Analysis of the Association of Two SNPs in the Promoter Regions of the PPP2R5C and SLC39A5 Genes with Litter Size in Yunshang Black Goats
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Samples
2.3. DNA and RNA Extraction, and Preparation of cDNA
2.4. Primer Design and Amplification for SNPs
2.5. Identification and Genotyping of Single Nucleotide Polymorphisms (SNPs)
2.6. RT-qPCR Validation
2.7. Protein Extraction and Western Blotting
2.8. Bioinformatics Analysis
2.9. Plasmid Construction and Dual Luciferase Assay
2.10. Statistical Analysis
3. Results
3.1. Identification and Typing of PPP2R5C and SLC39A5 SNPs
3.2. Polymorphism Analysis of PPP2R5C and SLC39A5
3.3. Analysis of the Associations between PPP2R5C g.65977743C>T and SLC39A5 g.50676693T>C and the Litter Size in Yunshang Black Goats
3.4. Expression of PPP2R5C and SLC39A5 with Different Genotypes in Goat Ovarian Tissues
3.5. SNPs Affect on the Promoter Activity of PPP2R5C and SLC39A5
3.6. Prediction of Transcription Factor binding Sites Affected by the PPP2R5C and SLC39A5 Polymorphisms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeder, M.A. Domestication and early agriculture in the Mediterranean Basin: Origins, diffusion, and impact. Proc. Natl. Acad. Sci. USA 2008, 105, 11597–11604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celozzi, S.; Battini, M.; Prato-Previde, E.; Mattiello, S. Humans and goats: Improving knowledge for a Better Relationship. Animals 2022, 12, 774. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Wang, X.; Li, M.; Li, Y.; Yang, Z.; Wang, X.; Pan, X.; Gong, M.; Zhang, Y.; Guo, Y.; et al. The origin of domestication genes in goats. Sci. Adv. 2020, 6, eaaz5216. [Google Scholar] [CrossRef] [PubMed]
- Kahi, A.K.; Wasike, C.B. Dairy goat production in sub-Saharan Africa: Current status, constraints and prospects for research and development. Asian-Australas J. Anim. Sci. 2019, 32, 1266–1274. [Google Scholar] [CrossRef] [Green Version]
- Loveday, C.; Tatton-Brown, K.; Clarke, M.; Westwood, I.; Renwick, A.; Ramsay, E.; Nemeth, A.; Campbell, J.; Joss, S.; Gardner, M.; et al. Mutations in the PP2A regulatory subunit B family genes PPP2R5B, PPP2R5C and PPP2R5D cause human overgrowth. Hum. Mol. Genet. 2015, 24, 4775–4779. [Google Scholar] [CrossRef] [Green Version]
- Wlodarchak, N.; Xing, Y. PP2A as a master regulator of the cell cycle. Crit. Rev. Biochem. Mol. Biol. 2016, 51, 162–184. [Google Scholar] [CrossRef]
- Yang, J.; Phiel, C. Functions of B56-containing PP2As in major developmental and cancer signaling pathways. Life Sci. 2010, 87, 659–666. [Google Scholar] [CrossRef] [Green Version]
- Shao, L.; Ma, Y.; Fang, Q.; Huang, Z.; Wan, S.; Wang, J.; Yang, L. Role of protein phosphatase 2A in kidney disease (Review). Exp. Ther. Med. 2021, 22, 1236. [Google Scholar] [CrossRef]
- Liu, S.; Shen, Q.; Chen, Y.; Zeng, C.; Cao, C.; Yang, L.; Chen, S.; Wu, X.; Li, B.; Li, Y. Alteration of gene expression profile following PPP2R5C knockdown may be associated with proliferation suppression and increased apoptosis of K562 cells. J. Hematol. Oncol. 2015, 8, 34. [Google Scholar] [CrossRef] [Green Version]
- Nobumori, Y.; Shouse, G.P.; Wu, Y.; Lee, K.J.; Shen, B.; Liu, X. B56γ tumor-associated mutations provide new mechanisms for B56γ-PP2A tumor suppressor activity. Mol. Cancer Res. 2013, 11, 995–1003. [Google Scholar] [CrossRef]
- Cheng, Y.S.; Seibert, O.; Klöting, N.; Dietrich, A.; Straßburger, K.; Fernández-Veledo, S.; Vendrell, J.J.; Zorzano, A.; Blüher, M.; Herzig, S.; et al. PPP2R5C couples hepatic glucose and lipid homeostasis. PLoS Genet. 2015, 11, e1005561. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.; Eide, D.J. The SLC39 family of zinc transporters. Mol. Asp. Med. 2013, 34, 612–619. [Google Scholar] [CrossRef] [Green Version]
- Schweigel-Röntgen, M. The families of zinc (SLC30 and SLC39) and copper (SLC31) transporters. Curr. Top. Membr. 2014, 73, 321–355. [Google Scholar] [CrossRef]
- Becares, E.R.; Pedersen, P.A.; Gourdon, P.; Gotfryd, K. Overproduction of human Zip (SLC39) Zinc Transporters in saccharomyces cerevisiae for biophysical characterization. Cells 2021, 10, 213. [Google Scholar] [CrossRef]
- Dong, S.; Tian, Q.; Zhu, T.; Wang, K.; Lei, G.; Liu, Y.; Xiong, H.; Shen, L.; Wang, M.; Zhao, R.; et al. SLC39A5 dysfunction impairs extracellular matrix synthesis in high myopia pathogenesis. J. Cell. Mol. Med. 2021, 25, 8432–8441. [Google Scholar] [CrossRef]
- Geiser, J.; De Lisle, R.C.; Andrews, G.K. The zinc transporter Zip5 (Slc39a5) regulates intestinal zinc excretion and protects the pancreas against zinc toxicity. PLoS ONE 2013, 8, e82149. [Google Scholar] [CrossRef]
- Jin, J.; Li, Z.; Liu, J.; Wu, Y.; Gao, X.; He, Y. Knockdown of zinc transporter ZIP5 (SLC39A5) expression significantly inhibits human esophageal cancer progression. Oncol. Rep. 2015, 34, 1431–1439. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Gao, H.; Wu, W.; Xie, E.; Yu, Y.; He, X.; Li, J.; Zheng, W.; Wang, X.; Cao, X.; et al. The zinc transporter SlC39A5 controls glucose sensing and insulin secretion in pancreatic β-cells via Sirt1- and Pgc-1α-mediated regulation of Glut2. Protein Cell 2019, 10, 436–449. [Google Scholar] [CrossRef] [Green Version]
- Xia, Z.; Bi, X.; Lian, J.; Dai, W.; He, X.; Zhao, L.; Min, J.; Wang, F. SlC39A5-mediated zinc homeostasis plays an essential role in venous angiogenesis in zebrafish. Open Biol. 2020, 10, 200281. [Google Scholar] [CrossRef]
- He, C.; Holme, J.; Anthony, J. SNP genotyping: The KASP assay. Methods Mol. Biol. 2014, 1145, 75–86. [Google Scholar] [CrossRef]
- Feng, T.; Geng, C.X.; Lang, X.Z.; Chu, M.X.; Cao, G.L.; Di, R.; Fang, L.; Chen, H.Q.; Liu, X.L.; Li, N. Polymorphisms of caprine GDF9 gene and their association with litter size in Jining Grey goats. Mol. Biol. Rep. 2011, 38, 5189–5197. [Google Scholar] [CrossRef]
- Mathelier, A.; Zhao, X.; Zhang, A.W.; Parcy, F.; Worsley-Hunt, R.; Arenillas, D.J.; Buchman, S.; Chen, C.Y.; Chou, A.; Ienasescu, H.; et al. JASPAR 2014: An extensively expanded and updated open-access database of transcription factor binding profiles. Nucleic Acids Res. 2014, 42, D142–D147. [Google Scholar] [CrossRef] [Green Version]
- Cortellari, M.; Barbato, M.; Talenti, A.; Bionda, A.; Carta, A.; Ciampolini, R.; Ciani, E.; Crisà, A.; Frattini, S.; Lasagna, E.; et al. The climatic and genetic heritage of Italian goat breeds with genomic SNP data. Sci. Rep. 2021, 11, 10986. [Google Scholar] [CrossRef]
- Yue, C.; Bai, W.L.; Zheng, Y.Y.; Hui, T.Y.; Sun, J.M.; Guo, D.; Guo, S.L.; Wang, Z.Y. Correlation analysis of candidate gene SNP for high-yield in Liaoning cashmere goats with litter size and cashmere performance. Anim. Biotechnol. 2021, 32, 43–50. [Google Scholar] [CrossRef]
- Hui, Y.; Zhang, Y.; Wang, K.; Pan, C.; Chen, H.; Qu, L.; Song, X.; Lan, X. Goat DNMT3B: An indel mutation detection, association analysis with litter size and mRNA expression in gonads. Theriogenology 2020, 147, 108–115. [Google Scholar] [CrossRef]
- Tao, L.; He, X.Y.; Jiang, Y.T.; Lan, R.; Li, M.; Li, Z.M.; Yang, W.F.; Hong, Q.H.; Chu, M.X. Combined approaches to reveal genes associated with litter size in Yunshang black goats. Anim. Genet. 2020, 51, 924–934. [Google Scholar] [CrossRef]
- Chang, Y.; Li, J.Y.; Jayakumar, T.; Hung, S.H.; Lee, W.C.; Manubolu, M.; Sheu, J.R.; Hsu, M.J. Ketamine, a clinically used anesthetic, inhibits vascular smooth muscle cell proliferation via PP2A-Activated PI3K/Akt/ERK inhibition. Int. J. Mol. Sci. 2017, 18, 2545. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yen, F.S.; Zhu, X.G.; Timson, R.C.; Weber, R.; Xing, C.; Liu, Y.; Allwein, B.; Luo, H.; Yeh, H.W.; et al. SLC25A39 is necessary for mitochondrial glutathione import in mammalian cells. Nature 2021, 599, 136–140. [Google Scholar] [CrossRef]
- Zhang, H.; Qin, F.; Liu, A.; Sun, Q.; Wang, Q.; Xie, S.; Lu, S.; Zhang, D.; Lu, Z. Electro-acupuncture attenuates the mice premature ovarian failure via mediating PI3K/AKT/mTOR pathway. Life Sci. 2019, 217, 169–175. [Google Scholar] [CrossRef]
- Zeng, J.; Sun, Y.; Li, X.; Zhu, J.; Zhang, W.; Lu, W.; Weng, Y.; Liu, J. 2,5-Hexanedione influences primordial follicular development in cultured neonatal mouse ovaries by interfering with the PI3K signaling pathway via miR-214-3p. Toxicol. Appl. Pharmacol. 2020, 409, 115335. [Google Scholar] [CrossRef]
- Bonuccelli, G.; Sotgia, F.; Lisanti, M.P. Matcha green tea (MGT) inhibits the propagation of cancer stem cells (CSCs), by targeting mitochondrial metabolism, glycolysis and multiple cell signalling pathways. Aging 2018, 10, 1867–1883. [Google Scholar] [CrossRef] [PubMed]
- Haberle, V.; Stark, A. Eukaryotic core promoters and the functional basis of transcription initiation. Nat. Rev. Mol. Cell Biol. 2018, 19, 621–637. [Google Scholar] [CrossRef] [PubMed]
- Pasanen, P.; Mäkinen, J.; Myllykangas, L.; Guerreiro, R.; Bras, J.; Valori, M.; Viitanen, M.; Baumann, M.; Tienari, P.J.; Pöyhönen, M.; et al. Primary familial brain calcification linked to deletion of 5′ noncoding region of SLC20A2. Acta Neurol. Scand. 2017, 136, 59–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Chaney, H.L.; Current, J.Z.; Yao, J. Identification of the core promoter of ZNFO, an oocyte-specific maternal effect gene in cattle. Gene 2021, 791, 145717. [Google Scholar] [CrossRef]
- Yu, S.; Wang, G.; Liao, J.; Chen, X. A functional mutation in the AMPD1 promoter region affects promoter activity and breast meat freshness in chicken. Anim. Genet. 2021, 52, 121–125. [Google Scholar] [CrossRef]
- Li, K.; Liu, Y.; He, X.; Tao, L.; Jiang, Y.; Lan, R.; Hong, Q.; Chu, M. A novel SNP in the promoter region of IGF1 associated with Yunshang black goat kidding number via promoting transcription activity by SP1. Front. Cell Dev. Biol. 2022, 10, 873095. [Google Scholar] [CrossRef]
- Serrote, C.M.L.; Reiniger, L.R.S.; Silva, K.B.; Rabaiolli, S.; Stefanel, C.M. Determining the polymorphism information content of a molecular marker. Gene 2020, 726, 144175. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, Z.; Li, W.; Zeng, T.; Zhang, X.; Li, J.; Zhang, W.; Yue, B. Isolation and strategies of novel tetranucleotide microsatellites with polymorphisms from different chromosomes of the rhesus monkey (Macaca mulatta). Mol. Biol. Rep. 2019, 46, 3955–3966. [Google Scholar] [CrossRef]
- Haesen, D.; Sents, W.; Lemaire, K.; Hoorne, Y.; Janssens, V. The basic biology of PP2A in hematologic cells and malignancies. Front. Oncol. 2014, 4, 347. [Google Scholar] [CrossRef] [Green Version]
- Eichhorn, P.J.; Creyghton, M.P.; Bernards, R. Protein phosphatase 2A regulatory subunits and cancer. Biochim. Biophys. Acta 2009, 1795, 1–15. [Google Scholar] [CrossRef]
- Dyson, J.J.; Abbasi, F.; Varadkar, P.; McCright, B. Growth arrest of PPP2R5C and PPP2R5D double knockout mice indicates a genetic interaction and conserved function for these PP2A B subunits. FASEB BioAdvances 2022, 4, 273–282. [Google Scholar] [CrossRef]
- Li, X.; Yao, X.; Xie, H.; Zhang, G.; Deng, M.; Deng, K.; Gao, X.; Bao, Y.; Li, K.; Wang, F. PPP2R2A affects embryonic implantation by regulating the proliferation and apoptosis of Hu sheep endometrial stromal cells. Theriogenology 2021, 176, 149–162. [Google Scholar] [CrossRef]
- Guo, H.; Jin, X.; Zhu, T.; Wang, T.; Tong, P.; Tian, L.; Peng, Y.; Sun, L.; Wan, A.; Chen, J.; et al. SLC39A5 mutations interfering with the BMP/TGF-β pathway in non-syndromic high myopia. J. Med. Genet. 2014, 51, 518–525. [Google Scholar] [CrossRef] [Green Version]
- Weaver, B.P.; Dufner-Beattie, J.; Kambe, T.; Andrews, G.K. Novel zinc-responsive post-transcriptional mechanisms reciprocally regulate expression of the mouse Slc39a4 and Slc39a5 zinc transporters (Zip4 and Zip5). Biol. Chem. 2007, 388, 1301–1312. [Google Scholar] [CrossRef] [Green Version]
- Raman, S.; Taylor, N.; Genuth, N.; Fields, S.; Church, G.M. Engineering allostery. Trends Genet. 2014, 30, 521–528. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.; Koh, G.Y. Taking aim at Sox18. eLife 2017, 6, e24238. [Google Scholar] [CrossRef]
- Zhang, Y.; Hou, L. Alternate roles of Sox transcription factors beyond transcription initiation. Int. J. Mol. Sci. 2021, 22, 5949. [Google Scholar] [CrossRef]
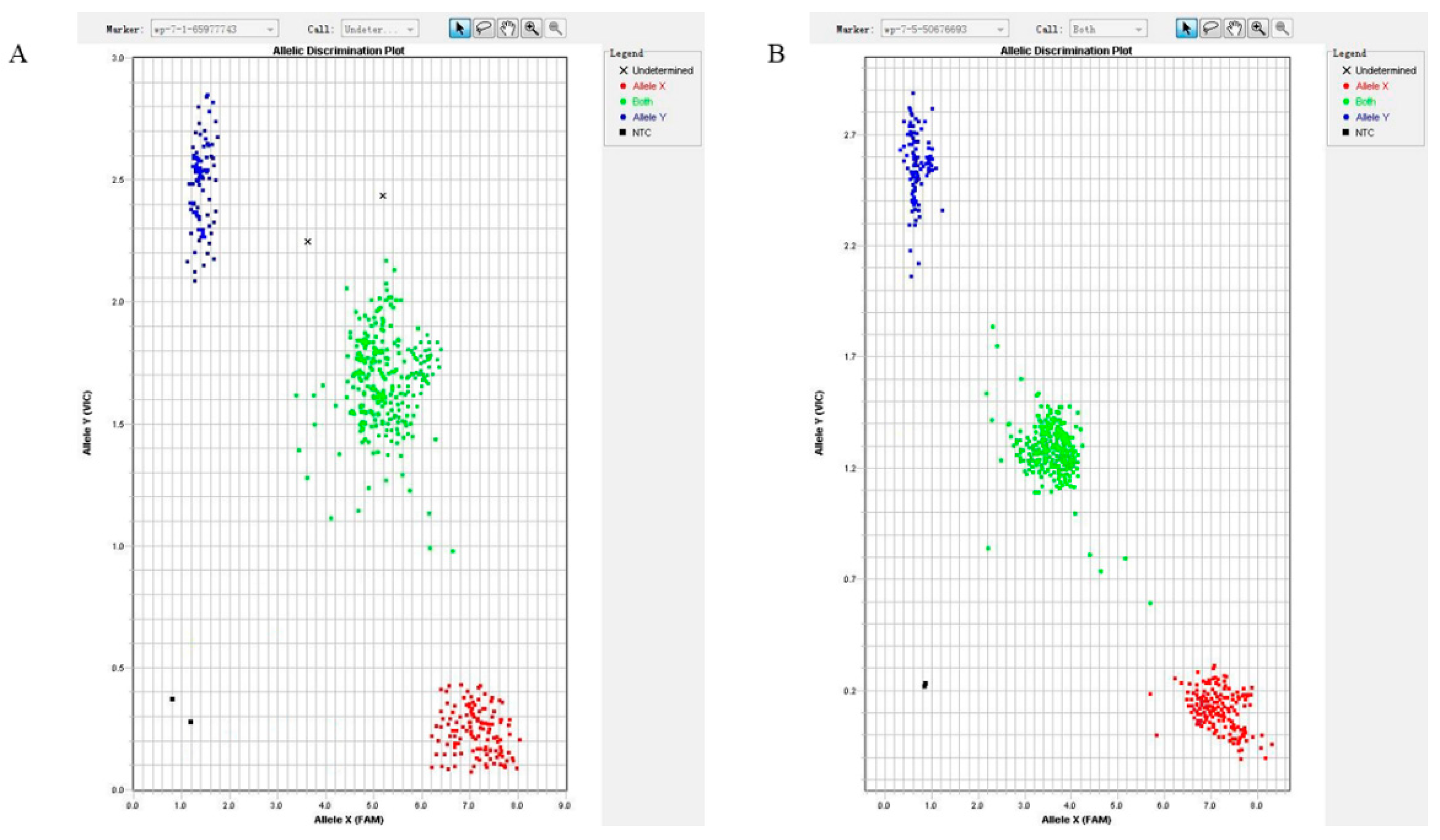
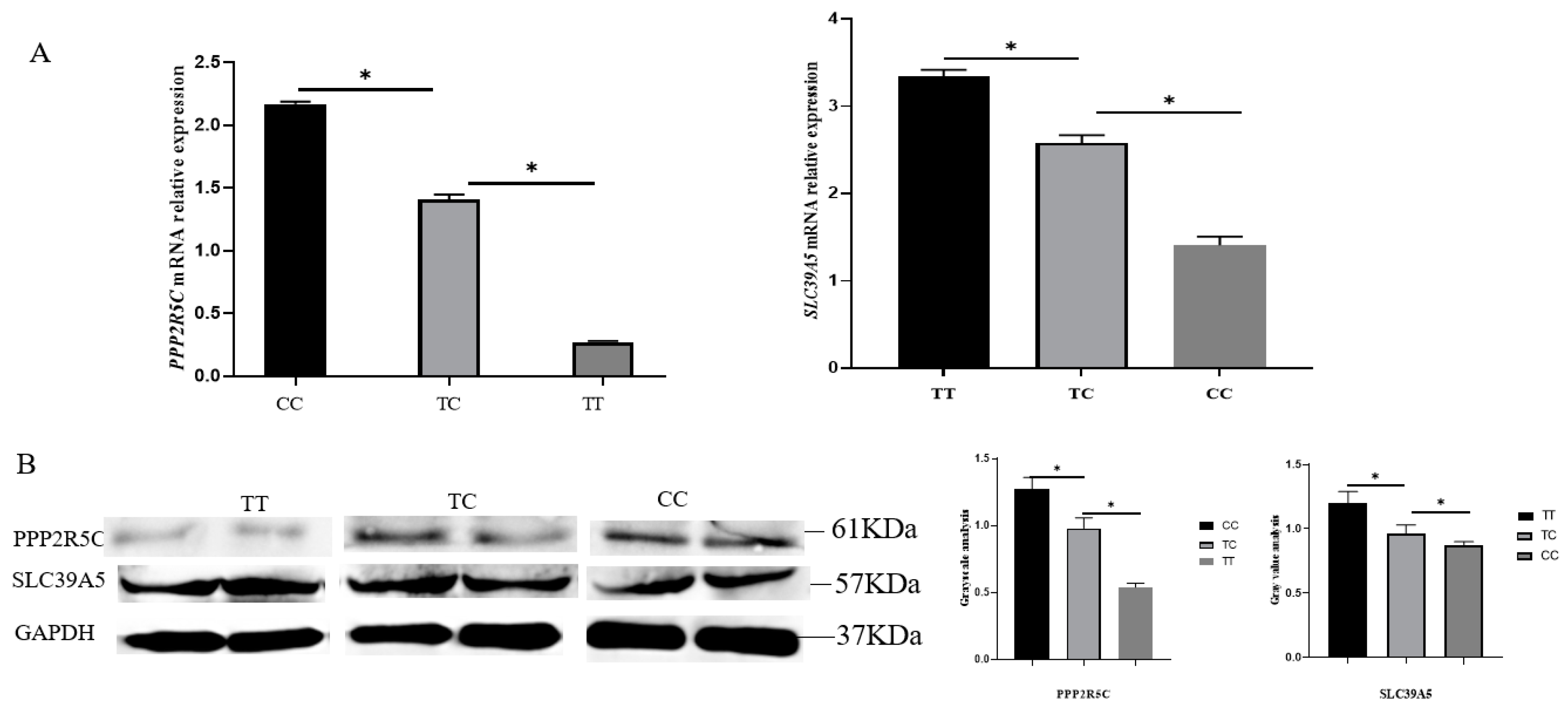

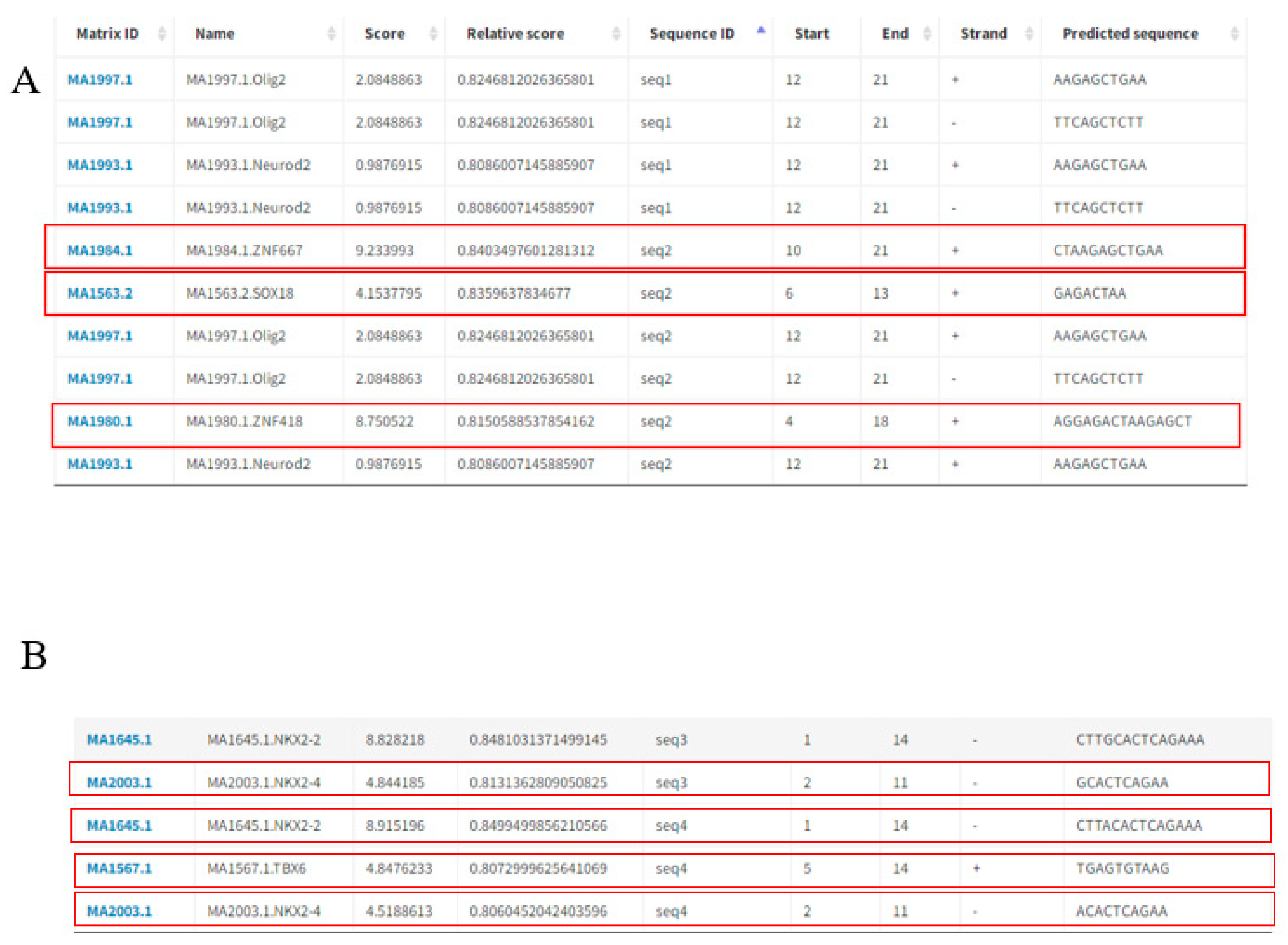
| Litter Size | Sample Size |
|---|---|
| single | 67 |
| double | 204 |
| triple | 298 |
| Gene Name | Primer Name | Sequence (5′-3′) | Length (bp) | Temperature (°C) |
|---|---|---|---|---|
| SLC39A5 | SLC39A5-1 | F: GGTGACCCTTGTCCACCTG | 1226 | 59 |
| R: TACTCGGGGGACAGGCTTAGAG | ||||
| SLC39A5-2 | F: AGGGGACCCTGGAAGAGAC | 350 | 59 | |
| R: CTTCCTGGGCTTGAGACCCAC | ||||
| SLC39A5-3 | F: GAGCCCCAGCCCCTTCC | 489 | 59 | |
| R: TGATGTCAGGAAGGCAGAGCTAG | ||||
| SLC39A5-4 | F: CTCATGGAGGTTGTAGAAAACT | 313 | 59 | |
| R: GGCAGAAAATAGGCAAAGAACA | ||||
| SLC39A5-5 | F: GGCCTCTAGTTACTGGGTGGG | 608 | 59 | |
| R: GGTGGCTCCCAGTGGAGG | ||||
| PPP2R5C | PPP2R5C-1 | F: CCCAGGATACTCTGATCAGAAATGATGT | 1024 | 59 |
| R: CTAGACTTCAGCGGGAAAGGCA | ||||
| PPP2R5C-2 | F: TTGCCTTTCCCGCTGAAGTCT | 173 | 59 | |
| R: CCATTTATTAACTAACCACAGAAGGTTCCGT | ||||
| PPP2R5C-3 | F: GAGTTAAGAAACATAGAAACTTTGTCATATGAAG | 629 | 59 | |
| R: TTCCCTCACTGTGGTCTAGGG | ||||
| PPP2R5C-4 | F: CCTGGCACAGAGACTTTTCTTA | 364 | 59 | |
| R: TATACCACCTGGCTGTGAAAAT | ||||
| PPP2R5C-5 | F: CACCACCTCATCTGTGGCT | 350 | 59 | |
| R: TTCAGATTACCCGAAGGGCAAGAAC | ||||
| PPP2R5C-6 | F: CCAGGCTTGGCTCGTCC | 425 | 59 | |
| R: AACTTGATCTAAGATGTACAGATGGGAGGT |
| Gene Name | Primer (5′-3′) | Primer_AlleleFAM (5′-3′) | Primer_AlleleHEX (5′-3′) |
|---|---|---|---|
| PPP2R5C g.65977743C>T | AATGATGTCCTTTCTTGGAGCAG | Gaaggtgaccaagttcatgct TAAAAGCTGTTATT CAGCTCTTA | Gaaggtcggagtcaacggatt TAAAAGCTGTTATT CAGCTCTTG |
| SLC39A5 g.50676693T>C | ACTGTGGACTTGTTCTCTGTTCTT | Gaaggtgaccaagttcatgct TGCTGAAGGCTGG GTGCA | Gaaggtcggagtcaacggatt TGCTGAAGGCTGG GTGCG |
| Gene Name | Sequence (5′-3′) | Length (bp) | Temperature (°C) |
|---|---|---|---|
| SLC39A5 | F: CAGCACCACTGTAGCGGTCTTC | 162 | 59 |
| R: GGACTCCAGACACCAGGCTCAG | |||
| PPP2R5C | F: TCAAGCGAGCACCATCAGCATC | 140 | 59 |
| R: GCGAGCCTCTTCGTTCACTGTC | |||
| RPL19 | F: ATCGCCAATGCCAACTC | 154 | 60 |
| R: CCTTTCGCTTACCTATACC |
| Gene Name | SNP Position | Genotype Frequency | Allele Frequency | PIC | He | Ne | χ2 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| TT | TC | CC | T | C | ||||||
| PPP2R5C | g.65977743C>T | 0.19 | 0.39 | 0.42 | 0.38 | 0.62 | 0.37 | 0.47 | 1.89 | 0.01 |
| SLC39A5 | g.50676693T>C | 0.31 | 0.45 | 0.23 | 0.54 | 0.46 | 0.37 | 0.49 | 1.99 | 0.03 |
| Gene Name | Loci | Genotype | 1st Parity Litter Size | 2st Parity Litter Size | 3st Parity Litter Size | Average Litter Size |
|---|---|---|---|---|---|---|
| CC | 1.96 ± 0.620 | 2.28 ± 0.135 | 2.38 ± 0.096 a | 2.21 ± 0.070 | ||
| PPP2R5C | g.65977743C>T | TC | 1.95 ± 0.070 | 2.18 ± 0.069 | 2.30 ± 0.054 b | 2.16 ± 0.036 |
| TT | 1.93 ± 0.048 | 2.15 ± 0.138 | 2.28 ± 0.072 b | 2.14 ± 0.051 | ||
| TT | 1.97 ± 0.085 | 2.25 ± 0.094 | 2.47 ± 0.079 a | 2.21 ± 0.053 | ||
| SLC39A5 | g.50676693T>C | TC | 1.92 ± 0.0066 | 2.21 ± 0.084 | 2.23 ± 0.053 a | 2.14 ± 0.037 |
| CC | 1.90 ± 0.0495 | 2.17 ± 0.113 | 2.16 ± 0.104 b | 2.09 ± 0.028 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Li, W.; Liu, Z.; He, X.; Lan, R.; Liu, Y.; Chu, M. Analysis of the Association of Two SNPs in the Promoter Regions of the PPP2R5C and SLC39A5 Genes with Litter Size in Yunshang Black Goats. Animals 2022, 12, 2801. https://doi.org/10.3390/ani12202801
Wang P, Li W, Liu Z, He X, Lan R, Liu Y, Chu M. Analysis of the Association of Two SNPs in the Promoter Regions of the PPP2R5C and SLC39A5 Genes with Litter Size in Yunshang Black Goats. Animals. 2022; 12(20):2801. https://doi.org/10.3390/ani12202801
Chicago/Turabian StyleWang, Peng, Wentao Li, Ziyi Liu, Xiaoyun He, Rong Lan, Yufang Liu, and Mingxing Chu. 2022. "Analysis of the Association of Two SNPs in the Promoter Regions of the PPP2R5C and SLC39A5 Genes with Litter Size in Yunshang Black Goats" Animals 12, no. 20: 2801. https://doi.org/10.3390/ani12202801





