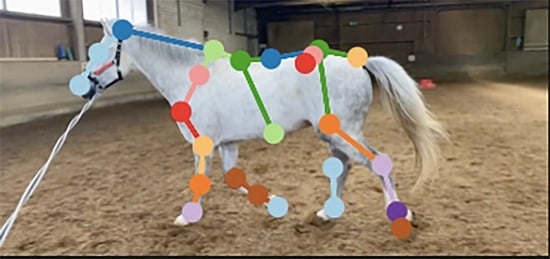
Artificial Intelligence for Lameness Detection in Horses—A Preliminary Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Technology
2.1.1. Deep Learning
2.1.2. Pose Estimation
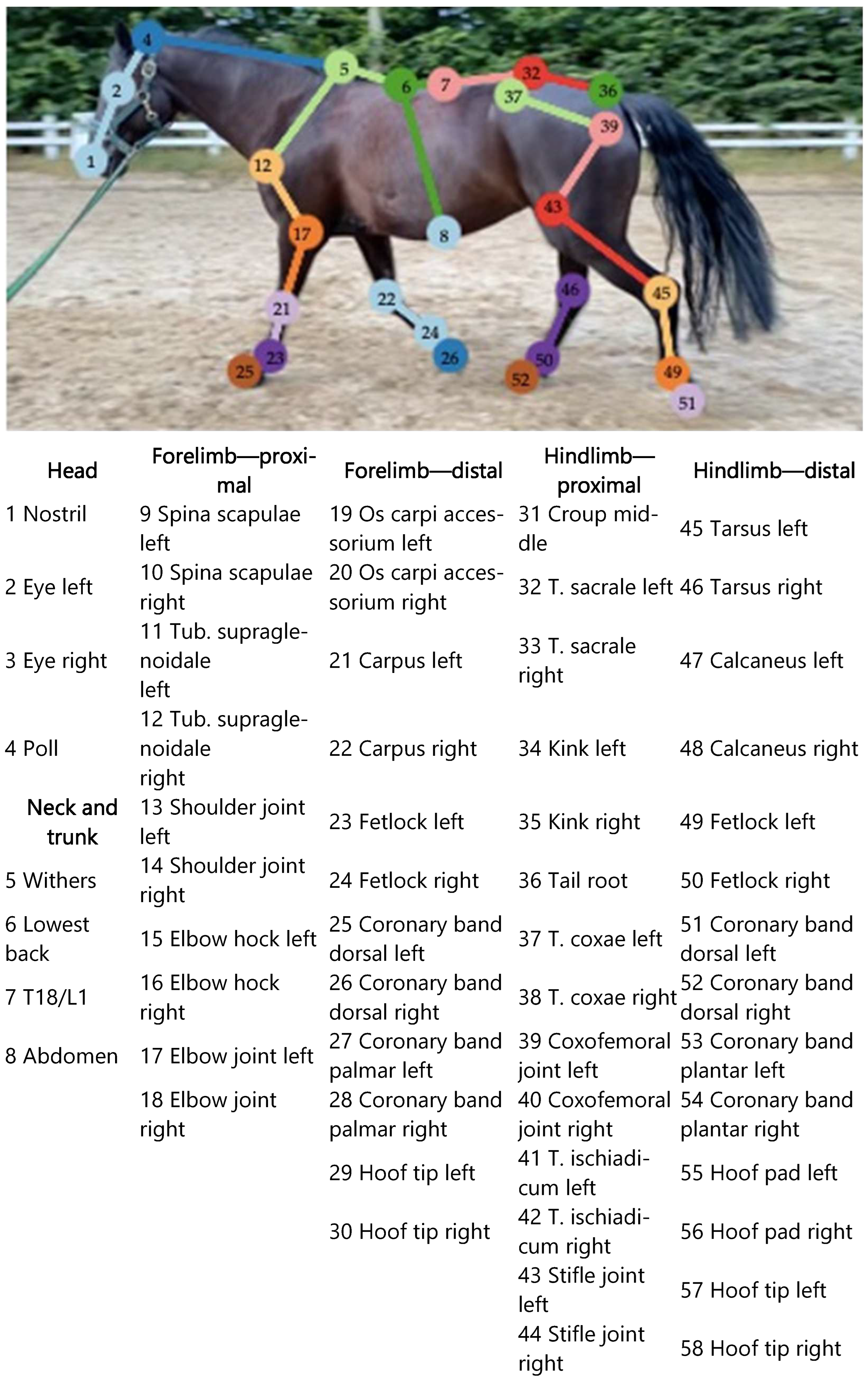
2.1.3. Reference Point Selection
2.2. Collection of Data in Investigated Groups
2.3. Training the Artificial Intelligence Tool Using Deep Learning
2.3.1. Data Processing and Training
2.3.2. Data Analysis and Measurements and Mathematical Calculations in Trot Videos
Forelimb Lameness
Hindlimb Lameness
Stifle Reference Point
Tuber coxae reference point
2.3.3. Statistical Analysis
3. Results
3.1. Forelimb Lameness
3.2. Hindlimb Lameness
3.2.1. Stifle Reference Point
3.2.2. Tuber Coxae Reference Point
4. Discussion
4.1. Forelimb Lameness
4.2. Hindlimb Lameness
4.2.1. Stifle
4.2.2. Tuber Coxae
4.3. Limitations
4.4. Outlook for the Future
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Reference Point in the Program | Anatomical Location | Reference Point in the Program | Anatomical Location |
|---|---|---|---|
| 1. Nostril | nostril | 30. Hoof tip right | hoof tip right forelimb |
| 2. Eye left | left eye | 31. Croup middle | midpoint between left and right tuber sacrale |
| 3. Eye right | right eye | 32. T. sacrale left | left tuber sacrale |
| 4. Poll | poll | 33. T. sacrale right | right tuber sacrale |
| 5. Withers | withers | 34. Kink left | midpoint between left tuber coxae and left tuber sacrale (view from behind) |
| 6. Lowest back | lowest part of the dorsal line | 35. Kink right | midpoint between right tuber coxae and right tuber sacrale (view from behind) |
| 7. T18/L1 | position of the 18th thoracic vertebra/first lumbar vertebra | 36. Tail root | tail root |
| 8. Abdomen | deepest part of the abdomen | 37. T. coxae left | left tuber coxae |
| 9. Spina scapulae left | scapular spine left | 38. T. coxae right | right tuber coxae |
| 10. Spina scapulae right | scapular spine right | 39. Coxofemoral joint left | left coxofemoral joint |
| 11 Tub. supraglenoidale left | supraglenoid tubercle left | 40. Coxofemoral joint right | right coxofemoral joint |
| 12. Tub. supraglenoidale right | supraglenoid tubercle right | 41. T. ischiadicum left | left ischial tuberosity |
| 13. Shoulder joint left | left shoulder joint | 42. T. ischiadicum right | right ischial tuberosity |
| 14. Shoulder joint right | right shoulder joint | 43. Stifle joint left | left stifle joint |
| 15. Elbow hock left | left elbow hock | 44. Stifle joint right | right stifle joint |
| 16. Elbow hock right | right elbow hock | 45. Tarsus left | left tarsus |
| 17. Elbow joint left | left elbow joint | 46. Tarsus right | right tarsus |
| 18. Elbow joint right | right elbow joint | 47. Calcaneus left | left calcaneus |
| 19. Os carpi accessorium left | left accessory carpal bone | 48. Calcaneus right | right calcaneus |
| 20. Os carpi accessorium right | right accessory carpal bone | 49. Fetlock left | fetlock left hindlimb |
| 21. Carpus left | left carpus | 50. Fetlock right | fetlock right hindlimb |
| 22. Carpus right | right carpus | 51. Coronary band dorsal left | dorsal part of the coronet band left hindlimb |
| 23. Fetlock left | fetlock left forelimb | 52. Coronary band dorsal right | dorsal part of the coronet band right hindlimb |
| 24. Fetlock right | fetlock right forelimb | 53. Coronary band plantar left | plantar part of the coronet band left hindlimb |
| 25. Coronary band dorsal left | dorsal part of the coronet band left forelimb | 54. Coronary band plantar right | plantar part of the coronet band right hindlimb |
| 26. Coronary band dorsal right | dorsal part of the coronet band right forelimb | 55. Hoof pad left | heel bulb left hindlimb |
| 27. Coronary band palmar left | palmar part of the coronet band left forelimb | 56. Hoof pad right | heel bulb right hindlimb |
| 28. Coronary band palmar right | palmar part of the coronet band left forelimb | 57. Hoof tip left | hoof tip left hindlimb |
| 29. Hoof tip left | hoof tip left forelimb | 58. Hoof tip right | hoof tip right hindlimb |
| Group 1 | Group 2 | Group 3 | ||
|---|---|---|---|---|
| Total Number | 65 | 22 | 8 | |
| Sex | Mare | 24 | 13 | 3 |
| Gelding | 41 | 9 | 5 | |
| Median Age (in years) | 13.8 | 11.6 | 12.4 | |
| Median Height (in meter) | 1.60 | 1.61 | 1.62 | |
| Breeds | Warmblood | 31 | 16 | 6 |
| Quarter Horse | 7 | |||
| PRE | 5 | |||
| Lusitano | 3 | |||
| Friese | 1 | |||
| Pinto | 2 | |||
| Knabstrupper | 1 | |||
| Arabian | 1 | 1 | ||
| Lewitzer | 1 | |||
| Haflinger | 1 | |||
| German Riding Pony | 12 | 5 | 2 | |
| Colours | Black | 8 | 1 | |
| Dark Bay | 10 | 7 | 3 | |
| Bay | 11 | 6 | 3 | |
| Chestnut | 15 | 5 | 2 | |
| Flaxen Chestnut | 3 | |||
| Buckskin | 1 | |||
| Palomino | 3 | |||
| Grey | 4 | |||
| White | 4 | 2 | ||
| Tobiano | 5 | |||
| Leopard | 1 | 1 | ||
| Classified by AI Non-Lame | Classified by AI Forelimb-Lame | Classified by AI Hindlimb-Lame Stifle | Total | |
|---|---|---|---|---|
| Clinically non-lame | 20 | 0 | 1 | 21 |
| Clinically forelimb-lame | 0 | 13 | 0 | 13 |
| Clinically hindlimb-lame stifle | 1 | 0 | 8 | 9 |
| Total | 21 | 13 | 9 | 43 |
Appendix B
| Forelimb Lameness | Clinically Forelimb-Lame | Clinically Non-Lame | Total | |
|---|---|---|---|---|
| AI classified as forelimb-lame | 13 | 0 | 13 | Positive predictive value |
| 1 | ||||
| AI classified as non-lame | 0 | 8 | 8 | Negative predictive value |
| 1 | ||||
| Total | 13 | 8 | 21 | |
| AI diagnostic test evaluation | Sensitivity of AI | Specificity of AI | Accuracy of AI | |
| 1 | 1 | 1 |
| Hindlimb Lameness Stifle | Clinically Hindlimb-Lame | Clinically Non-Lame | Total | |
|---|---|---|---|---|
| AI classified as hindlimb-lame | 8 | 1 | 9 | Positive predictive value |
| 0.888888889 | ||||
| AI classified as non-lame | 1 | 7 | 8 | Negative predictive value |
| 0.875 | ||||
| Total | 9 | 8 | 17 | |
| AI diagnostic test evaluation | Sensitivity of AI | Specificity of AI | Accuracy of AI | |
| 0.888888889 | 0.875 | 0.882352941 |
| Hindlimb Lameness Tuber Coxae | Clinically Hindlimb-Lame | Clinically Non-Lame | Total | |
|---|---|---|---|---|
| AI classified as hindlimb-lame | 3 | 3 | 6 | Positive predictive value |
| 0.5 | ||||
| AI classified as non- lame | 6 | 5 | 11 | Negative predictive value |
| 0.454545455 | ||||
| Total | 9 | 8 | 17 | |
| AI diagnostic test evaluation | Sensitivity of AI | Specificity of AI | Accuracy of AI | |
| 0.333333333 | 0.625 | 0.470588235 |
References
- Seitzinger, A.H. A comparison of the economic costs of equine lameness, colic, and equine protozoal myeloencephalitis (EPM). In Proceedings of the 9th International Symposium on Veterinary Epidemiology and Economics, Breckenridge, CO, USA, 6–11 August 2000; pp. 1–4. [Google Scholar]
- Nielsen, T.D.; Dean, R.S.; Robinson, N.J.; Massey, A.; Brennan, M.L. Survey of the UK veterinary profession: Common species and conditions nominated by veterinarians in practice. Vet. Rec. 2014, 174, 324. [Google Scholar] [CrossRef] [PubMed]
- USDA. Part I: Baseline Reference of 1998 Equine Health and Management; USDA: Washington, DC, USA, 1998; p. N280.898. [Google Scholar]
- Slater, J. National Equine Health Survey (NEHS) 2016; Blue Cross for Pets: Burford, UK, 2016. [Google Scholar]
- Muller-Quirin, J.; Dittmann, M.T.; Roepstorff, C.; Arpagaus, S.; Latif, S.N.; Weishaupt, M.A. Riding Soundness-Comparison of Subjective With Objective Lameness Assessments of Owner-Sound Horses at Trot on a Treadmill. J. Equine Vet. Sci. 2020, 95, 103314. [Google Scholar] [CrossRef] [PubMed]
- Dyson, S.; Pollard, D. Application of a Ridden Horse Pain Ethogram and Its Relationship with Gait in a Convenience Sample of 60 Riding Horses. Animals 2020, 10, 1044. [Google Scholar] [CrossRef] [PubMed]
- Starke, S.D.; May, S.A. Veterinary student competence in equine lameness recognition and assessment: A mixed methods study. Vet. Rec. 2017, 181, 168. [Google Scholar] [CrossRef]
- Keegan, K.G.; Dent, E.V.; Wilson, D.A.; Janicek, J.; Kramer, J.; Lacarrubba, A.; Walsh, D.M.; Cassells, M.W.; Esther, T.M.; Schiltz, P.; et al. Repeatability of subjective evaluation of lameness in horses. Equine Vet. J. 2010, 42, 92–97. [Google Scholar] [CrossRef]
- Fuller, C.J.; Bladon, B.M.; Driver, A.J.; Barr, A.R. The intra- and inter-assessor reliability of measurement of functional outcome by lameness scoring in horses. Vet. J. 2006, 171, 281–286. [Google Scholar] [CrossRef]
- Parkes, R.S.; Weller, R.; Groth, A.M.; May, S.; Pfau, T. Evidence of the development of ‘domain-restricted’ expertise in the recognition of asymmetric motion characteristics of hindlimb lameness in the horse. Equine Vet. J. 2009, 41, 112–117. [Google Scholar] [CrossRef]
- Arkell, M.; Archer, R.M.; Guitian, F.J.; May, S.A. Evidence of bias affecting the interpretation of the results of local anaesthetic nerve blocks when assessing lameness in horses. Vet. Rec. 2006, 159, 346–349. [Google Scholar] [CrossRef]
- Back, W.; Clayton, H.M. 1. History. In Equine Locomotion, 2nd ed.; van Weeren, P.R., Ed.; Saunders Elsevier: Edinburgh, UK; New York, NY, USA, 2013; pp. 1–30. [Google Scholar]
- Keegan, K.G. Evidence-based lameness detection and quantification. Vet. Clin. North Am. Equine Pract. 2007, 23, 403–423. [Google Scholar] [CrossRef]
- Bosch, S.; Serra Bragança, F.; Marin-Perianu, M.; Marin-Perianu, R.; van der Zwaag, B.J.; Voskamp, J.; Back, W.; van Weeren, R.; Havinga, P. EquiMoves: A Wireless Networked Inertial Measurement System for Objective Examination of Horse Gait. Sensors 2018, 18, 850. [Google Scholar] [CrossRef]
- Weishaupt, M.A.; Hogg, H.P.; Wiestner, T.; Denoth, J.; Stussi, E.; Auer, J.A. Instrumented treadmill for measuring vertical ground reaction forces in horses. Am. J. Vet. Res. 2002, 63, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Back, W.; Clayton, H.M. 9. Gait Adaption in Lameness. In Equine Locomotion; Buchner, H.H., Ed.; Saunders Elsevier: Edinburgh, UK; New York, NY, USA, 2013; pp. 175–197. [Google Scholar]
- Morris, E.; Seeherman, H. Redistribution of ground reaction forces in experimentally induced equine carpal lameness. In Equine Exercise Physiology; Wiley: Hoboken, NJ, USA, 1987; pp. 553–563. [Google Scholar]
- Byström, A.; Egenvall, A.; Roepstorff, L.; Rhodin, M.; Bragança, F.S.; Hernlund, E.; van Weeren, R.; Weishaupt, M.A.; Clayton, H.M. Biomechanical findings in horses showing asymmetrical vertical excursions of the withers at walk. PLoS ONE 2018, 13, e0204548. [Google Scholar] [CrossRef] [PubMed]
- Oosterlinck, M.; Pille, F.; Huppes, T.; Gasthuys, F.; Back, W. Comparison of pressure plate and force plate gait kinetics in sound Warmbloods at walk and trot. Vet. J. 2010, 186, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Keegan, K.G. Objective measures of lameness evaluation. In Proceedings of the American College of Veterinary Surgeons Symposium, National Harbor, MD, USA, 1–3 November 2012; pp. 127–131. [Google Scholar]
- Serra Bragança, F.M.; Rhodin, M.; van Weeren, P.R. On the brink of daily clinical application of objective gait analysis: What evidence do we have so far from studies using an induced lameness model? Vet. J. 2018, 234, 11–23. [Google Scholar] [CrossRef]
- Keegan, K.G.; Yonezawa, Y.; Pai, P.F.; Wilson, D.A.; Kramer, J. Evaluation of a sensor-based system of motion analysis for detection and quantification of forelimb and hind limb lameness in horses. Am. J. Vet. Res. 2004, 65, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Barrey, E. Methods, applications and limitations of gait analysis in horses. Vet. J. 1999, 157, 7–22. [Google Scholar] [CrossRef][Green Version]
- Rhodin, M.; Persson-Sjodin, E.; Egenvall, A.; Serra Bragança, F.M.; Pfau, T.; Roepstorff, L.; Weishaupt, M.A.; Thomsen, M.H.; van Weeren, P.R.; Hernlund, E. Vertical movement symmetry of the withers in horses with induced forelimb and hindlimb lameness at trot. Equine Vet. J. 2018, 50, 818–824. [Google Scholar] [CrossRef]
- Keegan, K.G.; Kramer, J.; Yonezawa, Y.; Maki, H.; Pai, P.F.; Dent, E.V.; Kellerman, T.E.; Wilson, D.A.; Reed, S.K. Assessment of repeatability of a wireless, inertial sensor-based lameness evaluation system for horses. Am. J. Vet. Res. 2011, 72, 1156–1163. [Google Scholar] [CrossRef]
- Titterton, D.; Weston, J. 4 Gyroscope Technology 1. In Strapdown Inertial Navigation Technology; Institution of Engineering and Technology: London, UK, 2004; pp. 59–112. [Google Scholar]
- van Weeren, P.R.; Pfau, T.; Rhodin, M.; Roepstorff, L.; Serra Bragança, F.; Weishaupt, M.A. Do we have to redefine lameness in the era of quantitative gait analysis? Equine Vet. J. 2017, 49, 567–569. [Google Scholar] [CrossRef]
- van Weeren, P.R.; Pfau, T.; Rhodin, M.; Roepstorff, L.; Serra Bragança, F.; Weishaupt, M.A. What is lameness and what (or who) is the gold standard to detect it? Equine Vet. J. 2018, 50, 549–551. [Google Scholar] [CrossRef]
- Mathis, A.; Mamidanna, P.; Cury, K.M.; Abe, T.; Murthy, V.N.; Mathis, M.W.; Bethge, M. DeepLabCut: Markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci. 2018, 21, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Kil, N.; Ertelt, K.; Auer, U. Development and Validation of an Automated Video Tracking Model for Stabled Horses. Animals 2020, 10, 2258. [Google Scholar] [CrossRef] [PubMed]
- Banzato, T.; Wodzinski, M.; Burti, S.; Osti, V.L.; Rossoni, V.; Atzori, M.; Zotti, A. Automatic classification of canine thoracic radiographs using deep learning. Sci. Rep. 2021, 11, 3964. [Google Scholar] [CrossRef] [PubMed]
- May, A.; Gesell-May, S.; Muller, T.; Ertel, W. Artificial intelligence as a tool to aid in the differentiation of equine ophthalmic diseases with an emphasis on equine uveitis. Equine Vet. J. 2022, 54, 847–855. [Google Scholar] [CrossRef]
- Insafutdinov, E.; Andriluka, M.; Pishchulin, L. ArtTrack: Articulated Multi-Person Tracking in the Wild. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Honolulu, HI, USA, 21–26 July 2017; pp. 6457–6465. [Google Scholar] [CrossRef]
- Cao, Z.; Simon, T.; Wei, S.-E. Realtime Multi-person 2D Pose Estimation Using Part Affinity Fields. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Honolulu, HI, USA, 21–26 July 2017. [Google Scholar]
- Nath, T.; Mathis, A.; Chen, A.C.; Patel, A.; Bethge, M.; Mathis, M.W. Using DeepLabCut for 3D markerless pose estimation across species and behaviors. Nat. Protoc. 2019, 14, 2152–2176. [Google Scholar] [CrossRef]
- Andriluka, M.; Pishchulin, L.; Gehler, P. 2D Human Pose Estimation: New Benchmark and State of the Art Analysis. In Proceedings of the IEEE Conference on computer Vision and Pattern Recognition, Columbus, OH, USA, 23–28 June 2014. [Google Scholar]
- Keegan, K.G.; Wilson, D.A.; Kramer, J.; Reed, S.K.; Yonezawa, Y.; Maki, H.; Pai, P.F.; Lopes, M.A. Comparison of a body-mounted inertial sensor system-based method with subjective evaluation for detection of lameness in horses. Am. J. Vet. Res. 2013, 74, 17–24. [Google Scholar] [CrossRef]
- Baxter, G.M.; Adams, O.R.; Stashak, T.S. Adams and Stashak’s Lameness in Horses, 6th ed.; Wiley-Blackwell: Chichester, UK; Ames, IA, USA, 2011; p. xxviii. 1242p. [Google Scholar]
- Ross, M.W.; Dyson, S.J. Diagnosis and Management of Lameness in the Horse, 2nd ed.; Saunders: St. Louis, MO, USA, 2011. [Google Scholar]
- Buchner, H.H.; Savelberg, H.H.; Schamhardt, H.C.; Barneveld, A. Head and trunk movement adaptations in horses with experimentally induced fore- or hindlimb lameness. Equine Vet. J. 1996, 28, 71–76. [Google Scholar] [CrossRef]
- Kramer, J.; Keegan, K.G.; Wilson, D.A.; Smith, B.K.; Wilson, D.J. Kinematics of the hind limb in trotting horses after induced lameness of the distal intertarsal and tarsometatarsal joints and intra-articular administration of anesthetic. Am. J. Vet. Res. 2000, 61, 1031–1036. [Google Scholar] [CrossRef]
- Buchner, H.H.; Savelberg, H.H.; Schamhardt, H.C.; Barneveld, A. Limb movement adaptations in horses with experimentally induced fore- or hindlimb lameness. Equine Vet. J. 1996, 28, 63–70. [Google Scholar] [CrossRef]
- May, S.A.; Wyn-Jones, G. Identification of hindleg lameness. Equine Vet. J. 1987, 19, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Church, E.E.; Walker, A.M.; Wilson, A.M.; Pfau, T. Evaluation of discriminant analysis based on dorsoventral symmetry indices to quantify hindlimb lameness during over ground locomotion in the horse. Equine Vet. J. 2009, 41, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G. Practical Statistics for Medical Research; Chapman & Hall/CRC: London, UK, 1999; p. XII. 611p. [Google Scholar]
- Buchner, H.H.; Savelberg, H.H.; Schamhardt, H.C.; Merkens, H.W.; Barneveld, A. Kinematics of treadmill versus overground locomotion in horses. Vet. Q. 1994, 16 (Suppl. 2), S87–S90. [Google Scholar] [CrossRef]
- Audigié, F.; Pourcelot, P.; Degueurce, C.; Geiger, D.; Denoix, J.M. Kinematic analysis of the symmetry of limb movements in lame trotting horses. Equine Vet. J. Suppl. 2001, 33, 128–134. [Google Scholar] [CrossRef]
- Kramer, J.; Keegan, K.G.; Kelmer, G.; Wilson, D.A. Objective determination of pelvic movement during hind limb lameness by use of a signal decomposition method and pelvic height differences. Am. J. Vet. Res. 2004, 65, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Leelamankong, P.; Estrada, R.; Mählmann, K.; Rungsri, P.; Lischer, C. Agreement among equine veterinarians and between equine veterinarians and inertial sensor system during clinical examination of hindlimb lameness in horses. Equine Vet. J. 2020, 52, 326–331. [Google Scholar] [CrossRef]
- Hardeman, A.M.; Serra Bragança, F.M.; Swagemakers, J.H.; van Weeren, P.R.; Roepstorff, L. Variation in gait parameters used for objective lameness assessment in sound horses at the trot on the straight line and the lunge. Equine Vet. J. 2019, 51, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Rhodin, M.; Pfau, T.; Roepstorff, L.; Egenvall, A. Effect of lungeing on head and pelvic movement asymmetry in horses with induced lameness. Vet. J. 2013, 198 (Suppl. 1), e39–e45. [Google Scholar] [CrossRef]
- Whay, H.R.; Main, D.C.; Green, L.E.; Webster, A.J. Assessment of the welfare of dairy cattle using animal-based measurements: Direct observations and investigation of farm records. Vet. Rec. 2003, 153, 197–202. [Google Scholar] [CrossRef]
- Whay, H.R.; Shearer, J.K. The Impact of Lameness on Welfare of the Dairy Cow. Vet. Clin. N. Am. Food Anim. Pract. 2017, 33, 153–164. [Google Scholar] [CrossRef]
- Haubro Andersen, P.; Bech Gleerup, K.; Wathan, J. Can a Machine Learn to See Horse Pain?: An Interdisciplinary Approach Towards Automated Decoding of Facial Expressions of Pain in the Horse. Animals 2018, 11, 1643. [Google Scholar] [CrossRef]

| Horse | Lameness | Degree of Lameness (1–5) | CL/CR | Classified Lame Based on AI | |||
|---|---|---|---|---|---|---|---|
| 1–2 | 3–4 | ||||||
| 1 | LH | X | CL CR | 42.50 44.17 | 1.67 | No | |
| 2 | RH | X | CL CR | 42.17 34.32 | 7.85 | Yes | |
| 3 | RH | X | CL CR | 31.16 29.69 | 1.47 | Yes | |
| 4 | LH | X | CL CR | 47.68 54.61 | 6.93 | Yes | |
| 5 | RH | X | CL CR | 43.32 42.09 | 1.23 | Yes | |
| 6 | LH | X | CL CR | 36.20 38.21 | 2.01 | Yes | |
| 7 | LH | X | CL CR | 48.03 51.12 | 3.09 | Yes | |
| 8 | LH | X | CL CR | 47.36 49.60 | 2.24 | Yes | |
| 9 | RH | X | CL CR | 49.90 38.55 | 11.35 | Yes | |
| Horse | CL/CR | Classified Sound Based on AI | ||
|---|---|---|---|---|
| 1 | CL CR | 38.27 37.76 | 0.51 | Yes |
| 2 | CL CR | 35.82 34.95 | 0.87 | Yes |
| 3 | CL CR | 40.44 39.75 | 0.69 | Yes |
| 4 | CL CR | 46.58 46.51 | 0.07 | Yes |
| 5 | CL CR | 46.09 45.93 | 0.16 | Yes |
| 6 | CL CR | 42.35 41.53 | 0.82 | Yes |
| 7 | CL CR | 37.43 36.19 | 1.24 | No |
| 8 | CL CR | 40.18 40.18 | 0. | Yes |
| Horse | Lameness | Degree of Lameness (1–5) | CL/CR | Classified Lame Based on AI | |||
|---|---|---|---|---|---|---|---|
| 1–2 | 3–4 | ||||||
| 1 | LH | X | CL CR | 11.29 19.21 | 7.92 | No | |
| 2 | RH | X | CL CR | 13.18 12.17 | 1.01 | No | |
| 3 | RH | X | CL CR | 11.81 14.62 | 2.81 | Yes | |
| 4 | LH | X | CL CR | 15.68 20.89 | 5.21 | No | |
| 5 | RH | X | CL CR | 9.22 9.95 | 0.73 | Yes | |
| 6 | LH | X | CL CR | 11.53 12.13 | 0.60 | No | |
| 7 | LH | X | CL CR | 13.69 15.02 | 1.33 | No | |
| 8 | LH | X | CL CR | 7.98 10.36 | 2.38 | No | |
| 9 | RH | X | CL CR | 11.18 11.27 | 0.09 | Yes | |
| Horse | CL/CR | Difference | Classified Sound Based on AI | |
|---|---|---|---|---|
| 1 | CL CR | 11.13 11.82 | 0.69 | Yes |
| 2 | CL CR | 12.06 11.55 | 0.51 | Yes |
| 3 | CL CR | 14.28 19.06 | 4.78 | No |
| 4 | CL CR | 13.99 14.49 | 0.50 | Yes |
| 5 | CL CR | 11.38 11.81 | 0.43 | Yes |
| 6 | CL CR | 9.96 10.64 | 0.68 | Yes |
| 7 | CL CR | 8.45 9.59 | 1.14 | No |
| 8 | CL CR | 8.15 9.79 | 1.64 | No |
| Test | True Positive | False Positive | False Negative | True Negative | SE (%) | SP (%) | AC (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|---|---|
| Forelimb AI | 13 | 0 | 0 | 8 | 100 | 100 | 100 | 100 | 100 |
| Hindlimb AI stifle | 8 | 1 | 1 | 7 | 88.9 | 87.5 | 88.2 | 88.9 | 87.5 |
| Hindlimb AI tuber coxae | 3 | 3 | 6 | 5 | 33.3 | 62.5 | 47.1 | 50 | 45.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feuser, A.-K.; Gesell-May, S.; Müller, T.; May, A. Artificial Intelligence for Lameness Detection in Horses—A Preliminary Study. Animals 2022, 12, 2804. https://doi.org/10.3390/ani12202804
Feuser A-K, Gesell-May S, Müller T, May A. Artificial Intelligence for Lameness Detection in Horses—A Preliminary Study. Animals. 2022; 12(20):2804. https://doi.org/10.3390/ani12202804
Chicago/Turabian StyleFeuser, Ann-Kristin, Stefan Gesell-May, Tobias Müller, and Anna May. 2022. "Artificial Intelligence for Lameness Detection in Horses—A Preliminary Study" Animals 12, no. 20: 2804. https://doi.org/10.3390/ani12202804
APA StyleFeuser, A.-K., Gesell-May, S., Müller, T., & May, A. (2022). Artificial Intelligence for Lameness Detection in Horses—A Preliminary Study. Animals, 12(20), 2804. https://doi.org/10.3390/ani12202804