Calas, M.J.G.; Koch, H.A.; Dutra, M.V.P. Breast ultrasound: Evaluation of echographic criteria for differentiation of breast lesions. Radiol. Bras. 2007, 40, 1–7. [Google Scholar] [CrossRef]
- Metz, S.; Daldrup-Link, H.E.; Richter, T.; Räth, C.; Ebert, W.; Settles, M.; Rummeny, E.J.; Link, T.M.; Piert, M. Detection and Quantification of Breast Tumor Necrosis with MR Imaging: Value of the Necrosis-avid Contrast Agent Gadophrin-3. Acad. Radiol. 2003, 10, 484–490. [Google Scholar] [CrossRef]
- Forster, J.C.; Harriss-phillips, W.M.; Douglass, M.J.J.; Bezak, E. A review of the development of tumor vasculature and its effects on the tumor microenvironment. Hypoxia 2017, 5, 21–32. [Google Scholar] [CrossRef] [Green Version]
- Wan, C.; Du, J.; Fang, H.; Li, F.; Wang, L. Evaluation of breast lesions by contrast enhanced ultrasound: Qualitative and quantitative analysis. Eur. J. Radiol. 2012, 81, e444–e450. [Google Scholar] [CrossRef]
- Wan, C.F.; Du, J.; Fang, H.; Li, F.H.; Zhu, J.S.; Liu, Q. Enhancement Patterns and Parameters of Breast Cancers at Contrast-enhanced US: Correlation with Prognostic Factors. Radiology 2012, 262, 450–459. [Google Scholar] [CrossRef]
- Dunst, J.; Ahrens, S.; Paulussen, M.; Burdach, S.; Jürgens, H. Prognostic impact of tumor perfusion in MR-imaging studies in Ewing tumors. Strahlentherapie Onkol. 2001, 177, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Sasaki, N.; Murakami, M.; Kumara, W.R.B.; Ohta, H.; Yamasaki, M.; Takagi, S.; Osaki, T.; Takiguchi, M. Contrast-Enhanced Ultrasonography for Characterization of Focal Splenic Lesions in Dogs. J. Vet. Intern. Med. 2010, 24, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.L.; Bao, W.; Zhu, S.G.; Wang, L.H.; Sun, M.H.; Wang, L.; Men, Y.M.; Xue, J. Contrast-enhanced ultrasound characteristics of breast cancer: Correlation with prognostic factors. Ultrasound Med. Biol. 2014, 40, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Loizides, A.; Peer, S.; Plaikner, M.; Djurdjevic, T.; Gruber, H. Perfusion pattern of musculoskeletal masses using contrastenhanced ultrasound: A helpful tool for characterisation? Eur. Radiol. 2012, 22, 1803–1811. [Google Scholar] [CrossRef] [PubMed]
- Gruber, L.; Loizides, A.; Luger, A.K.; Glodny, B.; Moser, P.; Henninger, B.; Gruber, H. Soft-tissue tumor contrast enhancement patterns: Diagnostic value and comparison between ultrasound and MRI. Am. J. Roentgenol. 2017, 208, 393–401. [Google Scholar] [CrossRef]
- Hindi, A.; Peterson, C.; Barr, R.G. Artifacts in diagnostic ultrasound. Reports Med. Imaging 2013, 6, 29–48. [Google Scholar] [CrossRef] [Green Version]
- Steel, R.; Poepping, T.L.; Thompson, R.S.; MacAskill, C. Origins of the edge shadowing artefact in medical ultrasound imaging. Ultrasound Med. Biol. 2004, 30, 1153–1162. [Google Scholar] [CrossRef]
- Gonzalez De Bulnes, A.; Garcia Fernandez, P.; Mayenco Aguirre, A.M.; Sanchez De la Muela, M. Ultrasonographic imaging of canine mammary tumours. Vet. Rec. 1998, 143, 687–689. [Google Scholar] [CrossRef]
- Caproni, N.; Marchisio, F.; Pecchi, A.; Canossi, B.; Battista, R.; D’Alimonte, P.; Torricelli, P. Contrast-enhanced ultrasound in the characterisation of breast masses: Utility of quantitative analysis in comparison with MRI. Eur. Radiol. 2010, 20, 1384–1395. [Google Scholar] [CrossRef]
- Collivignarelli, F.; Tamburro, R.; Aste, G.; Falerno, I.; Del Signore, F.; Simeoni, F.; Patsikas, M.; Gianfelici, J.; Terragni, R.; Attorri, V.; et al. Lymphatic drainage mapping with indirect lymphography for canine mammary tumors. Animals 2021, 11, 1115. [Google Scholar] [CrossRef]

| Histological Type | Origin | Number | |
|---|---|---|---|
| Benign | Adenoma | Mammary gland | 11 |
| Complex adenoma | Mammary gland | 4 | |
| Mastocytoma Grade I | Hind leg | 1 | |
| Total | 16 | ||
| Malignant | Complex adenocarcinoma Grade I | Mammary gland | 2 |
| Adenocarcinoma Grade I | Mammary gland | 7 | |
| Adenocarcinoma Grade II | Mammary gland | 2 | |
| Adenocarcinoma Grade III | Mammary gland | 3 | |
| Adenocarcinoma NG | Anal gland, anal sac | 2 | |
| Mastocytoma Grade II | Abdomen (2), hind leg (3) | 5 | |
| Mastocytoma Grade III | Front limb, hind limb | 2 | |
| Mastocytoma NG | Head | 1 | |
| Fibrosarcoma | Intra-oral (1), maxilla (1), front limb (2) | 4 | |
| Neurofibrosarcoma | Peri-anal | 1 | |
| Schwannoma | Front leg | 4 | |
| Osteosarcoma | Rib (2), intra-oral (1) | 3 | |
| Extra skeletal osteosarcoma | Mammary gland | 2 | |
| Inflammatory carcinoma | Mammary gland | 2 | |
| Squamous cell carcinoma | Mammary gland, mandible | 2 | |
| Cystadenocarcinoma | Mammary gland | 1 | |
| Complex carcinoma | Mammary gland | 1 | |
| Melanoma | Intra-oral | 1 | |
| Chondrosarcoma | Front limb | 1 | |
| Histiocytic sarcoma | Nose | 1 | |
| Total | 47 |
| Benign Neoplastic | Malignant Neoplastic | p-Value | |
|---|---|---|---|
| Shape | 0.11 | ||
| Ovoid | 75.0% | 38.3% | |
| Round | 6.3% | 10.6% | |
| Multilobulated | 12.5% | 40.4% | |
| Irregular | 6.3% | 8.5% | |
| Other | 0.0% | 2.1% | |
| Border definition | 0.04 | ||
| Well-defined | 81.0% | 48.9% | |
| Ill-defined | 19.0% | 51.1% | |
| Echogenicity | 0.01 | ||
| Hyperechoic | 12.5% | 2.1% | |
| Hypoechoic | 56.3% | 27.7% | |
| Isoechoic | 18.7% | 19.1% | |
| Mixed | 12.5% | 51.1% | |
| Echotexture | 0.04 | ||
| Heterogeneous | 37.5% | 68.1% | |
| Homogeneous | 62.5% | 31.9% | |
| Edge shadowing | 0.06 | ||
| Present | 37.5% | 12.8% | |
| Absent | 62.5% | 87.2% | |
| Cystic areas | 0.74 | ||
| Present | 25.0% | 21.3% | |
| Absent | 75.0% | 78.7% | |
| Mineralization | 0.35 | ||
| Present | 19.0% | 34.0% | |
| Absent | 81.0% | 66.0% | |
| Benign Neoplastic | Malignant Neoplastic | p-Value | |
|---|---|---|---|
| Wash-in | 0.05 | ||
| Centripetal | 68.7% | 31.9% | |
| Centrifugal | |||
| Combined | 2.1% | ||
| Chaotic | 31.3% | 57.4% | |
| NE | 8.5% | ||
| Large vessels | 0.32 | ||
| Present | 12.5% | 25.5% | |
| Absent | 87.5% | 70.2% | |
| NE | 4.3% | ||
| Non-perfused areas | 0.25 | ||
| Present | 37.5% | 53.2% | |
| Absent | 62.5% | 42.6% | |
| Enhancement at wash-in | 1.00 | ||
| Heterogeneous | 93.7% | 83% | |
| Rim | 6.3% | 8.5% | |
| Homogeneous | |||
| NE | 8.5% | ||
| Enhancement at peak | 0.26 | ||
| Heterogeneous | 75% | 78.7% | |
| Rim | 6.3% | 8.5% | |
| Homogeneous | 18.7% | 4.3% | |
| NE | 8.5% | ||
| Enhancement at wash-out | 1.00 | ||
| Heterogeneous | 93.7% | 83% | |
| Rim | 6.3% | 8.5% | |
| Homogeneous | |||
| NE | 8.5% | ||
| Enhancement degree at wash-in | 0.12 * | ||
| Hyperechoic | 43.8% | 36.2% | |
| Hypoechoic | 18.8% | 23.4% | |
| Isoechoic | 19% | 2% | |
| NE | 18.8% | 38.3% | |
| Enhancement degree at peak | 0.31 * | ||
| Hyperechoic | 38% | 36% | |
| Hypoechoic | 18.8% | 19% | |
| Isoechoic | 25% | 6.4% | |
| NE | 18.8% | 38.3% | |
| Enhancement degree at wash-out | 0.22 * | ||
| Hyperechoic | 8.5% | ||
| Hypoechoic | 43.8% | 17% | |
| Isoechoic | 37.5% | 36.2% | |
| NE | 18.8% | 38.3% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hillaert, A.; Stock, E.; Duchateau, L.; de Rooster, H.; Devriendt, N.; Vanderperren, K. B-Mode and Contrast-Enhanced Ultrasonography Aspects of Benign and Malignant Superficial Neoplasms in Dogs: A Preliminary Study. Animals 2022, 12, 2765. https://doi.org/10.3390/ani12202765
Hillaert A, Stock E, Duchateau L, de Rooster H, Devriendt N, Vanderperren K. B-Mode and Contrast-Enhanced Ultrasonography Aspects of Benign and Malignant Superficial Neoplasms in Dogs: A Preliminary Study. Animals. 2022; 12(20):2765. https://doi.org/10.3390/ani12202765
Chicago/Turabian StyleHillaert, Amber, Emmelie Stock, Luc Duchateau, Hilde de Rooster, Nausikaa Devriendt, and Katrien Vanderperren. 2022. "B-Mode and Contrast-Enhanced Ultrasonography Aspects of Benign and Malignant Superficial Neoplasms in Dogs: A Preliminary Study" Animals 12, no. 20: 2765. https://doi.org/10.3390/ani12202765
APA StyleHillaert, A., Stock, E., Duchateau, L., de Rooster, H., Devriendt, N., & Vanderperren, K. (2022). B-Mode and Contrast-Enhanced Ultrasonography Aspects of Benign and Malignant Superficial Neoplasms in Dogs: A Preliminary Study. Animals, 12(20), 2765. https://doi.org/10.3390/ani12202765








